Abstract
Background and study aims In colonoscopy, preparation is often regarded as the most burdensome part of the intervention. Traditionally, specific diets have been recommended, but the evidence to support this policy is insufficient. The aim of this study was to evaluate the impact of the decision not to follow a restrictive diet on bowel preparation and colonoscopy outcomes.
Patients and methods This was a multicenter, controlled, non-inferiority randomized trial with FIT-positive screening colonoscopy. The subjects were assigned to follow the current standard (1-day low residue diet [LRD]) or a liberal diet. The allocation was balanced for the risk of inadequate cleansing using the Dik et al. score. All participants received the same instructions for morning colonoscopy preparation. The primary outcome was the rate of adequate preparations as defined by the Boston Bowel Preparation Scale. Secondary outcomes included tolerability and measures of colonoscopy performance and quality.
Results A total of 582 subjects were randomized. Of these, 278 who received the liberal diet and 275 who received the 1-day LRD were included in the intent-to-treat analysis. Non-inferiority was demonstrated with adequate preparation rates of 97.8% in the 1-day LRD and 96.4% in the liberal diet group. Tolerability was higher with the liberal diet (94.7% vs. 83.2%). No differences were found with respect to cecal intubation time, aspirated volume, or length of the examination. Global and right colon average adenoma detection rates per colonoscopy were similar.
Conclusions The liberal diet was non-inferior to the 1-day LRD, and increased tolerability. Colonoscopy performance and quality were not affected. (NCT05032794)
Keywords: Endoscopy Lower GI Tract, CRC screening, Preparation, Quality and logistical aspects, Quality management
Introduction
Flexible colonoscopy was first performed more than 50 years ago 1 . Since then, the technique has improved notably 1 2 , but some of the practices established in the early days remain in place without any direct evidence to support them.
The growing demand for colonoscopies all over the world 3 4 has led to a progressive increase in workload at endoscopy units. In this situation, it is crucial to enhance the efficiency of interventions. Guidelines from various societies include quality parameters designed to improve efficiency, the most significant being adenoma detection rate (ADR) and cecal intubation rate 5 6 7 , Bowel cleansing is closely related to these important quality factors 7 8 9 . Poor colon preparation can lead to missed lesions and low ADRs 7 8 , increased procedure time, and higher risks 9 . In contrast, adequate bowel cleansing enhances colonoscopy quality and reduces opportunity costs 7 . Improving the safety and tolerability of bowel preparation may potentially increase uptake and enhance the performance of colorectal cancer (CRC) screening programs and thus, their efficacy as well 10 11 .
Diet restriction lowers participant satisfaction, has a negative effect on quality of life, and discourages patients from repeating the procedure 12 13 . Previous studies have already proven that a low-residue diet (LRD) is tolerated better than the traditionally recommended clear liquid diet and does not compromise cleansing quality 14 15 . Moreover, most recent trials have shown that 1-day LRD is enough to achieve appropriate bowel preparation and is better tolerated than the conventional 3-day LRD 16 17 18 . Nonetheless, current cleansing solutions and split-dosing regimens are also highly effective for bowel preparation. Therefore, we hypothesized that prescribing a LRD using state-of-the-art colonoscopy preparation may not actually offer any benefit. The aim of the present study was to evaluate the non-inferiority of a liberal diet (LD)-based cleansing protocol versus a 1-day LRD before colonoscopy with regard to achieving adequate bowel preparation.
Patients and methods
Design and setting
This multicenter, randomized, controlled, parallel-group clinical trial was performed at five centers across Spain between October 2021 and September 2022. The trial protocol followed the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines and the principles of the Declaration of Helsinki. 19 20 The study protocol was approved by the Ethical Commissions for Scientific Research at each center and is registered at Clinicaltrials.gov (NCT05032794). Written informed consent was obtained from all participants. All the co-authors had access to the study data and had reviewed and approved the final manuscript.
Participants and interventions
Consecutive participants enrolled on an organized average-risk CRC screening program with a positive fecal immunochemical test (FIT) were assessed for eligibility. Enrollment was performed by the nursing staff in charge of the CRC screening program. Patients of either sex aged 50 to 69 years participating in the CRC screening program with a positive FIT were eligible for inclusion. Subjects unable to understand the instructions properly or to give informed consent or who had contraindications for taking the cleansing solution or bisacodyl were excluded.
