Abstract
Background
Mechanisms of synaptic plasticity in retinal ganglion cells (RGCs) are complex and the current knowledge cannot explain. Growth and regeneration of dendrites together with synaptic formation are the most important parameters for evaluating the cellular protective effects of various molecules. The effect of ginsenoside Rg1 (Rg1) on the growth of retinal ganglion cell processes has been poorly understood. Therefore, we investigated the effect of ginsenoside Rg1 on the neurite growth of RGCs.
Methods
Expression of proteins and mRNA were detected by Western blot and qPCR. cAMP levels were determined by ELISA. In vivo effects of Rg1 on RGCs were evaluated by hematoxylin and eosin, and immunohistochemistry staining.
Results
This study found that Rg1 promoted the growth and synaptic plasticity of RGCs neurite by activating the cAMP/PKA/CREB pathways. Meanwhile, Rg1 upregulated the expression of GAP43, Rac1 and PAX6, which are closely related to the growth of neurons. Meantime, H89, an antagonist of PKA, could block this effect of Rg1. In addition, we preliminarily explored the effect of Rg1 on enhancing the glycolysis of RGCs, which could be one of the mechanisms for its neuroprotective effects.
Conclusion
Rg1 promoted neurite growth of RGCs through cAMP/PKA/CREB pathways. This study may lay a foundation for its clinical use of optic nerve diseases in the future.
Keywords: Ginsenoside Rg1, Synaptic plasticity, Neurite growth, Retinal ganglion cells, cAMP/PKA/CREB pathways
Graphical abstract
1. Introduction
Synaptic plasticity refers to the adjustable strength of synaptic connections between nerve cells and the ability of nerve cells to change their structure in order to adapt to changes in the external environment [1]. The morphology and function of synapses may occur lasting changes, and synapses strengthen and weaken when their activity increases and decreases, which play an important role in the cellular mechanisms of learning and memory in the human brain [2]. Synaptic plasticity is affected by many factors such as calcium channels [1], brain-derived neurotrophic factor (BDNF) [3] and synaptic growth-related regulatory proteins including growth associated protein-43 (GAP-43) [4], paired box protein 6 (PAX6) [5] and small GTPase Ras-related C3 botulinum toxin substrate 1 (Rac1) [6]. Many neurological diseases are associated with a decrease in synaptic plasticity, such as depressive disorder [7], autism [8], and Alzheimer's disease [9]. These diseases often accompany with the decrease of the regulatory proteins like GAP-43 [9].
Synaptic plasticity is controlled by complex regulation of diverse signaling pathways. The PI3K-Akt-mTOR pathway is closely related to the aluminum-induced synaptic plasticity impairment [10]. The activation of nuclear factor kappa B (NF-κB) may be required for the induction of synaptic plasticity and memory formation [11]. Dendritic spines are the major loci of synaptic plasticity. Molecular signaling pathways of dendritic spines are mainly Rho and Ras family small GTPases pathways that converge on actin cytoskeleton, modulate the spine morphology during synaptic activity [12]. Meanwhile, synaptic plasticity is bound up with the energy metabolism of nerve cells, especially glycolysis. Inhibition of the glycolysis might impair the neurite architecture [13].
Although there are plenty of researches on synaptic plasticity in brain, research on synaptic plasticity of eye diseases are a few. Irreversible damages or death of retinal ganglion cells (RGCs) are one of main causes of vision loss in patients with glaucoma. Nevertheless, a growing number of studies have revealed that RGCs might enter periods of dysfunction featuring the synaptic plasticity decrease which is prior to death. Vision improvement in glaucoma patients with clinically treatment may occur before the death of RGCs [14]. Studies have found that structural plasticity is important to human retinal tissues in response to age-related macular degeneration (AMD), having the capacity to form new synapses. It sheds a new light on repairing functionally abnormal human retina [15]. However, there is a few safe, effective and economical drugs or candidates for clinical use to regulate synaptic plasticity.
Ginsenoside Rg1 (Rg1) is one of Dammarane-type saponins, which can be isolated from Panax notoginseng (Burk.) F. H. Chen with rich pharmacological activities, such as anti-inflammatory, anti-oxidative stress, anti-apoptosis, and anti-neurodegeneration [[16], [17], [18]]. The effect of Rg1 by inject administration on synaptic plasticity has also been reported [19]. Rg1 enhances neurite growth by activating Akt and ERK1/2 signaling, increases the expression of GAP-43, and prevents Aβ 25-35-induced injury [20]. It has also been reported that Rg1 can achieve neuroprotective and antidepressant effects by activating the expression of synaptic plasticity related proteins like camp-response element binding protein (CREB) and BDNF [21]. There are reports that Rg1 can regulate the PI3K/Akt pathway to promote synaptic transmission and increase the complexity of dendritic spines [22]. Nevertheless, the effects of Rg1 on ophthalmology are rarely studied. Therefore, in this study, we focused on the effects of Rg1 on neurite growth of RGCs.
