Abstract
Background
Radiation is one of the most important stressors related to missions in space beyond Earth’s orbit. Epidemiologic studies of exposed workers have reported elevated rates of Parkinson’s disease. The importance of cognitive dysfunction related to low-dose rate radiation in humans is not defined. A meta-analysis was conducted of six cohorts in the Million Person Study (MPS) of low-dose health effects to learn whether there is consistent evidence that Parkinson’s disease is associated with radiation dose to brain.
Materials and methods
The MPS evaluates all causes of death among U.S. radiation workers and veterans, including Parkinson’s disease. Systematic and consistent methods are applied to study all categories of workers including medical radiation workers, industrial radiographers, nuclear power plant workers, atomic veterans, and Manhattan Projects workers at the Los Alamos National Laboratory and at Rocky Flats. Consistent methods for all cohorts are used to estimate organ-specific doses and to obtain vital status and cause of death.
Results
The meta-analysis include 6 cohorts within the MPS, consisting of 517,608 workers and 17,219,001 person-years of observation. The mean dose to brain ranged from 6.9 to 47.6 mGy and the maximum dose from 0.76 to 2.7 Gy. Five of the 6 cohorts revealed positive associations with Parkinson’s disease. The overall summary estimate from the meta-analysis was statistically significant based on 1573 deaths due to Parkinson’s disease. The summary excess relative risk at 100 mGy was 0.17 (95% CI: 0.05; 0.29).
Conclusions
Parkinson’s disease was positively associated with radiation in the MPS cohorts indicating the need for careful evaluation as to causality in other studies, delineation of possible mechanisms, and assessing possible implications for space travel as well as radiation protection guidance for terrestrial workers.
Keywords: Parkinson’s disease, Radiation epidemiology, Million Person Study
Introduction
The U.S. National Aeronautics and Space Administration (NASA) has developed detailed objective-based approaches to its human deep space exploration efforts as it plans missions to the moon and to Mars [1], [2], [3]. The lunar plan is to establish the deep space transportation systems for crew and cargo along with the first Artemis moonwalks followed by early human missions to Mars. Exposure to radiation is a primary risk factor to human health that remains a barrier to the safe exploration of space [4], [5], [6], [7]. There exists a complex radiation environment beyond Earth’s protective magnetic field [8], specifically comprised of galactic cosmic rays (GCR) and solar particle events (SPEs) that consist of high energy charged particles and resulting particle fragments from collisions with materials in spacecraft and the human body [7], [9], [10]. Depending on the type and length of mission, radiation exposures for astronauts during space exploration are expected to be relatively high with expected doses for a 180-day lunar mission to be about 60 mGy or so [7] and missions to Mars of about 600–1000 days estimated to be of the order of 300–450 mGy [11]. Beyond low earth orbit, space radiation consists of continuous exposure to GCR and sporadic eruptions from the sun that produce SPEs. The primary health risks associated with such exposures are cancer, cardiovascular disease, and central nervous system diseases [12], [13].
The National Council on Radiation Protection and Measurements (NCRP), at the request of NASA, evaluated the available literature on radiation exposures relevant to space missions and the potential for central nervous system (CNS) effects [13], [14], [15]. The scientific committee noted that some experimental studies using small animals showed CNS effects (both early and late neurological disorders) following relatively brief exposures to high-velocity heavy ions [16], as well as certain higher-dose human medical procedures (e.g., radiation therapy administration) delivering significant radiation exposures to the CNS resulting in significant acute and delayed effects. Exposure to ionizing radiation has also been linked to changes in cognitive functions affecting neurological integrity and damage to the central nervous system [17], [18], [19], [20]. In addition, high-linear energy transfer (LET) radiation exposures (e.g., from GCR simulations) in rodents have been associated with the potential to accelerate the development of CNS disorders including dementia, Alzheimer’s, and cognitive impairment [5], [14], [16]. Thus, there is concern about possible effects on astronauts that might impact mission performance and completion as well as potential risks for late occurring cognition-related outcomes. NCRP stressed the importance of assessing CNS outcomes in terrestrial analogs in humans, specifically suggesting additional studies of workers exposed to polonium, radium, plutonium, uranium, and americium. Such workers with intakes of radionuclides with potential to expose brain tissue to high-LET alpha particles (helium nuclei) should be studied for CNS effects including cognitive function and dementia-related disorders [14], [15], [21]. High-LET neutrons also were noted as a significant component of secondary radiation [14].
As described above, the types of radiation experienced in space as astronauts or as tourists are complex and include GCR, i.e., high-energy heavy ions, low-LET gamma radiation, protons and high-LET neutrons. The exposures can be continuous for several years for lengthy ventures beyond earth orbit. Although alpha-particle exposure to brain tissue is an imperfect analogue of high-energy heavy particles in space, terrestrial based epidemiologic studies provide another line of evidence that can be considered when making judgments for radiation protection guidance for flight crews on long missions in space [14], [15]. The strengths of human epidemiologic studies of high-LET exposures to brain and CNS effects include: high-LET radiation is received at a low dose rate (over years) in humans and not rodents; the exposure is to mixed fields of high-LET radiation and low-LET radiation (somewhat similar to the space environment); the energy deposition is similar for a wide range of particle types and energies; and human outcomes can be directly evaluated (e.g., the occurrence of Parkinson’s disease as well as quantitative measures of cognitive impairment) [6], [15]. While there are some similarities between high-LET alpha-particle exposure to brain tissue and high-LET exposures in space, there are significant dissimilarities [14]. Perhaps most importantly, while high energy ions and alpha particles emitted from radionuclides may share the same LET values, their track structures and energies are distinct with GCR energies and ranges being magnitudes higher. Nonetheless, while alpha particles emitted from radionuclides in brain as well as high-LET neutron exposures are an imperfect analogue for the high-energy heavy ions experienced in space, the results in human populations can be used in conjunction with animal studies, radiation response and concept models, and underlying assumptions related to human circumstances and space radiation for protection guidance when embarking on long-term missions [14], [15].
Following Alzheimer’s disease, Parkinson’s disease is the second-most common chronic and progressive, neurodegenerative disorder in the United States (U.S.). It is estimated that the prevalence for Parkinson’s disease is approximately 1 million people in the U.S. and 10 million people worldwide while about 90,000 new cases are diagnosed with the disease every year in the U.S. [22]. Individuals with Parkinson's often experience motor symptoms like tremor, muscle rigidity, slow movement, and difficulty with balance [23], [24]. Additionally, non-motor symptoms like depression, insomnia, constipation, and memory loss may also occur [24], [25]. Symptoms usually begin gradually and worsen over time. Although the causes of Parkinson's disease are unknown, people with Parkinson's disease typically show a decrease in the number of dopaminergic neurons located in a part of the brain called the substantia nigra [26]. When dopamine levels decrease, it results in abnormal brain activity, which can lead to the signs and symptoms associated with Parkinson's disease [27]. In addition, cholinergic neurons may also be involved in Parkinson’s disease [28], [29] with associated dysfunction displaying as cognitive decline, gait problems, falls, sleep disruption, and potential neuropsychiatric manifestations. Parkinson’s disease is characterized by the presence of Lewy bodies, which are clumps of proteins inside brain cells, and the aggregation of the alpha-synuclein protein within the Lewy bodies [26].