Participants were randomly assigned (1:1 ratio) to receive either no dietary restrictions (intervention group) or the 1-day LRD regimen (control group) and stratified for risk of inadequate cleansing. Allocation concealment was guaranteed by using a computer-based central randomization system integrated in a web-based electronic data capture system (REDCap) hosted at the Spanish Society for Gastroenterology 21 . The random allocation sequence in blocks of six was generated using software. Both groups were instructed to follow the same cleansing protocol with the exception of the diet. All the colonoscopies were scheduled in the morning and preparation included the intake of 1L polyethylene glycol plus ascorbic acid (PEG+Asc 1L) in split-dose regimens following the local institution protocol. The Dik score, a validated prediction score with an area under the curve of 0.72, was used to assess the risk of inadequate cleansing 22 23 . In case of risk of inadequate cleansing (Dik score ≥2), bisacodyl 5 mg bid for 3 days was added to the preparation scheme.
Dietary instructions were given during the enrollment visit (supplementary material). Participants receiving the LRD regimen were instructed to follow the diet 1 day before the examination, while those in the LD group received no dietary restrictions. The basal dietary habits of both groups were recorded. All participants were instructed to complete a dietary logbook for 3 days prior to their colonoscopy. All colonoscopies were performed by experienced endoscopists who routinely assessed the quality of cleansing using the Boston Bowel Preparation Scale (BBPS). In addition, all endoscopists had completed the BBPS Educational Program before their initial interaction with participants 24 . Given the study design, blinding of the participants was not feasible, but the researchers conducting the study interviews and performing the colonoscopies were blinded to the group assignation. The researcher responsible for recruitment and group assignation was aware of the treatment arm but did not take part in the colonoscopies. Alcohol consumption was assessed using the AUDIT-C questionnaire, a brief screening test validated for the Spanish population.
Outcomes
The primary outcome was adequacy of bowel cleansing, which was assessed with the BBPS. In addition, as a control variable, we also used a modified BBPS called “intubation BBPS,” which was assessed during endoscope advancement without taking into account any further improvement achieved with aspiration or flushing water. Both were recorded by the endoscopists immediately after the examination. In accordance with the BBPS, bowel cleansing was classified as excellent (9 points), adequate (all segments ≥2 points, total score: 6–8) or inadequate (one or more segments ≤1) 25 . Diet and cleansing solution tolerability and also satisfaction with the instructions received for bowel cleansing were assessed using a 5-point Likert scale, on which lower scores indicated better outcome. Tolerability also was assessed using the Hatoum et al.validated Patient Satisfaction Scale in Patients Undergoing Bowel Preparation Prior to Colonoscopy as an exploratory endpoint 26 . Prespecified secondary outcomes also included ADR, rates of right-sided adenoma, serrated adenoma, polyps and flat polyps, mean number of adenoma per colonoscopy, cecal intubation rate, withdrawal and entry times, volume of aspirate, time in minutes from the end of preparation to the colonoscopy, and time in days from inclusion in the study to colonoscopy. Endoscopic findings were recorded at the end of the procedure.
Sample size calculation
Calculation of the sample size was based on results obtained with the 1-day LRD regimen in a previous study by our group 17 , an unpublished feasibility trial, and with reference to our colonoscopy screening program, in which excellent or adequate preparation was achieved in about 95% of examinations. Assuming a power of 80%, an α error (also known as false positive) of 0.05 and 95% of adequate preparations in both groups, a sample size of 236 patients per arm was required to be sure that the upper limit of a one-sided 95% confidence interval (CI) excluded a difference in favor of the 1-day LRD regimen group of more than 5% (non-inferiority margin). Expecting a dropout rate of 5%, we calculated that a total of 248 participants per group was needed.
Statistical methodology
Descriptive statistics for quantitative variables are expressed as means±standard deviation (SD), or as medians and ranges when the distribution was asymmetrical. Categorical variables are expressed as absolute and relative frequencies.