2. Materials and methods
2.1. Reagents
Ginsenoside Rg1 (Rg1, C42H72O14, MW: 801.01) was isolated from Panax notoginseng (Burk.) F. H. Chen in our laboratory.
Forskolin (66575-29-9) was purchased from Aladdin (Shanghai, China); and H89 (127243-85-0) was purchased from GLPBIO (Montclair, CA, USA). Antibodies GAP-43 (16971-1-AP) and PAX6 (12323-1-AP) were obtained from Proteintech (Rosemont, IL, USA); antibodies CREB (ab32515), phospho-CREB (ab32096) and phospho-PKA (ab75991) were obtained from Abcam (Cambridge, UK); antibodies PKA (#4782) and active β-catenin (#19807) were obtained from Cell Signaling Technology (Boston, MA, USA).
2.2. Cell culture
RGC-5s cells were obtained from the BaiYe Biotechnology (Shanghai, China) and were cultured in MEM medium supplemented with 1% (v/v) penicillin-streptomycin (Biological Industries, Kibbutz Beit-Haemek, Israel) and 10% (v/v) fetal bovine serum (FBS) at 37 °C in a humidified atmosphere with 5% CO2.
2.3. Isolation and culture of primary RGCs
Eyes of day 1–3 mice were euthanized, and retinas were dissected in PBS. After dissection, retinas were grinded at 80 screen mesh. Retinas were digested with trypsin for about 25 min at 37 °C. Cells were separated by centrifugation and resuspended in DMEM/F12 with B27 (1x), 1% (v/v) penicillin and streptomycin and 10% (v/v) fetal bovine serum, after grinding at 400 mesh. Cells were plated on a poly-d-lysine (0.1 mg/mL) coated 24 well plate. The cells were incubated in a humidified atmosphere with 5% CO2 at 37 °C. After 24 h, 10 μL Ara-C (final concentration: 10 μmol/L) was added to cells.
2.4. Western blot analysis
Total protein from RGC-5s was extracted by using RIPA lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100 and 1 mM EDTA) with PMSF on ice. Then the cell lysates were determined by Detergent Compatible Bradford Protein Assay Kit (Beyotime Biotechnology, Beijing, China). They were subjected to SDS-PAGE electrophoresis and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). Next, the membranes were blocked with 5% BSA dissolved in double diluted water for 60 min. Next, primary antibodies were incubated with the protein overnight at 4 °C, then HRP-conjugated secondary antibodies were incubated with the protein for 1–2 h at room temperature. The protein was visualized with Tanon system.
2.5. Quantitative real-time PCR
Total RNA was extracted from RGC-5s by using RNA Plus (Takara, Otsu, Shiga, Japan). RNA samples were reverse-transcribed to cDNA by using commercial cDNA synthesis kits (Vazyme Biotech, R223-01) according to the manufacturer's protocol. Then they were subjected to a StepOne Real-Time PCR System (Applied Biosystems). Primer sets were in Table S1.
2.6. ELISA assay
The cAMP levels were determined by protocol together with some modifications [23]. For the cAMP extraction, the cells were quick-frozen at −80 °C for 10 min and then unfrozen at room temperature, which was repeated for 3 times. The supernatants were collected for measuring the content of cAMP by using a cAMP Elisa Kit (Elabscience Biotechnology, China) according to the manufacturer's instructions.
2.7. Extracellular acidification
Extracellular acidification rate (ECAR) was detected with the XF Glycolysis Stress Test by using a Seahorse Bioscience XFp Extracellular Flux Analyzer (Seahorse Bioscience, USA). Cells were incubated on the XF cell culture plates. For the ECAR assay, 10 mM glucose, 1.0 μM oligomycin (complex V inhibitor), and 50 mM 2-DG were added in sequence operating according to the manufacturer's instructions.
