Abstract
Since its outbreak in late 2019, the Coronavirus disease 2019 (COVID-19) pandemic has profoundly caused global morbidity and deaths. The COVID-19 pandemic caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has major complications in cardiovascular and pulmonary system. The increased rate of mortality is due to delayed detection of certain biomarkers that are crucial in the development of disease. Furthermore, certain proteins and enzymes in cellular signaling pathways play an important role in replication of SARS-CoV-2. Most cases are mild to moderate symptoms, however severe cases of COVID-19 leads to death. Detecting the level of biomarkers such as C-reactive protein, cardiac troponin, creatine kinase, creatine kinase-MB, procalcitonin and Matrix metalloproteinases helps in early detection of the severity of disease. Similarly, through downregulating Renin-angiotensin system, interleukin, Mitogen-activated protein kinases and Phosphoinositide 3-kinases pathways, COVID-19 can be effectively controlled and mortality could be prevented. Ginseng and ginsenosides possess therapeutic potential in cardiac and pulmonary complications, there are several studies performed in which they have suppressed these biomarkers and downregulated the pathways, thereby inhibiting the further spread of disease. Supplementation with ginseng or ginsenoside could act on multiple pathways to reduce the level of biomarkers significantly and alleviate cardiac and pulmonary damage. Therefore, this review summarizes the potential of ginseng extract and ginsenosides in controlling the cardiovascular and pulmonary diseases by COVID-19.
Keywords: Ginseng, Ginsenoside, COVID-19, Biomarkers, Cardiac and pulmonary complications
Graphical abstract
1. Introduction
Recently, Coronavirus disease 2019 (COVID-19) started around 2019 and is spreading very severely. There are at least 690 million cases of COVID-19 reported around the world. There were also 662 million people recovered from the disease, thanks to the rapid treatment and widespread vaccination around the globe. Vaccination has hindered the development of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and has reduced the mortality drastically. Several studies have reported that COVID-19 is not only a respiratory disease but can spread to other organs as well. Most cases are asymptomatic or with mild symptoms only, which does not help in early detection to control the spread of disease. Pulmonary injury is the first target for SARS-CoV-2, and symptoms begin with a mild flu and could leave to severe lung damage and multiple organ damage, then eventually leading to death. Through angiotensin converting enzyme (ACE) receptors, SARS-CoV-2 affect the heart and lungs, and develop diseases such as pneumonia, acute respiratory distress syndrome (ARDS), and multiple organ damage. The immune response from pulmonary infection by COVID-19 will compromise the activity of lungs and induce acute to severe lung injury [1]. Autopsies revealed pulmonary and airway-associated lesions and pathological studies found diffuse alveolar damage in patients [2]. ARDS will lead to fluid accumulation in the lungs and the inflammatory response will induce ground-glass opacification in lungs [3]. SARS-CoV-2 causes major damage to lungs, however there were several complications on heart and other organs when the disease is severe. In COVID-19 patients, about 69% had comorbidities specifically with cardiovascular disease, among them particularly males with hypertension exhibited cardiac damage with severity of COVID-19 [4]. High levels of cardiac troponin is reported on patients with cardiac injury, and this occurs up to 12% of all the patients, involvement of virus on cardiomyocytes and systemic inflammation are the major mechanisms for cardiac injury [5]. Cardiopulmonary symptoms and cardiovascular abnormalities reported from patients after the acute phase of COVID-19 [6], also cardiac disease is the major comorbidity in COVID-19 patients [7].