Because of a primary focus on cancer and cardiovascular outcomes, few epidemiologic studies have been conducted to explicitly explore the possible correlation of radiation exposure and Parkinson's disease or any neurological condition. In the few studies to date, however, there is growing evidence that Parkinson's disease may be associated with ionizing radiation [6], [30], [31], [32] and this has increased the interest in this area [33], [34].
The Study of One Million Radiation Workers and Veterans, known as the Million Person Study (MPS), includes over one million U.S. workers and veterans who had occupational monitoring for radiation exposure [6], [35], [36]. The MPS is designed to evaluate the level of health effects (cancer and non-cancer outcomes) on workers who receive protracted, chronic occupational exposures [36]. These workers are defined by occupational or service groups and were exposed to radiation at varying times from 1913 to the present, including: workers involved in the Manhattan project and at nuclear facilities of the U.S. Department of Energy [37], atomic veterans of the U.S. Department of Defense [38], nuclear power plant workers [39], industrial radiographers [6], [40], medical radiation workers [41], nuclear submariners and other U.S. Navy personnel [6], [42], and radium dial workers [43]. The project is a U.S. national effort with active cooperation of several federal agencies for support [44]. All MPS studies are now evaluating Parkinson’s disease as an outcome for dose-response evaluation [6], [31].
The purpose of the current study is to collate and describe MPS results on Parkinson’s disease risks and summarize Excess Relative Risk (ERR) estimates at 100 mGy brain dose for individual MPS cohorts using meta-analytic techniques. Such improved understanding of radiation risks in humans can provide benefit to NASA’s risk projection and mitigation approaches [5] as well as being relevant to radiation safety for workers in general [6].
Materials and methods
Million person study epidemiologic methods
The MPS applies harmonized and standardized radiation epidemiologic methodologies for each cohort for all aspects of the project with the ultimate aim in the future of pooling of all cohort data into one large analytic data resource [6]. Until then, results from individual MPS cohort studies are published as they are completed.
Cohort identification relies upon previously conducted studies [36] and the availability of central dosimetry repositories [6], [40], [45], [46], [47], [48], [49], [50]. Vital status determination and cause of death are obtained following a multistage approach with multiple sources of information that include state maintained vital statistics files and national databases of the Social Security Administration Death Master File (SSA-DMF), the National Death Index (NDI) and the SSA Service for Epidemiological Researchers (SSA-SER), along with publicly available data, ancestry services, and death certificates [51]. Vital status has been confirmed for over 90% of all study subjects in cohorts analyzed to date, and the ascertainment of cause of death is typically over 96%. Statistical adjustments are made for smoking status or a reliable surrogate such as area-level derivation of education [42], self-reported education, military rank, and/or worker job categories as measures of socioeconomic status. Underlying, and when available, contributing causes of death are coded according to the International Classification of Diseases (ICD) edition in place at the time of death, spanning six ICDs, from ICD5 to ICD10. For presentation purposes and analyses, cause of death (COD) for each worker is mapped to the comparable COD in ICD9 [51]. In early MPS studies a combined neurodegenerative disease outcome which included dementia, Alzheimer’s disease, Parkinson’s disease, and motor neuron disease and was assigned ICD9 290.0-290.4, 331.0, 332, 335.2 [52], [53]. Later MPS studies have evaluated each outcome separately with Parkinson’s disease (ICD9 332) specifically assessed [6], [38], [39], [40], [54], [55].
High-quality radiation epidemiology requires comprehensive organ dose assessments and reconstructions for each individual under study. The NCRP has provided specific guidance for deriving organ doses and their uncertainty for epidemiologic studies, with a focus on the MPS. This guidance was consistently employed for each study cohort in the MPS [56], [57]. Annual organ dose estimates are assigned based on defined exposure scenarios, exposure pathways, radiation monitoring devices using personal dosimeters, individual bioassay data, and by employing the latest guidance and dose coefficients from the NCRP and the International Commission on Radiological Protection [21], [49], [50], [56], [58], [59], [60], [61], [62]. In addition, the NCRP has recently developed improved kinetic and anatomical models for brain dosimetry for internally deposited radionuclides [15], [63].
Standardized mortality ratios (SMR) are used to compare observed deaths with the numbers expected in the U.S. general population accounting for age, sex, and calendar year of observation. Exact 95% Poisson confidence intervals (CIs) for the SMRs [64] and for the ratio of SMRs [65], [66] are computed.
Internal (within-cohort) analyses are conducted to account in part for the healthy worker effect that is often present in occupational studies when comparisons are made with the general population [67], [68]. These analyses were conducted using Cox proportional hazards models [69] and Poisson regression models [70], [71] with Parkinson’s disease mortality as the outcome. The dose-response models were based on the estimated brain doses in mGy with the primary analysis using categories of radiation dose to brain as the exposure. While a radiation weighting factor (DWF) of 1 was assumed for all alpha-particle related doses, DWFs of 2.5 and 16 were assumed for thermal and non-thermal neutrons, respectively [55]. Dose-response functions for continuous measurements of radiation dose were also modeled as linear ERR functions. All models included adjustment for sex, year of birth, and a measure or surrogate measure of socioeconomic status (SES) [42], [70]. Age was used as the timescale for the hazard function. Cox analyses were conducted using SAS/STAT software (version 9.4 of the SAS System for Windows, SAS Institute Inc., Cary, NC). Cox and Poisson ERR models were also constructed using EPICURE software [70], [71]. The PEANUTS program for ERRs and corresponding 95% confidence intervals (CI) included the same covariates as the final Cox models (sex, year of birth, and SES).
Meta-analysis
To quantitatively combine the ERR estimates for the association between Parkinson’s disease and radiation brain dose from six MPS cohorts, a meta-analysis was conducted. The meta-analysis combines the results of individual studies to obtain a summary estimate of risk that is assumed to be more informative than from a single study. A meta-analysis takes into account the sample size and precision of the included individual studies providing a summary estimate that is a weighted average of the individual study results. The quality of the studies included in a meta-analysis is an important consideration when interpreting the validity of the summary result. For the MPS, the quality of each study is similar and high with regard to dosimetry, vital status determination, population identification and completeness, and transparent statistical methods.