2.8. Animals and drug administration
A total of 20–24 g adult male ICR mice were supplied by the Laboratory Animal Center of Nanjing Qinglongshan (Nanjing, Jiangsu, China). All of the animals were kept in a facility at standard environment (22 ± 2 °C) with a 12 h light-dark cycle, given free access to water and food. Animal care and treatment were strictly conducted on the basis of the Provision and General Recommendation of Experimental Animals Administration Legislation of China Pharmaceutical University. The mice were randomly divided into four groups: the sham group (Sham, n = 12), the Rg1 group (Rg1, n = 12), the forskolin (cAMP agonists) group (Fors, n = 12), the H89 (PKA inhibitor) group (H89, n = 12). The sham group was subjected to saline. 1% (mg/100 mL) Rg1 was sterilely dissolved in saline and was used as eye drops once a day for 30 days. Forskolin (2 mg/kg) and H89 (2 mg/kg) was dissolved in 5% DMSO, 40% PEG300, 5% tween-80 and 50% saline. The forskolin group was taken via intraperitoneally (i.p.) once a week for a month. The H89 group was treated with both H89 and Rg1, which was received H89 via i. p. once a week for 30 days (first time before eye drop) and Rg1 by eye drops once a day for 30 days. All groups after the last administration, eye balls of mice were collected for various measurement.
2.9. Hematoxylin and eosin (H&E) staining
Eye balls were treated with 4% paraformaldehyde (PFA). After dewaxing in xylene and rehydrating in ethanol, a pathology slicer (Leica RM2016, Wetzlar, Heessen, Germany) was used to cut eye balls into pieces. Sections were stained with hematoxylin for 3–5 min, washed again with running water, and then differentiated in 80% alcohol. Finally, sections were stained with eosin dye for 5 min and dehydrated in alcohol according to the routine procedure [24]. Sections were observed with microscope (Leica DMi8, Wetzlar, Heessen, Germany).
2.10. Immunohistochemistry (IHC) staining
Eye balls were obtained from mice, and immersed in 4% PFA. They were stained by primary antibody following the standard protocols. Images were captured by the indicated microscope (Leica DMi8, Wetzlar, Heessen, Germany).
2.11. Axon quantification by CTB anterogradely labeling
RGC axons were measured by a previously described protocol with some modulations [25]. Briefly, Cholera toxin β subunit (CTB) with a conjugated Alexafluor 488 (Thermo Fisher Scientific, Waltham, MA) was intravitreously injected 2 days before tissue obtaining, which anterogradely labeled RGC axons. Then, optic nerves were embedded in OCT Compound (Tissue Tek, Sakura, Japan) for cryosectioning. Sections were observed with microscope (Leica DMi8, Wetzlar, Heessen, Germany).
2.12. Scanning electron microscopy (SEM)
SEM was performed as the standard protocol. Briefly, retinal cross sections were fixed, dehydrated, and dried. Specimens were attached to metallic stubs by using carbon stickers and then sputter-coated with gold. Then they were observed and taken images with scanning electron microscope (Hitachi SU8100, Tokyo, Japan).
2.13. Statistical analysis
All the data were shown as mean ± SD. The student's t-test was employed for statistical analysis using GraphPad Prism 7.0. The p value < 0.05 was considered statistically significant.
3. Results
3.1. Rg1 promoted neurite growth of RGC-5s and primary RGCs in vitro
We assessed the neurotrophic effects of Rg1 on RGCs in vitro by measuring neurite outgrowth. After Rg1 stimulation for 2 days, the synaptic length and complexity of RGC-5s increased significantly by a concentration dependent manner (Fig. 1A and C). Meantime similar results have been obtained from the primary RGCs (Fig. 1B and D), which were incubated with Rg1 for 1 week.
Fig. 1.
Rg1 promoted neurite growth of RGCs in vitro. (A) RGC-5s were observed under microscope after treatment with 1, 10, 30 μM Rg1 for 2 days respectively. (B) Primary RGCs were observed under microscope after treatment with 1, 10, 30 μM Rg1 for 1 week respectively. (C) Neurite length was measured in RGC-5s. (D) Neurite length was measured in primary RGCs. Data were mean ± SEM. Student's t-test, ∗p < 0.05, ∗∗p < 0.01.
3.2. Rg1 promoted the neurite growth of RGC-5s in a concentration and time dependent manner
The results in 3.1 demonstrated that Rg1 could effectively promote the neurite growth of RGCs. Herein we investigated the effects of Rg1 on regulatory proteins involved in nerve elongation such as GAP-43, PAX6, Rac1 and whether the effects varied with time and concentration. We found that Rg1 can increase the expression of GAP-43, PAX6 and Rac1 in a concentration dependent manner (Fig. 2A and B). In addition, the mRNA expression of GAP-43, PAX6 and CREB was significantly increased by Rg1 (Fig. 2C). GAP-43, a reliable marker of axon growth, was highly increased at both transcriptional and translational levels by Rg1 in a time dependent manner (Fig. 2D–F). Taken together, these results demonstrated that Rg1 can promote the neurite growth of RGCs in a concentration and time dependent manner.