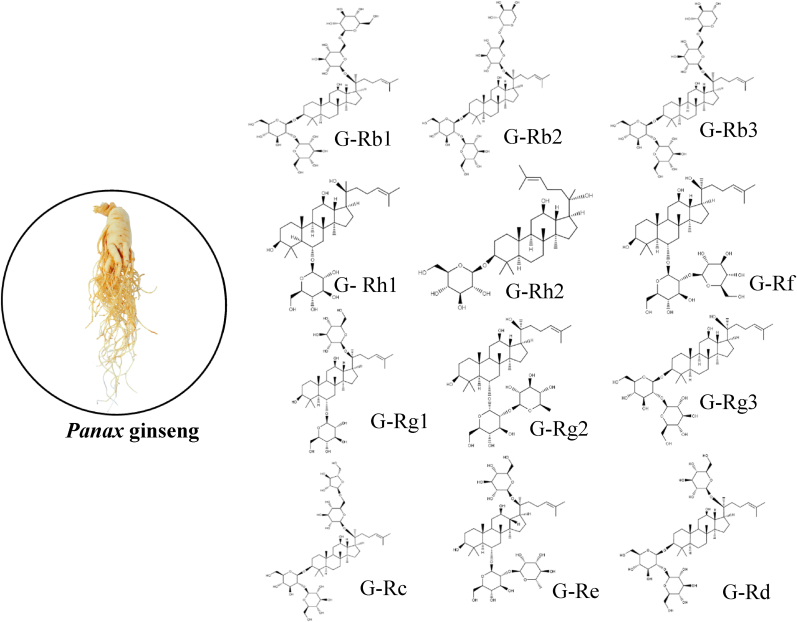
Ginseng has been used as a medicine for over centuries traditionally, mainly in asian countries like Korea and China. Korean ginseng (Panax ginseng) contains the bioactive components ginsenosides, which has potential therapeutic effect against several disease. Structurally each ginsenoside are different, but most commonly ginsenoside have a sugar molecule attached to steroid skeleton and hence they are amphiphilic in nature[Fig. 1]. Ginseng and its components exert anti-inflammatory effect by targeting inflammasome stimulation, Panax ginseng inhibit NLRP3 inflammasome stimulation that might lead to reduced inflammatory responses [8]. Supplementation of ginseng can prevent thrombosis and reduced mortality in COVID-19 patients [9]. In pulmonary system, ginseng possess protective and therapeutic activity that is evident from multiple research. American ginseng on induced chronic obstructive pulmonary disease (COPD) exhibited preventive effect and ameliorated lung function through tumor necrosis factor alpha (TNF-α) and interleukin pathways (IL) in mice [10]. Panaxydol (a component of Panax ginseng), ameliorated acute lung injury in lipopolysaccharide (LPS)-induced injury in mice [11]. Ginseng and ginsenosides have potential benefits in cardiovascular diseases and can help in healing cardiac damage induced by COVID-19. Prophylactic supplementation of ginseng protected from cardiac dysfunction in chemotherapy, similarly ginsenoside Rg1 ameliorated cardiac remodeling in mice suggesting the potential effect of ginsenosides against cardiac damage [12]. Also, ginsenoside Rg5 inhibited cardiac inflammation and protected the hearts of angiotensin II induced mice in hypertensive heart failure [13]. SARS-CoV-2 primarily affects the pulmonary system, but it could spread to heart and other organs. Based on the severity of these diseases, the virus will continue to spread, however it targets the respiratory system first followed by heart and other organs. This review is to focus on specific activities of ginseng and ginsenosides in preventing and ameliorating cardiac and pulmonary complications that arises due to COVID-19. In this review, we emphasize on significance of biomarkers and specific pathways that are involved in the pathogenesis of COVID-19 and the significance of ginseng or ginsenosides in ameliorating the disease by controlling these parameters.
Fig. 1.
List of the representative ginsenosides from Panax ginseng. Ginsenosides are expressed by Rx, where x is determined by the distance from the origin of thin-layer chromatography. Ginsenosides are generally divided into three groups: protopanaxadiols, protopanaxatriols, and oleanane (ginsenoside Ro). Protopanaxadiols have sugar moieties on the C-3 position of dammarane-type triterpene, such as ginsenosides Rb1(G-Rb1), Rb2(G-Rb2), Rb3(G-Rb3), Rc(G-Rc), Rd(G-Rd), Rg3(G-Rg3), and Rh2(G-Rh2). Protopanaxatriols have sugar moieties on the C-6 position of dammarane-type triterpene, such as ginsenosides Re(G-Re), Rf(G-Rf), Rg1(G-Rg1), Rg2(G-Rg2), and Rh1(G-Rh1).