A non-inferiority analysis was performed to compare adequate preparations between groups, carried out on both an intention-to-treat (ITT) and a per protocol basis. The non-inferiority margin was set at 5%. One-sided 95% CIs were obtained. We also performed an “as treated” analysis considering the actual diet recorded in the logbook. For this analysis, subjects with extended days of LRD were included in the 1-day LRD group.
Bivariate analyses using contingency tables (Chi-square statistics or Fisher's exact test for 2×2 tables) were used to compare secondary outcomes and colonoscopy efficiency parameters between the diet groups. Quantitative variables were dichotomized using the median. Relative risks (RRs) with 95% CIs were obtained for each variable. Confidence limits for differences in proportions were calculated following the Farrington-Manning method.
Statistical analyses were performed with the SAS system version 9.4 (SAS Institute Inc., Cary, North Carolina, United States). The level of statistical significance was set at α=0.05.
Results
Characteristics of the subjects
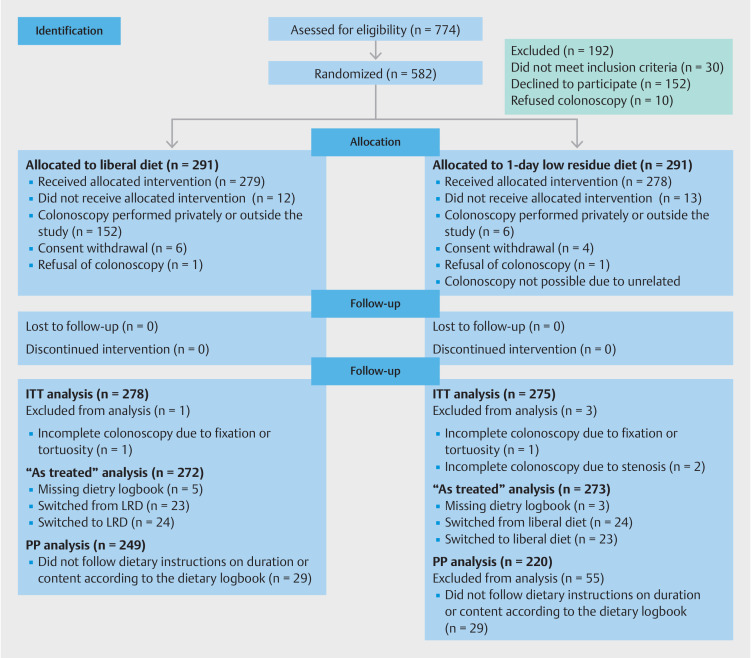
From October 2021 through June 2022, a total of 773 subjects were assessed for eligibility. In all, 582 subjects were randomly assigned to the LD group or 1-day LRD group (291 each) and 557 underwent the allocated intervention. Finally, 553 underwent ITT analysis after excluding those without a complete colonoscopy for any reason unrelated to the quality of the preparation. A detailed flow chart of the participants is shown in Fig. 1 . Participants were evenly distributed in the two groups, without any notable differences in their characteristics ( Table 1 ). Most subjects in both groups were overweight or obese (>70%).
Fig. 1.
Consort flowchart of participants.
Table 1 Characteristics of the subjects and colonoscopy preparation.