Fig. 2.
Rg1 promoted the neurite growth of RGC-5s in a concentration and time dependent manner. (A) Rg1 upregulated the expression of GAP-43, PAX6 and Rac1. The cells were treated with 1, 10, 30 μM Rg1 for 2 days respectively and the proteins were measured by western blotting. (B) Expression levels of GAP-43, PAX6, and Rac1. (C) The mRNA expression of GAP-43, PAX6 and CREB. The cells were treated with 1, 10, 30 μM Rg1 for 2 days respectively and the mRNA was measured by qPCR. (D) The mRNA expression of GAP-43. The cells were treated with 10 μM Rg1 for 1, 2, 3, 5 days respectively and the mRNA was measured by qPCR. (E) Rg1 upregulated the expression of GAP-43. The cells were treated with 10 μM Rg1 for 1, 2, 3 days. (F) Expression levels of GAP-43. Data were mean ± SEM. Student's t-test, ∗p < 0.05, ∗∗p < 0.01.
3.3. Rg1 enhanced the synaptic plasticity of RGCs by activating cAMP/PKA/CREB pathway
The synaptic plasticity is an important parameter for evaluating neuron function. To further investigate the potential mechanism of Rg1 influencing the neurite growth of RGCs, we detected the levels of cAMP with the ELISA tests in RGC-5s incubating with 10 μM Rg1 for 15 min. Rg1 increased cAMP levels in a concentration dependent manner (Fig. 3A). The classic intracellular target of cAMP is protein kinase A (PKA), which is believed to mediate many events induced by this intracellular second messenger [26]. Therefore, we observed the changes of PKA, phospho-PKA (Thr197), CREB, phospho-CREB (Ser133) in RGC-5s after treatment with Rg1. We found that Rg1 upregulated the relative expression of phospho-PKA and phospho-CREB (Fig. 3B and C). Next, we used forskolin, a cAMP agonist, and H89, a PKA inhibitor, to observe changes of RGC-5s and primary RGCs. Forskolin showed in a similar phenomenon as Rg1 did, which promoted the neurite growth of RGC-5s, but the effect caused by Rg1 was reversed by H89 (Fig. 3D and E). Furthermore, we found that the expression of GAP-43, PAX6 and active β-catenin were upregulated by Rg1 and forskolin, but this effect was reversed by H89 (Fig. 3F and G). And we also confirmed these similar results in primary RGCs (Fig. 3H–K). Therefore, these results demonstrated that Rg1 could enhance the synaptic plasticity of RGCs by activating cAMP/PKA/CREB pathway.
Fig. 3.
Rg1 enhanced the synaptic plasticity of RGCs by activating cAMP/PKA/CREB pathway. (A) Expression of cAMP in RGC-5s incubating with 1, 10, 30 μM Rg1 for 15 min by ELISA. (B) The expression of PKA, phospho-PKA (Thr197), CREB, phospho-CREB (Ser133) in RGC-5s. The cells were treated with 1, 10, 30 μM Rg1 for 2 days respectively and the proteins were measured by western blotting. (C) Expression levels of PKA, phospho-PKA (Thr197), CREB, phospho-CREB (Ser133). (D) RGC-5s were observed under microscope after treatment with 10 μM Rg1, 1 μM forskolin for 2 days respectively, and 10 μM Rg1 adding 1 μM H89 for 2 days. (E) Neurite length was measured in RGC-5s. (F) The expression of GAP-43, PAX6 and active β-catenin in RGC-5s. The cells were treated with 10 μM Rg1, 1 μM forskolin for 2 days respectively, 10 μM Rg1 adding 1 μM H89 for 2 days, and the proteins were measured by western blotting. (G) Expression levels of GAP-43, PAX6 and active β-catenin in RGC-5s. (H) Primary RGCs were observed under microscope after treatment with 10 μM Rg1, 1 μM forskolin for 2 days respectively, and 10 μM Rg1 adding 1 μM H89 for 1 week. (I) Neurite length was measured in primary RGCs. (J) The expression of GAP-43 in primary RGCs and the proteins were measured by western blotting. (K) Expression levels of GAP-43. Data were mean ± SEM. Student's t-test, ∗p < 0.05, ∗∗p < 0.01.