2. Ginseng and ginsenosides on the biomarkers of COVID-19
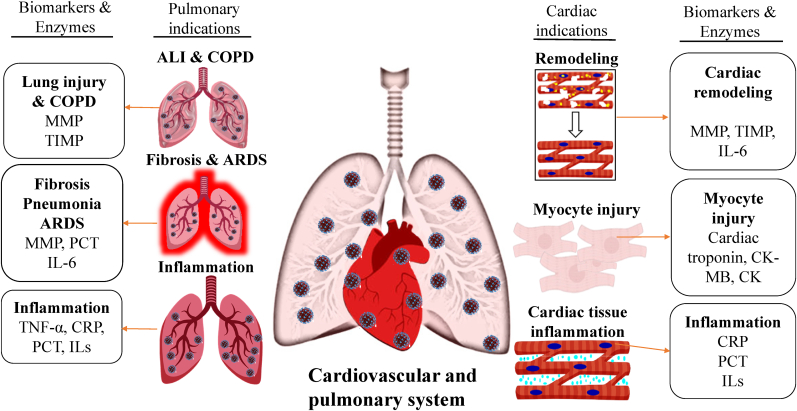
It is highly known that SARS-CoV-2 majorly affects the lungs, but can also affect the heart based on its severity. The virus attacks through the pulmonary system first by preventing the oxygen reaching from the heart muscle, which might lead to damage in heart tissue. The earlier stage of the disease might be due to binding of ACE receptor 2 to the virus, leading to lung damage. Further clinical examination revealed that several patients had declined cardiac health who never had history of cardiac illness[Fig. 2]. Biomarkers such as cardiac troponin, creatine kinase (CK), creatine kinase-MB (CK-MB) and myoglobin can help in examining cardiac health specifically to heart attack and coronary artery blockage [14]. A meta-analysis study reported that severity and mortality of COVID-19 patients could be reduced by identifying the level of these biomarkers [15]. In another study, Korean Red Ginseng (KRG) suppressed the biomarkers such as creatine kinase-MB and cardiac troponin I, thus preventing myocardial ischemia in guinea pigs [16]. A clinical study performed on 14 male adults in Taiwan, showed that supplementation with American ginseng has attenuated plasma CK activity after downhill running exercise and there were no muscle soreness for any of the subjects studied [17]. Earlier, we also reported that supplementation of red ginseng (Panax ginseng) in porcine has reduced the level of cardiac troponin I compared to the disease control (Isoproterenol induced myocardial infarction (MI)), indicating the protective effects of ginseng [18]. Similarly, in doxorubicin-induced myocardial dysfunction the cardiac troponin I level were ameliorated dose dependently by treatment with KRG signifying the protective effects [19]. These studies on cardiac injury, suggests that targeting biomarkers of heart throughout the treatment and effective supplementation with ginseng could weaken the symptoms of cardiac injuries and improve the function of heart for COVID-19 patients.
Fig. 2.
Biomarkers & enzymes involved in cardiac and pulmonary complications due to COVID-19. SARS-CoV-2 targets the respiratory system first with mild symptoms and then spread to cardiovascular system. The severe infection of the virus is stimulated by the over expression of biomarkers and enzymes. Abbreviation; Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Acute Lung Injury (ALI), Chronic Obstructive Pulmonary Disease (COPD), Acute Respiratory Distress Syndrome (ARDS), Matrix metalloproteinase (MMP), Tissue inhibitors of metalloproteinase (TIMP), Interleukin-6 (IL-6), Creatine kinase-myoglobin (CK-MB), Creatine kinase (CK), C-reactive protein (CRP), procalcitonin (PCT), Interleukins (ILs).
In COVID-19 patients and patients with lung injury C-reactive protein (CRP), procalcitonin (PCT), and creatine kinase (CK) help in detecting the severity of the disease. Several studies have reported that COVID-19 patients have reported the elevated levels of PCT and require emergency treatment [20]. Likewise, CRP is a protein that could be associated with the overproduction of inflammatory cytokines in COVID-19 patients. In another study, there was elevated levels of CRP up to 86% in patients with severe symptoms of COVID-19 [21]. Several findings suggest that estimation of CRP levels could predict the prognosis and severity of the disease even before the disease progression and the indication of clinical symptoms [22]. A meta-analysis revealed that ginseng supplementation might help in reducing the CRP level significantly in patients with elevated CRP levels [23]. Another study performed on 90 participants revealed that after 12 weeks of low-dose supplementation with fermented ginseng powder has significantly reduced the high-sensitivity CRP (hs-CRP) compared to the placebo group [24]. A clinical study conducted on pneumonia patients revealed that there was significant decrease in the serum PCT level for patients who received two times of 100 mg/day of ginseng extract for 14 days. Furthermore, there were reduced levels of neutrophils and length of stay in hospital were reduced [25]. A study performed on male Wistar rats with both alcohol and aqueous extract of ginseng (300 mg/kg/day) for 14 days, has shown substantial reduction in plasma CK levels and able to ameliorate eccentric exercise-induced muscle damage and inflammation [26]. In another study, supplementation of Panax ginseng along with salvia miltiorrhiza on healthy adult males had