| Participants characteristics | Liberal diet (n=278) | 1-day LRD (n=275) | Number of patients with missing data |
| Values for±are means±SD. †Interval between last drink of purgative and the beginning of colonoscopy. *Values are medians and interquartile ranges (IQR). LRD, low-residue date; ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range. | |||
| Female sex – no. (%) | 138 (49.6) | 130 (47.3) | 0 |
| Age – yr | 58.97±5.86 | 58.89±5.82 | 0 |
| Smokers – no. (%) | 66 (23.7) | 66 (24) | 0 |
| Ex-smokers – no. (%) | 100 (36) | 96 (34.9) | 0 |
| Risky alcohol use – no. (%) | 48 (17.3) | 58 (21.1) | 0 |
| Following a special diet – no. (%) | 13 (4.7) | 18 (6.5) | 0 |
| Morbidity and inadequate cleansing risk factors | |||
| Diabetes – no. (%) | 25 (9) | 22 (8) | 0 |
| Constipation – no. (%) | 31 (11.2) | 35 (12.7) | 0 |
| Abdominal or pelvic surgery – no. (%) | 22 (7.9) | 22 (8) | 0 |
| Other antidepressants – no. (%) | 29 (10.4) | 29 (10.5) | 0 |
| Statins – no. (%) | 53 (19.1) | 44 (16) | 0 |
| Aspirin and other anti-aggregants – no. (%) | 19 (6.8) | 13 (4.7) | 0 |
| None known – no. (%) | 150 (54) | 163 (59.3) | 0 |
| ASA ≥3 – no. (%) | 3 (1.1) | 3 (1.1) | 0 |
| Previous inadequate cleansing – no. (%) | 6 (2.2) | 1 (0.4) | 0 |
| Limited mobility – no. (%) | 2 (0.7) | 2 (0.7) | 0 |
| Parkinson's disease – no. (%) | 1 (0.4) | 1 (0.4) | 0 |
| Multiple sclerosis – no. (%) | 2 (0.7) | 0 (0) | 0 |
| Dementia – no. (%) | 0 (0) | 0 (0) | 0 |
| Cerebrovascular accident – no. (%) | 4 (1.4) | 1 (0.4) | 0 |
| Chronic kidney disease mild-moderate – no. (%) | 1 (0.4) | 1 (0.4) | 0 |
| Cirrhosis – no. (%) | 0 (0) | 1 (0.4) | 0 |
| Opioids – no. (%) | 4 (1.4) | 5 (1.8) | 0 |
| Tricyclic antidepressant – no. (%) | 3 (1.1) | 4 (1.5) | 0 |
| Oral anticoagulants – no. (%) | 10 (3.6) | 3 (1.1) | 0 |
| Calcium antagonist – no. (%) | 1 (0.4) | 5 (1.8) | 0 |
| Mean body mass index | 27.76±4.69 | 27.75±4.76 | 24 |
| BMI ≥25 – no. (%) | 191 (72.6) | 188 (70.7) | 24 |
| At risk of inadequate cleansing (Dik ≥2) – bisacodyl administration – no. (%) | 38 (13.7) | 40 (14.5) | 0 |
| Dik score points – no. (%) | 0 | ||
| 0 | 201 (72.3) | 198 (72) | |
| 1 | 39 (14) | 38 (13.8) | |
| 2 | 24 (8.6) | 24 (8.7) | |
| 3 | 11 (4) | 11 (4) | |
| 4 | 1 (0.4) | 1 (0.4) | |
| 5 | 2 (0.7) | 3 (1.1) | |
| Preparation and colonoscopy factors | |||
| Cleansing solution adherence – no. (%) | 0 | ||
| 100% | 254 (91.4) | 261 (94.9) | |
| ≥50% | 23 (8.3) | 14 (5.1) | |
| <50% | 1 (0.4) | 0 (0%) | |
| Days until colonoscopy – days (IQR)* | 27 (19) | 28 (20) | 0 |
| Waiting time ≥8 weeks | 27 (9.7) | 36 (13.1) | 0 |
| Runway time – hours * † | 3:08 (1:06) | 3:09 (1:15) | 6 |
| Followed diet as by the dietary logbook | |||
| Unrestricted diet | 249 (91.2) | 23 (8.4) | 8 |
| 1-day LRD | 22 (8.0) | 220 (80.8) | |
| 2-day LRD | 1 (0.4) | 8 (2.9) | |
| 3-day LRD | 1 (0.4) | 21 (7.7) | |
Colonoscopy preparation
The satisfaction with the information received for preparation received similar ratings across the groups, with 95.7% in the LD and 96.7% in the LRD groups rated as “good.” The median time from inclusion to colonoscopy was 27 days (interquartile range [IQR] 19 days) for LD and 28 days (IQR 20 days) for LRD. Colonoscopies were performed by a total of 39 endoscopists, each of whom received a similar proportion of subjects from both groups. Adherence and tolerability of PEG+Asc 1L were similar. The results of the Hatoum et al. survey can be found in Supplementary Table 1. The runway time from the end of preparation until the colonoscopy was also similar in the groups (average of 3:25 hours for LD) (SD 0:59 hours) for LD and (average of 3:24 hours for LRD) (SD 1:06 hours).