This meta-analysis was conducted to summarize the ERRs at 100 mGy brain dose from 6 MPS cohorts. The ERR at 100 mGy brain dose and 95% confidence intervals (CIs) were used with and without the assumption of heterogeneity of risks, and statistical tests for heterogeneity were computed. In practical terms, this means that a Fixed Effects meta-analysis model which considered only variations in risk within cohorts was applied, as was a Random Effects model which considers within cohort variation plus between cohort variations. The meta-analysis was done on the original scale (i.e., with no conversion to the logarithm of the relative risk before pooling) because all the included risks at 100 mGy have highly symmetrical confidence intervals about the central risk estimate. A summary estimate, which is the pooled, inverse-variance weighted mean risk, was calculated from the 6 cohorts. Cochran’s Q statistic (and corresponding p-value) and the I2 index were calculated to test for heterogeneity, and the DerSimonian-Laird method [72], [73] for the Random Effects model was applied for pooling heterogeneous groups of studies and for obtaining the overall variance on the summary risk estimate from the meta-analysis. These methods have been used previously for radiation-related risk assessments across various epidemiology study cohorts [74], [75], [76], [77], [78], [79]. All meta-analyses statistics were conducted using Microsoft® Excel software (version 16.57, Microsoft® Excel for Mac, Microsoft, Redmond, WA).
Results
MPS cohorts evaluating Parkinson’s disease
There are 8 MPS cohort studies that have evaluated Parkinson’s disease mortality to date. Two of the cohorts, Mound workers and Mallinckrodt uranium processing workers, had few deaths attributable to Parkinson’s disease, so that only a combined neurodegenerative category was evaluated that included dementia, Alzheimer’s disease, Parkinson’s disease, and motor neuron diseases [52], [53]. Accordingly, the Mound and Mallinckrodt studies were not included in the meta-analysis. Table 1 lists the six cohorts included in the Parkinson’s disease mortality meta-analysis: nuclear power plant workers [39], industrial radiography workers [6], [40], medical radiation workers [41], Los Alamos National Laboratory (LANL) workers [55], Rocky Flats workers [54], and atomic veterans [38]. These represent 517,608 workers followed from as early as 1945 through as late as 2019 with a combined total of 17,219,001 person-years of follow-up.
Table 1.
Million Person Study cohorts evaluating Parkinson’s disease mortality as a distinct outcome.
| MPS cohort | No. workers | Study follow-up | Person-years of follow-up |
|---|---|---|---|
| Nuclear Power Plant Workers (NPP) | 135,193 | 1957–2011 | 4,079,620 |
| Industrial Radiography Workers (IR) | 123,401 | 1969–2019 | 3,416,647 |
| Medical Radiation Workers (MRW) | 109,019 | 1965–2016 | 2,779,838 |
| Los Alamos National Laboratory Workers (LANL) | 26,328 | 1943–2017 | 1,181,472 |
| Rocky Flats Workers (RF) | 9,397 | 1951–2017 | 391,118 |
| Atomic Veterans (A-Vets)* | 114,270 | 1945–2010 | 5,370,306 |
| Total | 517,608 | – | 17,219,001 |
The total number of veterans in the A-Vets cohort was 114,270, however, the total number utilized in the dose response was 113,806, because of the exclusion of veterans with unknown doses.
Dose to brain by MPS cohort
The 6 MPS cohorts that evaluated Parkinson’s disease mortality included 4 cohorts that received low-LET gamma and x-ray exposures and 2 cohorts characterized by low-LET and high-LET exposures. The high-LET exposures included intakes of radionuclides and external neutrons. The internal intakes of radionuclides included plutonium, americium, polonium, and uranium, along with some potential for fission- and activation-products [6], [14], [40], [55], [80]. Resulting doses from all components of exposure were summed to estimate annual brain doses (mGy) for each individual in each cohort. Table 2 lists the mean (mGy), median (mGy), standard deviation (mGy), percent of workers with 100 mGy, and maximum (Gy) cumulative brain dose estimates for cohorts included in the meta-analysis. Across the cohorts, mean cumulative brain doses ranged from 6.9 mGy for the atomic veterans to 47.6 mGy for Rocky Flats workers. Maximum cumulative brain doses ranged from 0.76 to 2.7 Gy.
Table 2.
Estimated mean (mGy), median (mGy), and other brain dose statistics for 6 cohorts within the Million Person Study.
| MPS cohort | Mean (mGy) | Median (mGy) | STD (mGy) | Percent 100 mGy | Maximum (mGy) |
|---|---|---|---|---|---|
| Low-LET | |||||
| Nuclear Power Plant Workers (NPP) | 33.2 | 17.2 | 45.5 | 6.58 | 834 |
| Industrial Radiography Workers (IR) | 11.9 | 1.1 | 31.2 | 2.10 | 977 |
| Medical Radiation Workers (MRW) | 18.9 | 9.8 | 27.7 | 1.15 | 1080 |
| Atomic Veterans (A-Vets) | 6.9 | 2.6 | 17.7 | 0.05 | 2654 |
| High-LET and Low-LET | |||||
| Los Alamos National Laboratory Workers (LANL) | 11.6 | 0.8 | 39.4 | 1.78 | 760 |
| Rocky Flats Workers (RF) | 47.6 | 13.2 | 89.0 | 11.7 | 831 |
LANL and Rocky Flats workers received both high-LET and low-LET exposures that contributed to their cumulative brain dose. The high-LET component ranged from 10 to 15 percent of total brain dose assuming a dose weighting factor of 1 for alpha particles. For LANL workers, the contribution of high-LET radiation to total cumulative brain dose was 14.6%. The contributions were 13.6% from high-LET neutrons, 1.0% from high-LET plutonium alpha particles, 84.0% from low-LET photons, and 1.4% from low-LET tritium. For Rocky Flats workers, the contribution of high-LET radiations to total cumulative brain dose was 9.6%. The contributions were 9.1% from high-LET neutrons, 0.45% from high-LET plutonium (238Pu, 239Pu, and 241Pu) alpha particles, 0.01% from high-LET americium and progeny alpha particles, 0.03% from uranium alpha particles, and 90.4% from low-LET photons.
Radiation dose response and Parkinson’s disease mortality
Table 3 lists the number of Parkinson’s disease deaths, SMRs and ERRs at 100 mGy brain dose for Parkinson’s disease among 6 cohorts within the MPS. There were 1573 workers with an underlying cause of death coded as Parkinson’s disease. SMRs comparing observed deaths with the numbers expected in the general population in the U.S. ranged from as low as 0.82 (95% CI: 0.66;1.02; n=87) for the medical radiation worker cohort to as high as 1.16 (95% CI: 1.00;1.34; n=197) for the Los Alamos National Laboratory workers. While none of the SMRs were significantly different from 1.0 (all p > 0.05), 5 of the 6 cohorts had a positive ERR at 100 mGy even though, similarly, none were statistically significant. The ERR at 100 mGy for the atomic veteran cohort was negative but not statistically different from the other study estimates because of the wide confidence interval. For comparison, the ERR at 100 mGy brain dose for the combined neurodegenerative disease category (dementia, Alzheimer’s disease, Parkinson’s disease and motor neuron diseases) was 0.23 (95% CI: −0.01; 0.54) for Mound workers (4977 workers, 22 cases) and −0.06 (95% CI: −0.18; 0.06) for Mallinckrodt uranium processing workers (2514 workers, 93 cases). Mound workers had the potential for high-LET intakes of polonium and plutonium [52] as well as neutron exposure, and Mallinckrodt workers had the potential for high-LET intakes of uranium and radium [53].