significantly reduced plasma concentration of CRP levels and ameliorated arterial stiffening induced by eccentric exercise [27]. Estimation of CRP levels are very crucial for cardiac and pulmonary related problems. Correspondingly, PCT and CK level are equally important to determine the proliferation of SAR-CoV-2. Ginseng and ginsenosides can augment the therapy for COVID-19 by effectively suppressing these biomarkers[Table 1]. Matrix metalloproteinases (MMPs) are calcium-dependent zinc-containing endopeptidases that play an important role in tissue remodeling and serve as a biomarker for several lung related diseases. These enzymes act in the extracellular environment of cells and involved in degrading extracellular matrix through cleavage of matrix and non-matrix proteins. Additionally, they play an important role in pathological process of tissue repair and remodeling, inflammation and angiogenesis [43]. Furthermore, in COVID-19 patients, MMP3 was drastically higher and served as a tool for measuring the severity of disease in the COVID-19 patients [44]. Similarly, in another study, levels of MMPs MMP-2, MMP-3, MMP-9, and MMP-12 were significantly increased in cerebrospinal fluid of patients with neurological syndrome [45]. As previously, described ginseng and its active component ginsenosides exhibit therapeutic activity on regulation on certain enzymes and downregulating the gene expressions. KRG and its active components could potentially inhibit 3T3-L1 adipocytes through inhibition of MMP-2 and MMP-9. Ginsenosides such as Rc, Rd, Rf, Rg1, Rg3 and F4 are found to inhibit expression of MMP-13 in interleukin-1β-treated chondrocytes [37]. In another study, ginsenoside Rb2 treated cells suppressed the ultraviolet-B-induced ROS level and expression of MMP-2 activity, indicating the therapeutic potential of ginsenoside Rb2 [38]. The disease progression of COVID-19 depends upon the level of above mentioned biomarkers, ginsenoside acts on these biomarkers in ameliorating the development of disease and might assist in the treatment for COVID-19 related cardiac and pulmonary complications.
Table: 1.
Role of Ginseng and Ginsenoside With Biomarkers Involved in Cardiac and Pulmonary Diseases
| Biomarker | Ginseng extract/ ginsenosides | Therapeutic role | Reference |
|---|---|---|---|
| Cardiac troponin | KRG | Myocardial (I/R) Injury | [16] |
| Panax ginseng | Isoproterenol induced MI | [18] | |
| KRG | Doxorubicin induced MI | [19] | |
| G-Rc | M/I | [28] | |
| G-F1 (metabolite of Re & Rg1) | Myocardial oxidative stress and apoptosis | [29] | |
| Saponins from Panax quinquefolius | Cisplatin-induced cardiotoxicity | [30] | |
| Panax ginseng | Isoproterenol induced MI | [18] | |
| G-Re | Isoproterenol induced M/I injury | [31] | |
| G-Rc | Myocardial (I/R) Injury | [32] | |
| CK | American ginseng | Exercise induced muscle damage | [17] |
| North American ginseng | Exercise induced muscle damage and inflammation | [26] | |
| Saponins from Panax quinquefolius | Cisplatin-induced cardiotoxicity | [30] | |
| Total ginseng | M/I | [33] | |
| CK-MB | KRG | M/I | [16] |
| G-Rc | Cold-exposure induced myocardial injury | [34] | |
| G-Rc | M/I | [28] | |
| Saponins from Panax quinquefolius | Cisplatin-induced cardiotoxicity | [30] | |
| G-Re | Isoproterenol induced M/I injury | [31] | |
| G-Rc | Myocardial (I/R) Injury | [32] | |
| G-Rb3 and G-Rb2 combined treatment | Myocardial (I/R) Injury | [35] | |
| CRP | Red ginseng | Inflammation | [23] |
| Panax ginseng with salvia miltiorrhiza | Eccentric exercise-induced vascular stiffening | [27] | |
| Fermented ginseng powder | Fatigue and renal protection | [24] | |
| PCT | Ginseng extract G155 | Pneumonia | [25] |
| Ginseng extract | Community acquired pneumonia | [36] | |
| MMP | KRG | Adipogenesis | [37] |
| G-Rb2 | Ultraviolet-B-induced Reactive oxygen species | [38] | |
| G-Rg1 | Sepsis-induced cardiac dysfunction | [39] | |
| Total ginsenoside | Bleomycin induced pulmonary fibrosis | [40] | |
| G-Rh2 | Hypoxic tumor in lung adenocarcinoma cells | [41] | |
| G-Rg3 | Nasopharyngeal carcinoma | [42] |
Abbreviation; Ischemia and reperfusion (I/R), myocardial ischemia (M/I), myocardial infarction (MI), ginsenoside Rb2 (G-Rb2), ginsenoside Rb3 (G-Rb3), ginsenoside Rc (G-Rc), ginsenoside Re (G-Re), ginsenoside F1 (G-F1), ginsenoside Rg1 (G-Rg1), ginsenoside Rg3 (G-Rg3), ginsenoside Rh2 (G-Rh2), Korean Red Ginseng (KRG), Creatine kinase-myoglobin (CK-MB), Creatine kinase (CK), C-reactive protein (CRP), procalcitonin (PCT), Matrix metalloproteinases (MMP).