Main outcome
Preparation was adequate in 96.4% of the LD group and 97.8% of the LRD group. The difference between the groups was thus 1.4%, with a one-sided 95% CI of 4.1%, a value that did not exceed the prespecified inferiority limit. With regard to the assessment of colon cleanliness during intubation, preparations were adequate in 83.5% of the LD group and 86.5% of the LRD group. More details and results per segment are provided in Supplementary Table S2 and Supplementary Table S3.
Tolerability and subject preferences
The LD was better tolerated than the LRD, with more participants in the LD group reporting the highest level of tolerability (94.7% versus 83.2%; RR 1.14, 95% CI 1.07–1.21). In the LRD group 11.3% would have preferred to be assigned to the LD, compared with 3.6% in the other direction. Analysis of the dietary logbooks showed that 9% of subjects in the LD followed a LRD, while 8.5% of those assigned to LRD did not follow the diet and 10.3% extended the diet for more days. Thus, 90.8% of the LD group and 81.8% of the LRD group complied with their prescribed diet. An additional “as treated” non-inferiority analysis was conducted and can be found in the Supplementary Table S4.
Quality and efficiency parameters
Total examination times, withdrawal times, and volume aspirated did not differ between the two groups ( Table 2 ). The polyp detection rate and ADR and the average number per colonoscopy were also similar in the two groups: the ADR was 61.5% in the LD group and 55.3% in the LRD group with a RR of 1.11 (95% CI 0.97–1.28). In terms of quality of preparation, the LRD group had a higher proportion of excellent preparations (56.8% vs. 65.1%; RR 0.87; 95% CI 0.76–1.00). There were 11 complications registered in each study group (4%), most of them were resection related bleeding and none was related to the cleansing quality.
Table 2 Principal outcomes.
| Primary outcome | Liberal diet | 1-day LRD | Relative risk (95% CI)* | Non-inferiority risk difference † | One-sided 95%CI † | Number of patients with missing data |
| *The confidence intervals for the secondary outcomes have not been corrected for multiple comparisons, and no clinical conclusions can be drawn from these data. For quantitative variables, the relative risks were calculated for high values (above the median) as compared with low values (at or below the median). †Values shown are percentages. ‡Values shown are medians and interquartile ranges (IQRs) | ||||||
| ITT analysis; adequate cleansing – no. (%) n=553 | 268/278 (96.4) | 269/275 (97.8) | 0.99 (0.96–1.01) | 1.42 | 4.12 | 0 |
| Secondary outcomes | ||||||
| Diet tolerability – Likert Scale 1 and 2 – no. (%) | 282 (94.7) | 228 (83.2) | 1.14 (1.07–1.21) | 13 | ||
| Cleansing solution tolerability – Likert Scale 1 and 2 – no. (%) | 187 (67.8) | 186 (67.9) | 1.00 (0.89–1.12) | 3 | ||
| Satisfaction with the instructions received – Likert Scale 1 and 2 – no. (%) | 264 (95.7) | 265 (96.7) | 0.99 (0.96–1.02) | 3 | ||
| Would have preferred to be assigned to the other study group | 10 (3.6) | 31 (11.3) | 0.32 (0.16–0.64) | 5 | ||
| Excellent cleansing, BBPS 9 – no. (%) | 158 (56.8) | 179 (65.1) | 0.87 (0.76–1.00) | 0 | ||
| Colonoscopy efficiency | ||||||
| Cecal intubation time – minutes, median (IQR) ‡ | 6 (3) | 6 (4) | 0.96 (0.80–1.16) | 2 | ||
| Withdrawal time – minutes, median (IQR) ‡ | 14 (10) | 13(9) | 1.13 (0.94–1.37) | 2 | ||
| Total colonoscopy time – minutes median (IQR) ‡ | 21 (12) | 20 (11) | 1.03 (0.86–1.23) | 0 | ||
| Aspirate volume – mL, median (IQR) ‡ | 400 (200) | 400 (280) | 1.06 (0.90–1.24) | 1 | ||
| Cecal intubation – no. (%) | 276 (99.3) | 274 (99.6) | 1.00 (0.98–1.01) | 0 | ||
| Polyp detection rate – no. (%) | 198 (71.2) | 183 (66.5) | 1.07 (0.96–1.20) | 0 | ||