Table 3.
Standard mortality ratio (SMR) and Excess Relative Risk (ERR]) at 100 mGy brain dose) for Parkinson’s disease among 6 cohorts within the Million Person Study.
| MPS cohort* | No. workers | No. Parkinson’s disease deaths | SMR (95% CI) | ERR at 100 mGy (95% CI) |
|---|---|---|---|---|
| NPP | 135,193 | 140 | 0.90 (0.76; 1.06) | 0.24 (−0.02; 0.50) |
| IR | 123,401 | 235 | 0.96 (0.84; 1.09) | 0.24 (−0.13; 0.61) |
| MRW | 109,019 | 87 | 0.82 (0.66; 1.02) | 0.17 (−0.20; 0.54) |
| LANL | 26,328 | 193 | 1.16 (1.00; 1.34) | 0.16 (−0.07; 0.40) |
| RF | 9397 | 57 | 1.06 (0.80; 1.38) | 0.13 (−0.11; 0.37) |
| A-Vets | 113,806 | 861 | 0.94 (0.88; 1.01) | −0.22 (−0.90; 0.46) |
| Total | 517,608 | 1573 | 0.96 (0.86; 1.07) | 0.17 (0.05; 0.29) |
Abbreviations given in Table 2.
Meta-analysis for Parkinson’s disease mortality risk
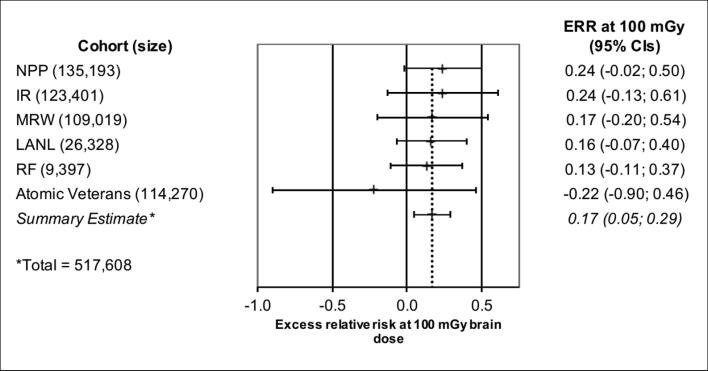
In the primary Random Effects model, the summary meta-analysis ERR at 100 mGy was 0.17 (95% CI: 0.05; 0.29), the I2 index was 0.0% (indicating that none of the variability in the radiation related effect size estimates is due to the differences between individual cohorts) and the p-value for the Cochran’s Q-test was 0.88 (indicating no statistically significant heterogeneity between the individual cohorts). A forest plot for the primary Random Effects model is shown in Fig. 1. The individual study weights in descending order were 27%, 26%, 22%, 11%, 11% and 3%, for weighting the risks from LANL, RF, NPP, IR MRW and A-Vets, respectively. In addition, an assessment of the individual impact of studies on the summary estimate (Table 4) shows that the findings are robust to the effects of any single study. For example, removing the A-Vets cohort had little effect on the overall summary meta-analysis estimate. The Fixed Effects model provided very similar results.
Figure 1.
Forest plot and result of the summary ERR at 100 mGy brain dose from a Random Effects meta-analysis for Parkinson’s disease mortality based on 6 of the Million Person Study (MPS) epidemiologic studies. Excess relative risks at 100 mGy brain dose with 95% confidence intervals for Parkinson’s disease mortality are plotted on rows corresponding with each MPS study cohort. The summary estimate is displayed in the last row (and is indicated by the vertical dotted line). The total number of veterans in the A-Vets cohort was 114,270, however, the total number utilized in the dose response was 113,806, because of the exclusion of veterans with unknown doses. Abbreviations: NPP, nuclear power plant; IR, industrial radiographer; MRW, medical radiation workers; LANL, Los Alamos Nuclear Laboratory; RF, Rocky Flats; ERR, excess relative risk.
Table 4.
Meta-analysis estimate of ERR at 100 mGy (95% CI) after removing the indicated individual study from the analysis.
| MPS cohort removed* | ERR at 100 mGy (95% CI) |
|---|---|
| None, i.e., overall summary estimate | 0.17 (0.05; 0.29) |
| NPP | 0.15 (0.01; 0.28) |
| IR | 0.16 (0.03; 0.29) |
| MRW | 0.17 (0.04; 0.29) |
| LANL | 0.17 (0.07; 0.31) |
| RF | 0.18 (0.04; 0.32) |
| A-Vets | 0.18 (0.05; 0.30) |
Abbreviations given in Table 2.
Discussion
The meta-analysis of 6 MPS cohort studies found a statistically significant ERR at 100 mGy brain dose for Parkinson’s disease mortality: 0.17 (95%CI: 0.05; 0.29) (Fig. 1). This provocative finding is based on over 50% of the workers in the MPS, i.e., over half a million workers, but needs to be confirmed in ongoing follow-up of these and other MPS cohorts such as Hanford, Savanah River, Fernald, and Oak Ridge Laboratories (X-10, Y-12, and K-25), and nuclear submariners. Future MPS work will include an overall pooled estimate of these harmonized MPS cohorts. This harmonization effort is underway with development of statistical software to enable assessments of up to 50 million person-years of workers with individual organ doses, job histories, demographic and clinical information, and vital status outcomes. The pooling is possible because the basic datasets for all cohorts provide the same or very similar categorizations of worker characteristics, time-dependent organ-dose reconstruction, and outcomes based on the same criteria [56].
A preliminary pooled analysis combining 3 MPS cohorts, i.e., nuclear power workers, industrial radiographers, and medical radiation workers (representing 367,722 total workers) resulted in an ERR at 100 mGy brain dose for Parkinson’s disease of 0.30 (95% CI: 0.08; 0.56) for 354 deaths [31], [40], a value consistent with the summary estimate in this present study as would be expected since these combined cohorts represent 3 of the 6 cohorts included in the meta-analysis and provided 60% of the total person-years of observation.
Other studies have recently reported associations of increased risk of CNS outcomes with radiation. A study among Russian Mayak workers for cumulative gamma-ray dose to the brain reported an evaluation of 300 incident Parkinson’s diagnoses with an ERR per Gy of 1.02 (95% CI:0.59; 1.63) [30]. A recent update of a French nuclear worker study [32] evaluated 124 deaths due to Parkinson’s disease with an ERR per Gy of -1.30 (95% CI: not estimated, 7.44). An overlapping meta-analysis of 3 MPS mortality studies along with the Mayak incidence study reported a significant summary relative risk of Parkinson’s disease at 100 mGy of 1.12 (95% CI 1.07, 1.17) [33].