3. Ginseng and ginsenoside on cardiac and pulmonary injury
Panax ginseng has been used for several illness and multiple studies were performed to understand its therapeutic effect. Ginsenoside have potential anti-oxidant and anti-inflammatory activities that is supported with several in vitro and in vivo studies. Namely, in a study performed on Sprague-dawley rats, pretreatment with ginsenoside Rb2 has attenuated lung injury including inflammation and pulmonary edema. And, this potential activity might be through inhibiting TNF-α signaling pathway [46]. In another study performed on mice, KRG has attenuated the symptoms of cadmium induced lung injury. This study has also found that KRG could inhibit the upregulation of inflammatory signal of Mitogen-activated protein kinases (MAPK) [47]. In lung cancer cell line (A549), fermented black color ginseng has shown potential anti-lung cancer properties, furthermore it has inhibited the replication of SARS-CoV-2-infected Vero E6 cell and reduced the amount of viral RNA copies in the extracellular environment [48]. In another study, virus infected mice fed with ginseng extract for 180 days showed lower viral titers in the lung compared to control. Based on this study, ginseng supplementation can enhance immune responses in human ACE 2-transgenic mice infected with SARS-CoV-2 [49]. In a study conducted on subjects with acute respiratory illness, KRG treatment group had shorter duration of symptoms and frequency of cough compared to the placebo group [50]. A study conducted with 11 isolated ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, and Rh2) from KRG extract, each of the ginsenoside showed reduction in total cell numbers in broncho-alveolar lavage fluid. Moreover, ginsenoside Rc, Re, Rg1 and Rh2 exhibited even more effect than others. Specifically, ginsenoside Re suppressed significantly on the lungs against inflammation possibly by blocking MAPKs, NF-kB/c-Fos signaling pathway [51]. In another study, modified KRG (Rh2-enriched KRG) demonstrated inhibition of lung cancer development and metastases by targeting cell death through multiple pathways [52]. Similarly, treatment with Rk1 and Rg5 could subside non-small cell lung carcinoma by suppressing the activation of Smad2/3, Nf-kB, ERK, p38 MAPK and c-Jun N-terminal kinase pathway [53]. Meanwhile, Panax notoginseng saponin (PNS) and the constituents ginsenosides Rg1 and R1 might aid in angiogenesis during cardiac injury [54]. Similarly, ginsenoside Rd ameliorated myocardial ischemia injury by modulating adipocytes and cardiomyocytes in isoproterenol induced heart failure in mice [55]. Ginsenoside Rc drastically improved cardiac function, by inhibiting enzymes such as lactate dehydrogenase, aspartate aminotransferase and creatine-kinase isoenzyme in rats. This study suggested that ginsenoside Rc could possibly protect from cold exposure-induced myocardial damage [34]. Stem-leaf saponins from PNS diminished autophagy and apoptosis in the myocardial cells, this cardiac protective activity studies on mice might be exerted through PI3K/Akt/mTOR pathway [56]. Against myocardial ischemia/reperfusion (I/R) injury in rats, ginsenoside Rb1 ameliorated the injury and improved cardiac function by inhibiting CK-MB, cardiac troponin I and cathepsin B through mTOR signaling pathway [57]. Also, in ginseng treated group, cardiac troponin I levels were significantly reduced compared to the non-treatment group and protected the cardiac tissue [58]. These findings implies the role of active constituents in ginseng towards defending cardiac injury. Either ginsenosides or the total extract of ginseng could be beneficial in treatment and enhance the cardiac activity in patients.