| Polyp in right colon detection rate – no. (%) | 95 (34.2) | 85 (30.9) | 1.10 (0.87–1.41) | 0 | ||
| Flat polyp detection rate – no. (%) | 35 (12.6) | 22 (8) | 1.57 (0.63–2.12) | 0 | ||
| Adenoma detection rate – no. (%) | 171 (61.5) | 152 (55.3) | 1.11 (0.97–1.28) | 0 | ||
| Adenoma in right colon detection rate – no. (%) | 80 (28.8) | 71 (25.8) | 1.11 (0.85–1.46) | 0 | ||
| Serrated adenoma detection rate – no. (%) | 35 (12.6) | 30 (10.9) | 1.15 (0.73–1.83) | 0 | ||
| Serrated adenoma in right colon detection rate – no. (%) | 21 (7.6) | 18 (6.5) | 1.15 (0.63–2.12) | 0 | ||
| Average detection of polyps – median (IQR) | 2 (3) | 2 (3) | 0.95 (0.75–1.20) | 0 | ||
| Average detection of polyps in the right colon – median (IQR) ‡ | 1 (1) | 1 (1) | 1.19 (0.85–1.68) | 0 | ||
| Average detection of adenomas – median (IQR) ‡ | 2 (2) | 2 (2) | 0.83 (0.61–1.15) | 0 | ||
| Average detection of adenomas in right colon – median (IQR) ‡ | 1 (1) | 1 (1) | 1.19 (0.80–1.77) | 0 | ||
Discussion
Main findings
This trial proves the non-inferiority of an LD before colonoscopy for achieving adequate bowel cleansing compared with the current standard of 1-day LRD under the terms for which it was designed. There was an increased proportion of good tolerability in the LD, and this diet was preferred by the participants. There was no effect on colonoscopy quality or performance outcomes.
These findings have significant clinical implications, given that eliminating dietary restrictions would considerably simplify the colonoscopy preparation protocol. It may also enhance the acceptability of colonoscopy and CRC screening and improve patient experience and satisfaction, which are factors associated with CRC screening uptake 11 . Because no previous diet is required, the preparation can be much faster, with same-day or day-before schemes allowing rapid rescheduling if necessary, at least in participants without risk of poor preparation.
These findings do not support the current guidelines recommending LRDs for colonoscopy cleansing. However, this does not mean that our results contradict the recommendations in guidelines, because those recommendations are based on the comparison of a low-fiber diet versus a liquid diet. Indeed, to our knowledge, no previous study has directly investigated the respective impacts of restrictive and liberal diets in this setting. Recent studies using split-dose PEG-based preparations have shown that shortening dietary restrictions from 3 days to 1 day is associated with improved tolerability and does not compromise bowel cleansing 16 17 18 . Few studies to date have used a LD in their cleansing protocol, although the ones that have been published have obtained favorable results 27 28 . However, these studies were unable to assess the impact of dietary restriction.
Certain aspects of this trial should be taken into account with regard to its internal and external validity. First, we applied strict selection criteria with regard to the centers to ensure the use of similar methodologies in terms of colonoscopy preparation and colonoscopy itself. Second, we observed current evidence-based preparation standards such as fractionated preparation and finishing the preparation as close as possible to 2 hours of fasting, procedures which have been shown to have a major impact on the adequacy of bowel cleansing. We also monitored for confounding factors associated with colon cleansing based on current knowledge, such as examination times (which may be prolonged in order to perform additional lavage) as well as ADR and other outcomes that act as quality markers. The groups were comparable and received exactly the same preparation except for the diet.