3.4. Rg1 strengthened the energy metabolism through glycolysis
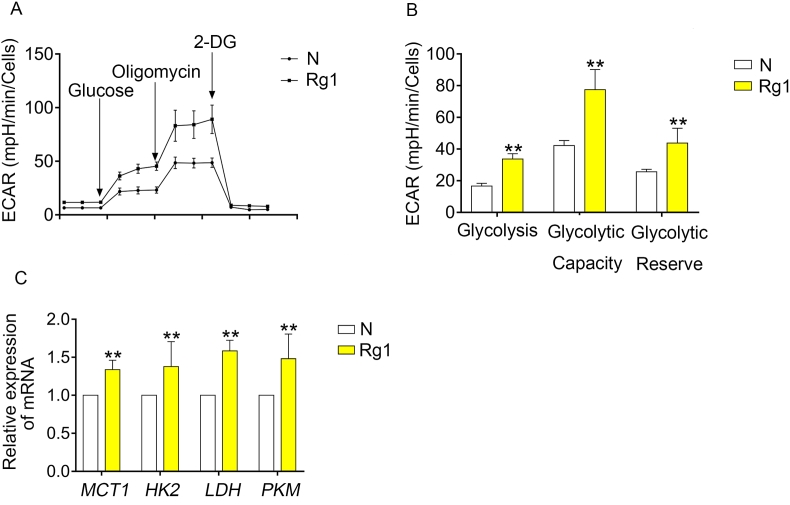
The energy metabolism in retina is very special, these “cancer-like” metabolism was called the Warburg effect, in which the retina produces energy from glycolysis even in the presence of oxygen in order to keep neurons away exciting for phototransduction and neurotransmission. This special energy metabolism may be closely relative to the neurite growth of RGCs, so we further investigate their relationship. To value the glycolytic capacities of RGCs, we detected the ECAR of RGC-5s. Rg1 significantly increased the glycolysis, glycolytic capacity and glycolytic reverse (Fig. 4A and B). Furthermore, we investigated the effect of Rg1 on enzymes in glycolysis. Rg1 upregulated the mRNA expression of hexokinase 2 (HK2), monocarboxylate transporter (MCT1), lactic dehydrogenase (LDH) and pyruvate kinase (PKM) (Fig. 4C). Together, we can conclude that Rg1 strengthen the energy metabolism by effecting glycolysis.
Fig. 4.
Rg1 strengthened the energy metabolism through glycolysis. (A) Normalized ECAR analysis of RGC-5s treated with 10 μM Rg1 for 2 days. (B) Normalized ECAR analysis of glycolysis, glycolytic capacity and glycolytic reverse. (C) The mRNA expression of HK2, MCT1, LDH, PKM. Data were mean ± SEM. Student's t-test, ∗∗p < 0.01.
3.5. Rg1 promoted neurite growth of primary RGCs in vivo
To determine the promotion of neurite growth of RGCs, Rg1 was administrated to male ICR mice through eye drops. We conduct forskolin as the positive drug control and H89 as the inhibitor. The RGCs axons were valued by retrograde labeling using CTB with a conjugated Alexafluor 488. Rg1 and forskolin increased the length and fluorescence intensity of the optic nerve, but this effect was reversed by H89 (Fig. 5A). Next, we used Golgi-Cox staining to observe the dendrite branches, dendritic spine density and dendritic length of RGCs. We found that Rg1 enhanced the complexity of dendritic of RGCs as the positive control group did, which was reversed by H89 (Fig. 5B). We conducted H&E staining to observe the ganglion cell layer (GCL). We found that the thickness of the GCL was upregulated by Rg1 and forskolin, but there is no significant difference in H89 group (Fig. 5C). Moreover, we observed the expression of GAP43, PAX6, phospho-PKA and phospho-CREB on retina by IHC. And the expression of GAP43 (Fig. 5D), PAX6 (Fig. 5E), phospho-PKA (Fig. 5F) and phospho-CREB (Fig. 5G) were increased by Rg1 and foskolin, and there is no significant change in H89 group. Furthermore, we also conducted SEM scanning to observe the density of retina (Fig. 5H and I). The Rg1 group showed more dense nerve fibre. Together we found that Rg1 promotes neurite growth of primary RGCs in vivo.
Fig. 5.
Rg1 promoted neurite growth of primary RGCs in vivo. (A) Optic nerves retrograde labeled by CTB with a conjugated Alexafluor 488. (B) Golgi-Cox stained RGCs. (C) Representative images of H&E staining in the retina of ICR mice. (D–G) Representative imagines of GAP43, PAX6, phospho-PKA and phospho-CREB IHC staining in ICR mice retina. (H–I) Representative images for SEM of retinal sections.