4. Beneficial roles of ginseng and ginsenosides on signaling pathways involved in COVID-19
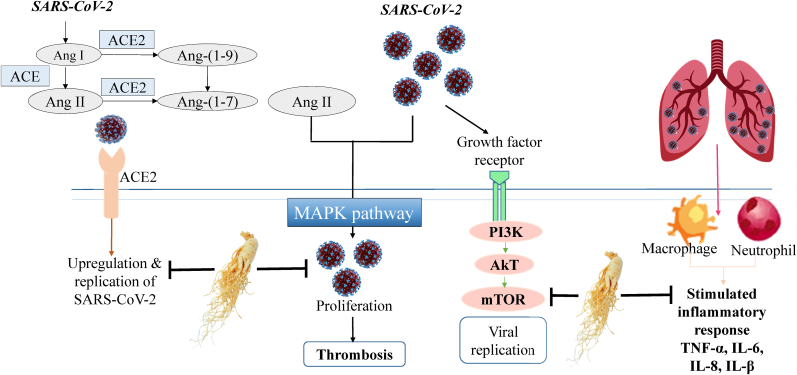
Signaling pathways are very crucial for any viral infection and replication, hyper-activation of the signaling pathway helps in rapid spread of SARS-CoV-2 and initiate a strong inflammatory response. There are several pathways involved in COVID-19 and targeting multiple pathways is required to control the clinical manifestations. The virus replicates rapidly in the host cells by deregulating PI3K/Akt/mTOR pathway, also increased pro-inflammatory response activates MAPK/ERK pathway. Pulmonary infections from SARS-CoV-2 might be binding to ACE2 receptor present in the surface on alveolar cells, coronavirus penetrates intracellularly through renin angiotensin pathway and causes severe pulmonary and cardiac malfunctions[Fig. 3]. Similarly, cytokines are significantly increased among the COVID-19 patients, the role of interleukin pathway is one of the most important factors in early detection of the disease.
Fig. 3.
Pathways involved in COVID-19 and potential effect of ginseng and ginsenosides. Ginseng and ginsenosides exerts therapeutic activity by downregulating ACE2 receptor PI3K/Akt/mTOR and MAPK pathway, and by reducing overexpression of inflammatory responses. Abbreviation; Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Angiotensin converting enzyme (ACE), Angiotensin (Ang), Mitogen-activated protein kinases (MAPK), phosphoinositide3-kinase (PI3K), Protein kinase B (Akt), mammalian target of rapamycin (mTOR), Tumor necrosis factor alpha (TNF-α), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin beta (IL-β).
4.1. Renin angiotensin pathway of ginseng and ginsenosides
Renin-angiotensin system (RAS) is a hormone system that plays an important role in regulating blood pressure, electrolyte balance and systemic vascular resistance. To maintain this role, RAS is controlled by the activity of renin, ACE, and angiotensin receptor. Hyperactivity of renin, ACE or angiotensin receptor is a usual sign of abnormal cardiac or pulmonary action such as hypertension, left ventricular hypertrophy, MI and congestive heart failure. In COVID-19 disease, ACE2 acts as the cellular receptor for the virus in lungs and heart. In a study performed on hospitalized patients angiotensin receptor blockers and ACE inhibitors is associated with reduced risk of in-hospital mortality [59]. Similarly, in another study it was shown that an imbalance in the RAS could be linked to the severity of COVID-19 [60] [Table 2]. When hypotensive components-enriched fraction of red ginseng (HCEF-RG) was administered orally to spontaneously hypertensive rats, there was decreased activity seen in renin, ACE and angiotensin II levels [61]. Likewise, Panax notoginseng (Burkill) F. H. Chen flower saponins (PNFS) inhibited the hyperactivity of RAS, while improving vascular endothelial function and reducing the blood pressure in rats with metabolic hypertension [62]. Besides, ginseng had cardio and renal protective effects on rats by downregulating NF-κB/PKC/AT1R, there was reduced content of angiotensin II type 1 receptor (AT1 receptor) in the heart tissue compared to the epinephrine treated group [63]. In another study, ginsenoside Rg5 had a ACE-inhibition action on wild-type AB line zebra fish, indicating ginsenoside Rg5 could enhance cardiac function in a dose-dependent manner [64].
Table: 2.