Furthermore, there were no differences in variables known to play an important role in cleansing quality, such as adherence to PEG+Asc, the waiting time from instruction to examination, or time elapsed from the completion of PEG+Asc intake until the start of colonoscopy. Third, the adequacy of the preparation was assessed using the BBPS, a consolidated tool for rating cleansing quality and a surrogate variable of the clinical impact of colonoscopy used in clinical practice. Because the BBPS is assessed upon withdrawal after performing lavage and aspirating all possible material, we feared that this might significantly mitigate the difference in cleansing. Although this would probably be reflected in longer examination times and volume aspirated, we decided to go a step further and added data about the condition of the colon before starting additional cleansing maneuvers. To do this, we used a modified BBPS that reflects the state of the colon during cecal intubation without considering additional lavage. This allowed us to confirm that the increase in adequate preparation after lavage and aspiration was similar in the two groups (12.9% vs. 11.3%) and we found a slight change in the difference from 3% before additional cleansing to 1.42%. We used a validated scale to balance the distribution of individuals at risk of poor preparation and thus, to prevent bias 22 .
We are aware of the limitations of the present study. First, despite its robust internal validity, its external validity is limited because the sample comprised only asymptomatic subjects, most of them with low morbidity, aged 50 to 69 years undergoing outpatient screening colonoscopy with no representation of hospitalized or elderly patients or patients with significant morbidity. The study design was set for the estimation of a non-inferiority unilateral 95% CI instead 97.5% CI, which is recommended by the European Medicine Agency for drug clinical trials. However, this clinical trial involved no drugs and this difference implies a very slight increase in the risk of a false non-inferiority. Taking into account that we are using a conservative non-inferiority margin compared with other studies, we can say that this increase is negligible.
Although 14.1% of the sample had an increased risk of poor preparation, there was no significant representation of American Society of Anesthesiologists Class ≥3 patients (1.1% of the sample) or elderly patients. Furthermore, a single, high-efficiency evacuant solution was used, which may have undermined the impact of the diet. However, the design of the study does not enable us to draw definite conclusions about this issue. In that regard, our findings cannot be universally generalized and they need to be reproduced in other studies involving different populations and cleansing solutions.
Conclusions
A LD is not inferior to a 1-day LRD for achieving and adequate bowel preparation. In this low-morbidity sample, the choice of diet had no impact on the efficiency of the exploration. We suggest conducting further studies to determine whether our findings are generalizable.
Acknowledgement
We want to thank all the volunteers for their participation. The following collaborators have participated in the development of the clinical trial: M. Vergara, A. Sánchez, C. Bueno, C. Díaz, S. Mayor, M. Ruiz, M. Chuecos, J. Serra, M. Gálvez, G. Vallejo, A. Franch, B. Marco, L. Pascual, R. Pastor, L. Hernández, J. Vives, A. Lira, J. Da Costa, F. Junquera, V. Puig, M. Miquel, I. Esteve, B. García, D. Nicolás, R. Romero, I. Alonso, Y. González, J. Ortega, De la Barreda, L. Ramos, Sánchez del Río, E. Rodríguez, M. Domínguez, M. Delgado, G. Iborra, M. Sampedro, S. Carrión, JM. Castellví, M. Martínez, A. Bonilla, C. Loras, M. Esteve, B. Arau, F. Fernández, X Andújar, Y. Sabana, R. Rifa, J. Profitós, S. Galter D. Zaffalón. Nùria Piqué is a Serra Hunter fellow.
Footnotes
Conflict of Interest Salvador Machlab speakers' fee from Norgine. None of the other authors has any other disclosure.