Death certificates do not capture all incident cases of Parkinson's disease which conceivably could lead to decreased statistical power [81] as well as any bias related to cause of death coding when there are multiple contributing causes. Further, the quality or accuracy of death certificate coding for Parkinson’s disease may not be as high as for incident diagnoses, suggesting the importance of conducting incidence studies. To this end, NASA has recently funded studies to link the MPS rosters with the U.S. Centers for Medicare and Medicaid Services (CMS) assessment and claims databases that include information on claims-based disease incidence as well most chronic conditions (e.g., dementia, Alzheimer’s, Parkinson’s), other potentially related medical factors (e.g., tobacco use, obesity, diabetes), and cognitive function scores from standardized tests for workers admitted to nursing home facilities [6], [82]. Linkages to these databases for the MPS cohorts are ongoing, and analyses for each cohort based on both incidence and mortality data will be forthcoming.
Specific mechanisms for Parkinson’s disease have not yet been determined but may be associated with inflammatory processes in general, neural inflammation specifically, autophagy, oxidative stress, mitochondrial dysfunction, and/or impaired protein aggregation may all be involved in the disease pathogenesis [83], [84]. However, no specific mechanism has been proposed or identified that definitively addresses the possible causes of a potential correlation of radiation exposure and Parkinson's disease mortality. Interestingly, cigarette smoking is consistently seen to reduce the risk of Parkinson's disease in large population studies [85], [86] which may, perhaps, be associated with nicotinic acetylcholine receptor functioning [28]. Within the MPS cohorts, separate analyses have shown that the SMR for Parkinson’s disease for workers or veterans with higher levels of education were higher than the SMR for those with lower levels of education (as a surrogate for socioeconomic status), consistent with the expectation that those who are unlikely to smoke are at higher risks of Parkinson’s disease than likely smokers [6], [38], [40], [85], [86]. The associations with genetic factors, tobacco use and environmental exposures, such as pesticides [84], suggest that there may be unknown lifestyle and environmental factors that could be contributing to or confounding these Parkinson’s disease mortality findings. While other forms of neurological disorders such as dementia, Alzheimer's, and motor neuron disease, such as ALS, have not been found to be associated with radiation exposure, they too remain areas of ongoing and future research.
In conclusion, Parkinson’s disease is a complex chronic and progressive neurodegenerative disease associated with dopamine deficiency and several other factors including environmental and genetic influencers of potential risk [84]. Evidence from occupational studies, including those performed as part of the MPS, have correlated chronic ionizing radiation exposures with increased mortality rates of Parkinson’s disease. Such CNS outcomes that have the potential for cognitive impairment and are, if substantiated, highly relevant for protection guidance for radiation workers and especially for astronauts. Research efforts should be initiated to elucidate putative radiation biologic and/or pathologic mechanisms, to identify potential hallmarks and disease biomarkers, and further evaluate the impact of smoking assessment. Additionally, epidemiologic studies should explore the inclusion of the incidence of Parkinson’s along with mortality. More statistically precise estimates of radiation risk following chronic worker exposures [87] are anticipated from future pooling of MPS cohorts for Parkinson’s disease and other CNS-related mortality and incidence outcomes associated with cognitive detriment [6].
Data Availability Statement
The code used to extract the data is distributed by the authors as open-source. The patient data can not be made available on request due to privacy/ethical restrictions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The content of this article does not necessarily represent the views of the National Council on Radiation Protection and Measurements, Vanderbilt University Medical Center, Memorial Sloan Kettering Cancer Center, U.S. Department of Energy, National Aeronautics and Space Administration, or the U.S. Navy.
Acknowledgments
The National Council on Radiation Protection and Measurements acknowledges the exceptional assistance on dosimetry reconstructions for these MPS cohorts from the Center for Radiation Protection Knowledge at Oak Ridge National Laboratories (Rich Leggett, Caleigh Samuels, Keith Eckerman), Memorial Sloan Kettering Cancer Center (Michael Bellamy, David Bierman) as well as staff support (Kathy Held, President NCRP; Laura Atwell, Director of Operations NCRP). This study, as part of the MPS, was supported in part by a research grant from the U.S. Department of Energy (DOE) (Grant No. DE-SC0008944) awarded to the National Council on Radiation Protection and Measurements, which included interagency support from the U.S. Nuclear Regulatory Commission (NRC), the U.S. Environmental Protection Agency and the National Aeronautics and Space Administration (NASA); and more recent DOE Grants (No. DE-AU0000042 and DE-AU0000046). Additional support included grants from the NRC (NRC-HQ‐60‐14‐G‐0011); the Centers for Disease Control and Prevention (5UE1EH000989, 5NUE1EH001315); NASA (NNX15AU88G, 80NSSC17M0016, 80NSSC17M0016, 80NSSC19M0161), and a Discovery Grant from the Vanderbilt-lngram Cancer Center (Center no. 404-357-9682). Additional funding was received from an NIH/NCI Cancer Center Support Grant (P30 CA008748) awarded to Sloan-Kettering Institute for Cancer Research. Contract support also was received from the Naval Sea Systems Command (N00024-17-C-4322) for dosimetry linkage.
References
- 1.National Aeronautics and Space Administration (NASA). Moon to Mars Objectives. Washington DC: NASA; 2022. https://www.nasa.gov/sites/default/files/atoms/files/m2m-objectives-exec-summary.pdf
- 2.National Aeronautics and Space Administration (NASA). NASA’s Moon to Mars Architecture: a summary of the 2022 architecture concept review process and results. Washington DC: NASA; 2023. https://www.nasa.gov/sites/default/files/atoms/files/m2m-architecture-executive-summary.pdf
- 3.National Aeronautics and Space Administration (NASA). NASA’s Moon to Mars Strategy and Objectives Development: a blueprint for sustained human presence and exploration throughout the Solar System. Washington DC: NASA; 2023. https://www.nasa.gov/sites/default/files/atoms/files/m2m_strategy_and_objectives_development.pdf
- 4.Boice J.D., Jr. Space: The final frontier – research relevant to Mars. Health Phys. 2017;112(4):392–397. doi: 10.1097/HP.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 5.Boice J.D. The Million Person Study relevance to space exploration and Mars. Int J Radiat Biol. 2022;98(4):551–559. doi: 10.1080/09553002.2019.1589020. [DOI] [PubMed] [Google Scholar]
- 6.Boice J.D., Jr, Quinn B., Al-Nabulsi I., Ansari A., Blake P.K., Blattnig S.R., et al. A million persons, a million dreams: a vision for a National Center of Radiation Epidemiology and Biology. Int J Radiat Biol. 2022;98(4):795–821. doi: 10.1080/09553002.2021.1988183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Academies of Sciences, Engineering, and Medicine (NASEM). Space radiation and astronaut health: managing and communcating cancer risks. Washington DC: NASEM; 2021. https://nap.nationalacademies.org/login.php?record_id=26155
- 8.Simonsen LC, Nealy JE. Radiation protection for human missions to the Moon and Mars. NASA Technical Paper 3079. Hampton, VA: NASA; 1991. https://ntrs.nasa.gov/api/citations/19910008686/downloads/19910008686.pdf
- 9.Cucinotta FA, Kim MY, Chappell LJ. Space radiation cancer risk projections and uncertainties-2012. NASA/TP-2013-217375. Houston, TX: NASA; 2013. https://three.jsc.nasa.gov/articles/TP_2013_CancerRisk.pdf
- 10.Kronenberg A., Cucinotta F.A. Space radiation protection issues. Health Phys. 2012;103(5):556–567. doi: 10.1097/HP.0b013e3182690caf. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen LC, Slaba TC. Ensemble methodologies for astronaut cancer risk assessment in the face of large uncertainties. NASA/TP-2020-5008710. Hampton, VA: NASA; 2020. https://spaceradiation.larc.nasa.gov/nasapapers/2020/5008710.pdf
- 12.National Council on Radiation Protection and Measurements (NCRP). Radiation protection for space activities: supplement to previous recommendations. NCRP Commentary 23. Bethesda MD: NCRP; 2014.