Pathways for Ginseng and Ginsenosides Involved in Cardiac and Pulmonary Diseases
| Pathway | Ginseng extract / ginsenosides | Therapeutic role | Reference |
|---|---|---|---|
| RAS | Panax notoginseng (Burkill) F. H. Chen flower saponins | Metabolic hypertension | [62] |
| G-Rb1 | Cardiac remodeling | [78] | |
| G-Rg5 | Heart failure | [64] | |
| KRG | Hypertension | [61] | |
| Panax ginseng extract | Epinephrine-induced MI | [63] | |
| G-Rg1 and G-Rg3 | Hypertension and cardio vascular protection | [79] | |
| MAPK/ERK | G-Rb3 | COPD | [80] |
| Notoginsenoside R1 | Enhancing cardiac function | [81] | |
| G-Rg3 | Lung injury | [82] | |
| Ginseng root extract | anti-inflammatory and anti-oxidative effect | [68] | |
| Ginsenoside Compound K | Anti-adipogenic effect | [83] | |
| Gintonin | Anti-inflammation activity | [70] | |
| G-Rb1 | Bacterial infection in bronchoalveolar cells | [69] | |
| G-Rb1 | Staphylococcus aureus-induced mouse | [84] | |
| Acute lung injury | |||
| G-Rh1 | Asthma and allergic inflammation | [85] | |
| PI3K/Akt/mTOR | G-Rg1 | Sepsis-induced cardiac dysfunction | [39] |
| G-Rb1 | Myocardial (I/R) Injury | [86] | |
| G-Rg1 | Non small cell lung cancer | [87] | |
| Notoginsenoside R1 | Enhancing cardiac function | [81] | |
| Total saponin from KRG | Platelet αIIb/β3-mediated thrombotic disease | [76] | |
| Ginsenoside Compound K | Anti-adipogenic effect | [83] | |
| G-Rh1 | Asthma and allergic inflammation | [85] | |
| Panax ginseng berry calyx | macrophage-mediated inflammatory response | [74] | |
| G-Rb3 | Cisplatin-induced nephrotoxicity | [30] | |
| G-Rd | Cardiac dysfunction | [88] | |
| G-Rg1 | alcohol-induced myocardial injury | [89] | |
| Interleukin signaling | Ginseng extract | Community acquired pneumonia | [90] |
| G-Rb1 | Cancer Cachexia | [91] | |
| KRG | Allergic rhinitis | [92] | |
| KRG | Anti-inflammatory and autophagy promoting activities | [93] | |
| Panax KRG extract | Respiratory syncytial virus | [94] | |
| G-Rg6 | Anti-inflammatory and lung damage | [95] | |
| G-Rb1 | Induced asthma | [96] |
Abbreviation; Chronic Obstructive Pulmonary Disease (COPD), myocardial infarction (MI), ginsenoside Rb1 (G-Rb1), ginsenoside Rb3 (G-Rb3), ginsenoside Rc (G-Rc), ginsenoside Rd (G-Rd), ginsenoside Rg1 (G-Rg1), ginsenoside Rg3 (G-Rg3), ginsenoside Rg5 (G-Rg5), ginsenoside Rg6 (G-Rg6), ginsenoside Rh1 (G-Rh1), renin angiotensin system (RAS) pathway, Mitogen-activated protein kinases (MAPK) pathway, extracellular signal-regulated kinases (ERK) pathway, Phosphoinositide 3-kinases (PI3K) pathway, protein kinase B (Akt) pathway, mammaliam target of rapamycin (mTOR).
4.2. Role of ginseng and ginsenosides in regulating MAPK/ERK pathway
Mitogen-activated protein kinases (MAPK) or extracellular signal-regulated kinases (ERK) pathway includes many activated proteins through increased pro-inflammatory cytokine production. SARS-CoV-2 has shown an increased level of MAPK-related biomarkers in the patients and these biomarkers might be helpful in identifying patients who are with risk of serious complications from the disease. Namely, the p38 MAPK pathway plays a key role with inflammation implicated in heart and pulmonary systems, and SARS-CoV-2 might directly upregulated this pathway [65]. Similarly, downregulating MAPK pathway and or NF-κB pathway might attenuate the implications of COVID-19 infection [66]. Myocardial inflammation associated with infection of SARS-CoV-2 upregulates MAPK pathways and complement system on patients [67]. Ginseng root extract fed to mice at 0.18g/kg for 10 days exerted anti-inflammatory and anti-oxidative activities through MAPK/NF-κB pathway and treatment of ginseng root extract had significantly inhibited phosphorylation of MAPKs [68]. Likewise, ginsenoside Rb1 increased the phosphorylation of p38 MAPK and Akt, along with macrophage phagocytosis in mouse lung cells [69]. Gintonin isolated from ginseng, has shown to mediate signal transduction pathway via MAPH and NF-κB pathways exerting its anti-inflammatory activity [70].