4. Discussion
Synaptic plasticity allows the continuous modification of connections and neural circuits between nerves to increase neurotransmission efficiency and adaptability of synapses to the environment [27]. GAP-43 is highly expressed during the growth of axons and at the growth cone end of new axons, and it is a reliable marker of axon growth [28]. Overexpression of GAP-43 can lead to spontaneous formation of new synapses and enhance sprouting after injury [29]. Studies showed the similar results in adult rat RGCs that GAP-43 increased the potential of injured RGCs to regenerate [30]. Furthermore, Rapid remodeling of the actin cytoskeleton is critical for structural plasticity. Rac1, a member of the Rho GTPase family, can affect a variety of nervous system functions including neurite growth as a key regulator of dendritic and cytoskeleton reorganization. Recent studies revealed that Rac1-cKO significantly reduced the complexity of the dendrites, resulting in a reduction in the total number of spinous branches of the dendrites. Rac1 affects excitatory synaptic transmission in RGCs by modulating dendritic complexity [31]. PAX6 is a novel downstream target of the Wnt/ß -catenin pathway, which plays an important role in neurogenesis [32]. It was demonstrated to be involved in the pathogenesis of glaucoma [33]. The posterior ganglion diseases are difficult to cure and RGCs cannot be repaired once damaged. The mechanisms of RGCs degeneration or death in various eye diseases are complex and current knowledge cannot explain the changes of RGCs. Therefore, dendritic growth, regeneration and synaptic formation are the most important parameters for evaluating the cellular protective effects of various chemical molecules [34]. Consistent with these reports, our results revealed that Rg1 not only promoted the neurite growth of RGCs and the expression of GAP-43, Rac1 and PAX6 both in vitro and in vivo, but also increased the dendritic complexity and dendritic length in vivo.
Studies have shown that the axon length, growth cone surface area and growth cone filamentous pseudopod number were significantly increased in highly purified primary RGCs by vitreous injection of forskolin or treatment with forskolin or cAMP analogues. The intraocular elevation of cAMP can promote the elongation of RGCs and directly affect RGCs [35]. In this study, we observed that Rg1 upregulated the expression levels of cAMP in RGCs. Wnt/β-catenin is an important pathway that regulates various physiological processes such as cell growth and development. β-catenin is also involved in the regulation and remodeling of neuronal synapses. PKA-mediated phosphorylation of β-catenin has been reported to enhance β-catenin transcriptional activity [36]. At the meantime, studies have shown that activation of Wnt/β-catenin signal can promote regeneration of injured RGCs axons [25]. We found that Rg1 increased the expression of active β-catenin. Together these, the effect of Rg1 on cAMP/PKA/CREB pathway has proved its good neural activity for RGCs.
RGCs are the only neurons in the retina that can generate action potential, the only visual output cells in the retina, and their axon are the main parts of the optic nerve. In addition to maintaining normal cellular functions, the retina requires a great deal of energy to keep neurons away for exciting phototransduction and neurotransmission. Therefore, the retina produces energy from glycolysis even in the presence of oxygen. This phenomenon is often observed in cancers and is known as aerobic glycolysis called the Warburg effect, and the molecular mechanisms of the retina and cancer's Warburg effect may be similar [37]. The metabolic reprogramming in the Warburg effect is a complex process in which PI3K/AKT pathway may be essential to the metabolic budget system [38]. In order to further study the mechanism of Rg1 influencing on the growth of RGCs, we also explored the relationship between the energy metabolism of RGCs and Rg1. We discovered Rg1 enhanced glycolysis and glycolysis capacity of RGCs adjusting to environmental variations, which need to be investigated in the future.
5. Conclusion
In summary, the present study, for the first time, demonstrated that Rg1 promoted the growth of RGCs both in vivo and in vitro by activating the cAMP/PKA/CREB pathway (Fig. 6). Rg1 upregulated the expression of GAP-43, Rac1, PAX6, but these effects could be reversed by H89. In addition, combined the special energy metabolism in retina and Rg1 upregulating RGCs synaptic plasticity, we proposed that Rg1 may play a role in the treatment of retinal ganglion diseases. This study may lay a foundation for the clinical use of diseases related to optic nerve injury in the future.
Fig. 6.
Schematic diagram of the neurite growth in the retina promoting effect of Rg1 by potentiating synaptic plasticity via cAMP/PKA/CREB signaling pathway.
Declaration of competing interest
All authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by the “Double First-Class” University Project (No. CPU2018GF05) of China Pharmaceutical University and the Program of Innovative Research Team of Jiangsu Province.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2022.05.002.
Contributor Information
Zhen Wang, Email: drwang@cpu.edu.cn.