- 13.National Council on Radiation Protection and Measurements (NCRP). Potential for central nervous system effects from radiation exposure in space activities, phase I: overview. NCRP Commentary 25. Bethesda MD: NCRP; 2016.
- 14.National Council on Radiation Protection and Measurements (NCRP). Radiation exposures in space and the potential for central nervous system effects: phase II. NCRP Report 183. Bethesda MD: NCRP; 2019.
- 15.National Council on Radiation Protection and Measurements (NCRP). Development of kinetic and anatomical models for brain dosimetry for internally deposited radionuclides. NCRP Commentary 31. Bethesda MD: NCRP; 2021.
- 16.Britten R.A., Wellman L.L., Sanford L.D. Progressive increase in the complexity and translatability of rodent testing to assess space-radiation induced cognitive impairment. Neurosci Biobehav Rev. 2021;126:159–174. doi: 10.1016/j.neubiorev.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Cuccurullo V., DiSasio G.D., Cascini G.L., Gatta G., Bianco C. The molecular effects of ionizing radiations on brain cells: radiaition necrosis vs. tumor recurrence. Diagnostics (Basel) 2019;9(4):127. doi: 10.3390/diagnostics9040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalchuk A., Kolb B. Low dose radiation effects on the brain – from mechanisms and behavioral outcomes to mitigation strategies. Cell Cycle. 2017;16(3):1266–1270. doi: 10.1080/15384101.2017.1320003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markesbery W.R., Lovell M.A. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol. 2007;64(7):954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- 20.Dropcho E.J. Central nervous system injury by therapeutic irradiation. Neurol Clin. 1991;9(4):969–988. [PubMed] [Google Scholar]
- 21.Leggett R.W., Eckerman K.F., Bellamy M. MPS dose reconstruction for internal emitters: some site-specific issues and approaches. Int J Radiat Biol. 2022;98(4):631–643. doi: 10.1080/09553002.2018.1558302. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson’s Foundation. Statistics. Miami, FL: Parkinson’s Foundation; 2023. https://www.parkinson.org/understanding-parkinsons/statistics?gad=1&gclid=Cj0KCQjwpPKiBhDvARIsACn-gzAUVZuHIhKdjopbsPzDs2qfIlO8lv3aXPjBGmEpAkENc7YAroL4UeQaAgrtEALw_wcB&utm_source=google&utm_medium=adgrant&utm_campaign=&utm_term=parkinson%27s%20disease%20statistics
- 23.World Health Organization (WHO). Parkinson disease. Geneva: WHO; 2022. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease.
- 24.Michael J. Parkinson’s 101. Michael J. Fox Foundation; New York, NY: 2023. Fox Foundation for Parkinson’s Research.https://www.michaeljfox.org/parkinsons-101 [Google Scholar]
- 25.Davis Phinney Foundation . David Phinney Foundation; Louisville CO: 2017. What are the non-motor symptoms of Parkinson’s?https://davisphinneyfoundation.org/what-are-the-non-motor-symptoms-of-parkinsons/ [Google Scholar]
- 26.Bates C.A., Zheng W. Brain disposition of alpha-Synuclein: roles of brain barrier systems and implications for Parkinson’s disease. Fluids Barriers CNS. 2014;11:17. doi: 10.1186/2045-8118-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo Clinic. Parkinson’s disease. Mayo Foundation for Medical Education and Research. Rochester MN: Mayo Clinic; 2023. https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055
- 28.Yu L.F., Zhang H.K., Caldarone B.J., Eaton J.B., Lukas R.J., Kozikowski A.P. Recent developments in novel antidepressants targeting α4β2-nicotinic acetylcholine receptors. J Med Chem. 2014;57(20):8204–8223. doi: 10.1021/jm401937a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquini J., Brooks D.J., Pavese N. The cholinergic brain in Parkinson’s disease. Mov Disord Clin Pract. 2021;8(7):1012–1026. doi: 10.1002/mdc3.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azizova T.V., Bannikova M.V., Grigoryeva E.S., Rybkina V.L., Hamada N. Occupational exposure to chronic ionizing radiation increases risk of Parkinson's disease incidence in Russian Mayak workers. Int J Epidemiol. 2020;49(2):435–447. doi: 10.1093/ije/dyz230. [DOI] [PubMed] [Google Scholar]
- 31.Zablotska L.B., Zupunski L., Leuraud K., Lopes J., Hinkle J., Pugeda T., et al. Radiation and CNS effects: summary of evidence from a recent symposium of the Radiation Research Society. Int J Radiat Biol. 2022 doi: 10.1080/09553002.2023.2142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent O., Samson E., Caër-Lorho S., Fournier L., Laurier D., Leuraud K. Updated mortality analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014. Cancers (Basel) 2022;15(1):79. doi: 10.3390/cancers15010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava T., Chirikova E., Birk S., Xiong F., Benzouak T., Liu J.Y., et al. Exposure to ionizing radiation and risk of dementia: a systematic review and meta-analysis. Radiat Res. 2023 doi: 10.1667/RADE-22-00153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes J., Leuraud K., Klokov D., Durand C., Bernier M.O., Baudin C. Risk of developing non-cancerous central nervous system diseases due to ionizing radiation exposure during adulthood: systematic review and meta-analyses. Brain Sci. 2022;12(8):984. doi: 10.3390/brainsci12080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boice J.D., Held K.D., Shore R.E. Radiation epidemiology and health effects following low-level radiation exposure. J Radiol Prot. 2019;39(4):S14–S27. doi: 10.1088/1361-6498/ab2f3d. [DOI] [PubMed] [Google Scholar]