Supplementary Material
References
- 1.Axon ATR. Fifty years of digestive endoscopy: Successes, setbacks, solutions and the future. Dig Endosc. 2020;32:290–297. doi: 10.1111/den.13593. [DOI] [PubMed] [Google Scholar]
- 2.Hayman CV, Vyas D. Screening colonoscopy: The present and the future. World J Gastroenterol. 2021;27:233–239. doi: 10.3748/wjg.v27.i3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan R, Rutter MD. Colorectal cancer screening—Who, how, and when? Clin Endosc. 2018;51:37–49. doi: 10.5946/ce.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comas M, Mendivil J, Andreu M et al. Long-term prediction of the demand of colonoscopies generated by a population-based colorectal cancer screening program. PLoS One. 2016;11:e0164666. doi: 10.1371/journal.pone.0164666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rembacken B, Hassan C, Riemann J et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Schoenfeld PS, Cohen J et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Burke CA, Johnson DA et al. The importance of colonoscopy bowel preparation for the detection of colorectal lesions and colorectal cancer prevention. Endosc Int Open. 2020;8:E673. doi: 10.1055/a-1127-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froehlich F, Wietlisbach V, Gonvers J-J et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Imperiale TF, Latinovich DR et al. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 10.The Lancet Gastroenterology and Hepatology . Improving uptake of colorectal cancer screening. Lancet Gastroenterol Hepatol. 2017;2:767. doi: 10.1016/S2468-1253(17)30298-4. [DOI] [PubMed] [Google Scholar]
- 11.Denters MJ, Deutekom M, Bossuyt PM et al. Patient burden of colonoscopy after positive fecal immunochemical testing for colorectal cancer screening. Endoscopy. 2013;45:342–349. doi: 10.1055/s-0032-1326238. [DOI] [PubMed] [Google Scholar]
- 12.Walter J, Patel A, Matro R et al. The impact of diet liberalization on bowel preparation for colonoscopy. Am J Gastroenterol. 2013;108:S162. doi: 10.1055/s-0043-101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Reyes E, Tepox-Padrón A, Cano-Manrique G et al. A low-residue diet before colonoscopy tends to improve tolerability by patients with no differences in preparation quality: a randomized trial. Surg Endosc. 2020;34:3037–3042. doi: 10.1007/s00464-019-07100-6. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen DL, Jamal MM, Nguyen ET et al. Low-residue versus clear liquid diet before colonoscopy: A meta-analysis of randomized, controlled trials. Gastrointest Endosc. 2016;83:499–5070. doi: 10.1016/j.gie.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Soweid AM, Dgayli K. The effect of fiber-free diet and splitting the dose of a low volume polyethylene glycol electrolyte solution on the quality of colonoscopy preparation. Gastrointest Endosc. 2015;81:Ab313–ab314. [Google Scholar]
- 16.Gimeno-García AZ, De La Barreda Heuser R, Reygosa C et al. Impact of a 1-day versus 3-day low-residue diet on bowel cleansing quality before colonoscopy: A randomized controlled trial. Endoscopy. 2019;51:628–636. doi: 10.1055/a-0864-1942. [DOI] [PubMed] [Google Scholar]
- 17.Machlab S, Martínez-Bauer E, López P et al. Comparable quality of bowel preparation with single-day versus three-day low-residue diet: Randomized controlled trial. Dig Endosc. 2020;33:797–806. doi: 10.1111/den.13860. [DOI] [PubMed] [Google Scholar]
- 18.Taveira F, Areia M, Elvas L et al. A 3-day versus 1-day low residue diet to improve colonoscopy preparation result and patient tolerability, a randomized, single-blinded, controlled trial. United Eur Gastroenterol J. 2019;7:547. doi: 10.1177/2050640619883176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AW, Tetzlaff JM, Gøtzsche PC et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki . ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dik VK, Moons LMG, Hüyük M et al. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: Development and validation of a prediction score. Gastrointest Endosc. 2015;81:665–672. doi: 10.1016/j.gie.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 23.Gkolfakis P, Kapizioni C, Tziatzios G et al. Comparative performance and external validation of three different scores in predicting inadequate bowel preparation among Greek inpatients undergoing colonoscopy. Ann Gastroenterol. 2023;36:25–31. doi: 10.20524/aog.2023.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderwood AH, Logan JR, Zurfluh M et al. Validity of a Web-based educational program to disseminate a standardized bowel preparation rating scale. J Clin Gastroenterol. 2014;48:856–861. doi: 10.1097/MCG.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai EJ, Calderwood AH, Doros G et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatoum HT, Lin S-J, Joseph RE et al. Validation of a patient satisfaction scale in patients undergoing bowel preparation prior to colonoscopy. Patient. 2016;9:27–34. doi: 10.1007/s40271-015-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoun E, Abdul-Baki H, Azar C et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without ietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62:213–218. doi: 10.1016/s0016-5107(05)00371-8. [DOI] [PubMed] [Google Scholar]
- 28.Chang HJ, Algar U, Chu K et al. Bowel preparation for colonoscopy: is diet restriction necessary? South African J Surg. 2020;58:217E–217F. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.