Ning-hua Tan, Email: nhtan@cpu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Mateos-Aparicio P., Rodríguez-Moreno A. Calcium dynamics and synaptic plasticity. Adv Exp Med Biol. 2020;1131:965–984. doi: 10.1007/978-3-030-12457-1_38. [DOI] [PubMed] [Google Scholar]
- 2.Mansvelder H.D., Verhoog M.B., Goriounova N.A. Synaptic plasticity in human cortical circuits: cellular mechanisms of learning and memory in the human brain? Curr Opin Neurobiol. 2019;54:186–193. doi: 10.1016/j.conb.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfenninger K.H., Becky A., Helmke S.M., Quiroga S. Growth-regulated proteins and neuronal plasticity. Mol Neurobiol. 1991;5(2–4):143–151. doi: 10.1007/BF02935543. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava K., Tripathi R., Mishra R. Age-dependent alterations in expression and co-localization of Pax6 and Ras-GAP in brain of aging mice. J Chem Neuroanat. 2018;92:25–34. doi: 10.1016/j.jchemneu.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bu F., Munshi Y., Furr J.W., Min J.W., Qi L., Patrizz A., Spahr Z.R., Urayama A., Kofler J.K., McCullough L.D., et al. Activation of neuronal Ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and axonal plasticity in mice. J Neurochem. 2021;157(4):1366–1376. doi: 10.1111/jnc.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W.N., Xue X.L., Xia J., Liu J.T., Qi Z.T. Swimming exercise reverses CUMS-induced changes in depression-like behaviors and hippocampal plasticity-related proteins. J Affect Disord. 2018;227:126–135. doi: 10.1016/j.jad.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Hansel C. Deregulation of synaptic plasticity in autism. Neurosci Lett. 2019;688:58–61. doi: 10.1016/j.neulet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Li S.M., Selkoe D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer's brain. J Neurochem. 2020;154(6):583–597. doi: 10.1111/jnc.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Xue X.L., Li L., Li Y.Q., Wang Y.N., Huang T., Wang Y.H., Meng H.X., Pan B.L., Niu Q. Aluminum-induced synaptic plasticity impairment via PI3K-Akt-mTOR signaling pathway. Neurotox Res. 2020;37(4):996–1008. doi: 10.1007/s12640-020-00165-5. [DOI] [PubMed] [Google Scholar]
- 11.Engelmann C., Haenold R. Transcriptional control of synaptic plasticity by transcription factor NF-κB. Neural Plast. 2016;2016 doi: 10.1155/2016/7027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chidambaram S.B., Rathipriya A.G., Bolla S.R., Bhat A., Ray B., Mahalakshmi A.M., Manivasagam T., Thenmozhi A.J., Essa M.M., Guillemin G.J., et al. Dendritic spines: revisiting the physiological role. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;92:161–193. doi: 10.1016/j.pnpbp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Segarra-Mondejar M., Casellas-Díaz S., Ramiro-Pareta M., Müller-Sánchez C., Martorell-Riera A., Hermelo I., Reina M., Aragonés J., Martínez-Estrada O.M., Soriano F.X. Synaptic activity-induced glycolysis facilitates membrane lipid provision and neurite outgrowth. EMBO J. 2018;37(9) doi: 10.15252/embj.201797368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry L.E., Fahy E., Chrysostomou V., Hui F., Tang J., Wijngaarden P.V., Petrou S., Crowston J.G. The coma in glaucoma: retinal ganglion cell dysfunction and recovery. Prog Retin Eye Res. 2018;65:77–92. doi: 10.1016/j.preteyeres.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan R.K.P., WoldeMussie E., Pow D.V. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Invest Ophthalmol Vis Sci. 2007;48(6):2782–2791. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- 16.Zu G., Guo J., Che N.W., Zhou T.T., Zhang X.W., Wang G.Z., Ji A.L., Tian X.F. Protective effects of ginsenoside Rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the Wnt/β-catenin pathway. Sci Rep. 2016;6 doi: 10.1038/srep38480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F.L., Wu X.Q., Li J., Niu Q.L. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer's disease model. Mol Med Rep. 2016;13(6):4904–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 18.Li G., Zhang N., Geng F., Liu G.L., Liu B., Lei X., Li G., Chen X. High-throughput metabolomics and ingenuity pathway approach reveals the pharmacological effect and targets of ginsenoside Rg1 in Alzheimer's disease mice. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-43537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X.Y., Zhang J.T. Effects of ginsenoside Rg1 on synaptic plasticity of freely moving rats and its mechanism of action. Acta Pharmacol Sin. 2001;22(7):657–662. [PubMed] [Google Scholar]