- 36.Boice J.D., Jr, Cohen S.S., Mumma M.T., Ellis E.D. The Million Person Study, from whence it came and why. Int J Radiat Biol. 2022;98(4):537–550. doi: 10.1080/09553002.2019.1589015. [DOI] [PubMed] [Google Scholar]
- 37.Ellis E.D., Girardi D., Golden A.P., Wallace P.W., Phillips J., Cragle D.L. Historical perspective on the department of energy mortality studies: Focus on the collection and storage of individual worker data. Int J Radiat Biol. 2022;98(4):560–567. doi: 10.1080/09553002.2018.1547851. [DOI] [PubMed] [Google Scholar]
- 38.Boice J.D., Jr, Cohen S.S., Mumma M.T., Chen H., Golden A.P., Beck H.L., et al. Mortality among US military participants at eight aboveground nuclear weapons test series. Int J Radiat Biol. 2022;98(4):679–700. doi: 10.1080/09553002.2020.1787543. [DOI] [PubMed] [Google Scholar]
- 39.Boice J.D., Jr, Cohen S.S., Mumma M.T., Hagemeyer D.A., Chen H., Golden A.P., et al. Mortality from leukemia, cancer and heart disease among U.S. nuclear power plant workers, 1957-2011. Int J Radiat Biol. 2022;98(4):657–678. doi: 10.1080/09553002.2021.1967507. [DOI] [PubMed] [Google Scholar]
- 40.Boice J.D., Jr, Cohen S.S., Mumma M.T., Walsh L., Hagemeyer D., Yoder R.C., et al. Mortality among industrial radiographers exposed to ionizing radiation, 1969–2019. Radiat Res. 2023 [Google Scholar]
- 41.Boice J.D., Jr, Cohen S.S., Mumma M.T., Howard S.C., Yoder C., Dauer L.T. Mortality among medical radiation workers in the United States, 1965–1994. Int J Radiat Biol. 2023;99(2):183–207. doi: 10.1080/09553002.2021.1967508. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S.S., Mumma M.T., Ellis E.D., Boice J.D., Jr. Validating the use of census data on education as a measure of socioeconomic status in an occupational cohort. Int J Radiat Biol. 2022;98(4):587–592. doi: 10.1080/09553002.2018.1549758. [DOI] [PubMed] [Google Scholar]
- 43.Martinez N.E., Jokisch D.W., Dauer L.T., Eckerman K.F., Goans R.E., Brockman J.D., et al. Radium dial workers: back to the future. Int J Radiat Biol. 2022;98(4):750–768. doi: 10.1080/09553002.2021.1917785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.United States Government Accountability Office (GAO). Low-dose radiation: interagency collaboration on planning research could improve information on health effects. GAO-17-546. Washington (DC): GAO; 2017. https://www.gao.gov/products/gao-17-546
- 45.Anzenberg V., Lewis D.E., Dickson E.D., Bush-Goddard S.P., The U.S. Nuclear Regulatory Commission Radiation Exposure Information Reporting System (REIRS) Radiat Res. 2010;173(2):254–255. doi: 10.1667/RR1958.1. [DOI] [PubMed] [Google Scholar]
- 46.U.S. Department of Energy (USDOE). Occupational radiation exposure. The Radiation Exposure Monitoring System (REMS). Washington DC: USDOE; 2021. http://energy.gov/ehss/occupational-radiation-exposure
- 47.U.S. Nuclear Regulatory Commission (USNRC). Radiation Exposure Information and Reporting System (REIRS). Washington DC: USNRC; 2021. https://www.reirs.com [DOI] [PubMed]
- 48.Hagemeyer D., Nichols G., Mumma M.T., Boice J.D., Jr, Brock T.A. 50 years of the Radiation Exposure Information and Reporting System and importance to the Million Person Study. Int J Radiat Biol. 2022;98(4):568–571. doi: 10.1080/09553002.2018.1540896. [DOI] [PubMed] [Google Scholar]
- 49.Yoder R.C., Balter S., Boice J.D., Grogan H., Mumma M., Rothenberg L., et al. Using personal monitoring data to derive organ doses for medical radiation workers in the Million Person Study – considerations regarding NCRP Commentary No. 30. J Radiol Prot. 2021;41(1):118–128. doi: 10.1088/1361-6498/abcfcb. [DOI] [PubMed] [Google Scholar]
- 50.Yoder R.C., Dauer L.T., Balter S., Boice J.D., Jr, Grogan H.A., Mumma M.T., et al. Dosimetry for the study of medical radiation workers with a focus on the mean absorbed dose to the lung, brain and other organs. Int J Radiat Biol. 2022;98(4):619–630. doi: 10.1080/09553002.2018.1549756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mumma M.T., Cohen S.S., Sirko S.S., Ellis E.D., Boice J.D., Jr. Obtaining vital status and cause of death on a million persons. Int J Radiat Biol. 2022;98(4):580–586. doi: 10.1080/09553002.2018.1539884. [DOI] [PubMed] [Google Scholar]
- 52.Boice J.D., Jr, Cohen S.S., Mumma M.T., Ellis E.D., Cragle D.L., Eckerman K.F., et al. Mortality among Mound workers exposed to polonium-210 and other sources of radiation, 1944–1979. Radiat Res. 2014;181(2):208–228. doi: 10.1667/RR13395.1. [DOI] [PubMed] [Google Scholar]
- 53.Golden A.P., Ellis E.D., Cohen S.S., Mumma M.T., Leggett R.W., Wallace P.W., et al. Updated mortality analysis of the Mallinckrodt uranium processing workers, 1942–2012. Int J Radiat Biol. 2022;98(4):701–721. doi: 10.1080/09553002.2019.1569773. [DOI] [PubMed] [Google Scholar]
- 54.Golden AP, Howard S, Dauer LT, Boice Jr. JD. Preliminary results from a mortality study of Rocky Flats nuclear workers:a Million Person Study cohort. International Society for Radiation Epidemiology and Dosimetry. ISORED Scientific Meeting, May 16-18, 2023, Sitges, Spain. https://radiation.isglobal.org/isored/HTML/index.html
- 55.Boice J.D., Jr, Cohen S.S., Mumma M.T., Golden A.P., Howard S.C., Girardi D.J., et al. Mortality among workers at the Los Alamos National Laboratory, 1943–2017. Int J Radiat Biol. 2022;98(4):722–749. doi: 10.1080/09553002.2021.1917784. [DOI] [PubMed] [Google Scholar]
- 56.National Council on Radiation Protection and Measurements (NCRP). Deriving organ doses and their uncertainty for epidemiologic studies with a focus on the one million U.S. workers and veterans study of low-dose radiation health effects. NCRP Report 178. Bethesda MD: NCRP; 2018.
- 57.Dauer L.T., Bouville A., Toohey R.E., Boice J.D., Jr, Beck H.L., Eckerman K.F., et al. Dosimetry and uncertainty approaches for the million-worker study of radiation workers and veterans: overview of the recommendations in NCRP Report No. 178. Int J Rad Biol. 2022;98(4):600–609. doi: 10.1080/09553002.2018.1536299. [DOI] [PubMed] [Google Scholar]
- 58.National Council on Radiation Protection and Measurements (NCRP). Uncertainties in the measurement and dosimetry of external radiation. NCRP Report No. 158. Bethesda MD: NCRP; 2008.
- 59.International Commission on Radiological Protection (ICRP) Conversion coefficients for radiological protection quantities for external radiation exposures. ICRP Publication 116. Ann ICRP. 2010;40(2–5):1–257. doi: 10.1016/j.icrp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Bouville A., Toohey R.E., Boice J.D., Beck H.L., Dauer L.T., Eckerman K.F., et al. Dose reconstruction for the Million Worker Study: status and guidelines. Health Phys. 2015;108(2):206–220. doi: 10.1097/HP.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Council on Radiation Protection and Measurements (NCRP). Dosimetry guidance for medical radiation workers with a focus on lung dose reconstruction. NCRP Commentary 30. Bethesda MD: NCRP; 2020.
- 62.Till J.E., Beck H.L., Aanenson J.W., Grogan H.A., Mohler H.J., Mohler S.S., et al. Dosimetry associated with veterans who participated in nuclear weapons testing. Int J Radiat Biol. 2022;98(4):610–618. doi: 10.1080/09553002.2018.1551639. [DOI] [PubMed] [Google Scholar]
- 63.Leggett R.W., Tolmachev S.Y., Avtandilashvili M., Eckerman K.F., Grogan H.A., Sgouros S., et al. Methods of improving brain dose estimates for internally deposited radionuclides. J Radiol Prot. 2022;42(3) doi: 10.1088/1361-6498/ac7e02. [DOI] [PubMed] [Google Scholar]
- 64.Rothman KJ, Boice JD. Epidemiologic analysis with a programmable calculator. NIH Publication 79–1649. Washington DC: US Government Printing Office; 1979.
- 65.Breslow NE, Day NE. Statistical methods in cancer research. Vol. 2. The design and analysis of cohort studies. Publication 82. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed]
- 66.Armstrong B.G. Comparing standardized mortality ratios. Ann Epidemiol. 1995;5(1):60–64. doi: 10.1016/1047-2797(94)00032-o. [DOI] [PubMed] [Google Scholar]
- 67.Monson R. Observations on the healthy worker effect. J Occup Med. 1986;28(6):425–433. doi: 10.1097/00043764-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Buckley J.P., Keil A.P., McGrath L.J., Edwards J.K. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26(2):204–212. doi: 10.1097/EDE.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 69.Cox D. Regression models and life-tables. J R Stat Soc Ser B. 1972;34(2):187–220. [Google Scholar]
- 70.Golden A.P., Cohen S.S., Chen H., Ellis E.D., Boice J.D., Jr. Evaluation of statistical modeling approaches for epidemiologic studies of low-dose radiation health effects. Int J Radiat Biol. 2022;98(4):572–579. doi: 10.1080/09553002.2018.1554924. [DOI] [PubMed] [Google Scholar]
- 71.Preston D.L., Lubin J., Pierce D., McConney M., Shilnikova N. Risk Sciences International; Ottawa, Canada: 2015. Epicure risk regression and person-year computation software: command summary and user guide. [Google Scholar]
- 72.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 73.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(PtA):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shore R., Walsh L., Azizova T., Rühm W. Risk of solid cancer in low dose-rate radiation epidemiological studies and the dose-rate effectiveness factor. Int J Radiat Biol. 2017;93(10):1064–1078. doi: 10.1080/09553002.2017.1319090. [DOI] [PubMed] [Google Scholar]
- 75.Daniels R.D., Schubauer-Berigan M.K. A meta-analysis of leukaemia risk from protracted exposure to low-dose gamma radiation. Occup Environ Med. 2011;68(6):457–464. doi: 10.1136/oem.2009.054684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Electric Power Research Institute (EPRI). Cardiovascular risks from low dose radiation exposure: review and scientific appraisal of the literature. Palo Alto, CA: EPRI; 2020. https://www.epri.com/research/products/000000003002018408
- 77.Hauptmann M., Daniels R.D., Cardis E., Cullings H.M., Kendall G., Laurier D., et al. Epidemiological studies of low-dose ionizing radiation and cancer: summary bias assessment and meta-analysis. J Natl Cancer Inst Monogr. 2020;56:188–200. doi: 10.1093/jncimonographs/lgaa010. Erratum in: J Natl Cancer Inst Monogr 2023;2023(61):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richardson D.B., Abalo K., Bernier M.O., Rage E., Leuraud K., Laurier D., et al. Meta-analysis of published excess relative risk estimates. Radiat Environ Biophys. 2020;59(4):631–641. doi: 10.1007/s00411-020-00863-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little M.P., Azizova T.V., Richardson D.B., Tapio S., Bernier M.O., Kreuzer M., et al. Ionising radiation and cardiovascular disease: systematic review and meta-analysis. BMJ. 2023;380 doi: 10.1136/bmj-2022-072924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boice J.D., Jr, Leggett R.W., Ellis E.D., Wallace P.W., Mumma M., Cohen S.S., et al. A comprehensive dose reconstruction methodology for former Rocketdyne/Atomics International radiation workers. Health Phys. 2006;90(5):409–430. doi: 10.1097/01.HP.0000183763.02247.7e. [DOI] [PubMed] [Google Scholar]
- 81.Shi H., Counsel C. Accuracy of death certificates for recording parkinsonian syndromes and associated dementia. J Neurol. 2022;268(1):140–146. doi: 10.1007/s00415-020-10113-0. [DOI] [PubMed] [Google Scholar]
- 82.Dauer L.T., Mumma M.T., Lima J.C., Cohen S.S., Andresen D., Bahadori A.A., et al. A million person study innovation: evaluating cognitive impairment and other morbidity outcomes from chronic radiation exposure through linkages with the Centers for Medicaid and Medicare Services assessment and claims data. Radiat Res. 2023 doi: 10.1667/RADE-23-00186.1. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao Z., Wood N.W. Cell death pathways in Parkinson’s disease: role of mitochondria. Antioxid Redox Signal. 2009;11(9):2135–2149. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- 84.Simon D.K., Tanner C.M., Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mappin-Kasirer B., Pan H., Lewington S., Kizza J., Gray R., Clarke R., et al. Tobacco smoking and the risk of Parkinson disease: a 65-year follow-up of 30,000 male British doctors. Neurology. 2020;94(20):e2132–e2138. doi: 10.1212/WNL.0000000000009437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloem B.R., Okun M.S., Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 87.Boice J.D. Uncertainties in studies of low statistical power. J Radiol Prot. 2010;30(2):115–120. doi: 10.1088/0952-4746/30/2/E02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The code used to extract the data is distributed by the authors as open-source. The patient data can not be made available on request due to privacy/ethical restrictions.