- 20.Huang L., Liu L.F., Liu J., Dou L., Wang G.Y., Liu X.Q., Yuan Q.L. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11(2):319–325. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H.L., Fan C.Q., Yang L.J., Yu S.Y., Song Q.Q., Wang P., Mao X.Q. Ginsenoside Rg1 prevents chronic stress-induced depression-like behaviors and neuronal structural plasticity in rats. Cell Physiol Biochem. 2018;48(6):2470–2482. doi: 10.1159/000492684. [DOI] [PubMed] [Google Scholar]
- 22.Zhu G., Wang Y., Li J., Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015;292:81–89. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Zou L.F., Yu K.H., Fan Y., Cao S.X., Liu L.S.M., Shi L.R., et al. The inhibition by Guanfu base a of neuropathic pain mediated by P2Y12 receptor in dorsal root Ganglia. ACS Chem Neurosci. 2019;10(3):1318–1325. doi: 10.1021/acschemneuro.8b00399. [DOI] [PubMed] [Google Scholar]
- 24.Song L., Li L., Liu B., Yu D.X., Sun F.G., Guo M.M., Ruan Z.M., Zhang F.X. Diagnostic evaluations of ultrasound and magnetic resonance imaging in mammary duct ectasia and breast cancer. Oncol Lett. 2018;15(2):1698–1706. doi: 10.3892/ol.2017.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A.K., Park K.K., Hackam A.S. Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience. 2017;343:372–383. doi: 10.1016/j.neuroscience.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J.K., Heckert L.L. Dmrt1 expression is regulated by follicle-stimulating hormone and phorbol esters in postnatal sertoli cells. Endocrinology. 2001;142(3):1167–1178. doi: 10.1210/endo.142.3.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G.L., An T.Y., Lei C., Zhu X.F., Yang L., Zhang L.X., Zhang R.H. Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134–mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression. J Ginseng Res. 2022;46(3):376–386. doi: 10.1016/j.jgr.2021.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M.M., Yin Z.Q., Zhang L.Y., Liao H. Quercetin promotes neurite growth through enhancing intracellular cAMP level and GAP-43 expression. Chin J Nat Med. 2015;13(9):667–672. doi: 10.1016/S1875-5364(15)30064-9. [DOI] [PubMed] [Google Scholar]
- 29.Aigner L., Arber S., Kapfhammer J.P., Laux T., Schneider C., Botteri F., Brenner H.R., Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83(2):269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 30.Doster S.K., Lozano A.M., Aguayo A.J., Willard M.B. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6(4):635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- 31.Li L.Z., Yin N., Li X.Y., Miao Y.Y., Cheng S., Li F., Zhao G.L., Zhong S.M., Wang X., Yang X.L., et al. Rac1 modulates excitatory synaptic transmission in mouse retinal ganglion cells. Neurosci Bull. 2019;35(4):673–687. doi: 10.1007/s12264-019-00353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan Q.N., Lee A., Suzuki R., Yamagami T., Stokes A., Nguyen B.C., Pleasure D., Wang J.J., Chen H.W., Zhou C.J.J. Pax6 mediates β-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells. Stem Cell. 2014;32(1):45–58. doi: 10.1002/stem.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng J., Xu J. Identification of pathogenic genes and transcription factors in glaucoma. Mol Med Rep. 2019;20(1):216–224. doi: 10.3892/mmr.2019.10236. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X.M., Liu D.T.L., Chiang S.W.Y., Choy K.W., Pang C.P., Lam D.S.C., Yam G.H.F. Immunopanning purification and long-term culture of human retinal ganglion cells. Mol Vis. 2010;16:2867–2872. [PMC free article] [PubMed] [Google Scholar]
- 35.Argaw A., Duff G., Boire D., Ptito M., Bouchard J.F. Protein kinase A modulates retinal ganglion cell growth during development. Exp Neurol. 2008;211(2):494–502. doi: 10.1016/j.expneurol.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Potasiewicz A., Hołuj M., Boire D., Ptito M., Bouchard J.F. 3-Furan-2-yl-Np-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic receptor, reverses schizophrenia-like cognitive and social deficits in rats. Neuropharmacology. 2017;113(Pt A):188–197. doi: 10.1016/j.neuropharm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Ng S.K., Wood J.P.M., Chidlow G., Han G.G., Kittipassorn T., Peet D.J., Casson R.J. Cancer-like metabolism of the mammalian retina. Clin Exp Ophthalmol. 2015;43(4):367–376. doi: 10.1111/ceo.12462. [DOI] [PubMed] [Google Scholar]
- 38.Courtnay R., Ngo D.C., Malik N., Ververis K., Tortorella S.M., Karagiannis T.C. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42(4):841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.