4.3. Role of ginseng and ginsenosides in regulating PI3K/Akt/mTOR pathway
SARS-CoV-2 infection induced the activation of Akt pathway, and treatment with an Akt inhibitor, MK-2206, eradicated interleukin expressions, indicating the affect of SARS-CoV-2 for cytokines prduction through Akt pathway [71]. In another study, increased activation of PI3K in infected cells could increase mTORC1 signaling, furthermore replication of the virus was decreased by applying mTORC1 inhibitors [72]. In hospitalized patients, there was increased phosphorylation of PI3K pathway and it shows that Akt signaling pathway plays a crucial role with platelet activation in severely affected patients [73]. In a study performed on RAW264.7 cells, an ethanolic extract of panax ginseng berry calyx reduced protein kinase B (AKT) 1 and AKT2 activities, and immunoblotting studies revealed that this ethanolic extract blocked the AKT enzymes and it could attenuate macrophage-mediated inflammatory response [74]. Similarly, Siberian ginseng extract inhibited mTORC1 pathway by downregulating phosphorylated S6K in brain, liver and muscle tissues on diabetes-induced obese mice [75]. In another study, KRG inhibited binding of fibrinogen and fibronectin to αIlb/β3 through phosphorylation of VASP and dephosphorylation of PI3K and Akt pathways, indicating the potential benefits of total saponin in prevention of platelet mediated thrombotic disease [76]. Pretreatment of ginsenoside Rb3 exhibited protective effects in HEK293 cells and renal injury through AMPK/mTOR signaling pathway, and ginsenoside Rb3 alleviated cisplatin-induced nephrotoxicity through the same pathway [77].
4.4. Role of ginseng and ginsenosides in regulating Interleukin signaling pathway on COVID-19
There are more than 50 interleukins involved in regulating immune response. Interleukin 2 (IL-2) controls the activities of lymphocytes and activated by CD4+T cells and CD8+ T cells. While Interleukin 6 (IL-6) functions as a pro-inflammatory cytokine, and could potentially serve as a marker for COVID-19 patients with severe infections. A meta-analysis study revealed that, IL-6 levels were drastically high in COVID-19 patients and resulted in unfavourable clinical outcome. This implies that determination of IL-6 levels could be a potential tool in COVID-19 patients [97]. Similarly, IL-6 levels when monitored daily basis on hospitalized patients, non-survivors group had about 10 fold higher values compared to the survivors group. In few days, because IL-6 reached a peak level in non-survivors group compared to survivors group, this data suggest that IL-6 could be a prognostic marker in COVID-19 patients [98]. In a study performed in Slovakia, the concentration of more than 24 pg/mL of IL-6 predicted the development of hypoxemia requiring oxygen therapy. With IL-6 above 24 pg/mL can put a patient at risk of respiratory failure. Therefore, detection of IL-6 levels at early stage is crucial in predicting the severity of disease [99]. Aqueous extract of ginseng and ginsenoside Rb1 (Rb1) on cancer cachexia mice reduced the level of TNF-α and IL-6 levels, thereby ameliorating the induced inflammation in mouse model [91]. In aged mice, aqueous extract of KRG suppressed the mRNA expression in genes such as (IL)-1β, IL-8, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein-1, and IL-6 in several organs. Furthermore, KRG inhibited NF-kB and AP1 protein levels, specifically in lung and kidney [93]. In another study, oral administration of KRG suppressed the IL-6 and IL-8 levels in respiratory syncytial virus (RSV) infected murine model. Namely, ginseng extract enhanced the survival rate in human lung epithelial cells against RSV and its replication [94]. Similarly, in formalin-inactivated-RSV mouse model, ginseng lowered IL-4 cells and reduced the levels of CD4+T (CD3+CD8-) cells, thereby increasing the ratio of CD8+/CD4+ T Cells [95]. Likewise, in asthma model of mice, ginsenoside Rb1 suppressed IL-4 level and ameliorated airway inflammation and progression of asthma [96].
5. Summary
Several studies indicate that ginseng and ginsenosides can increase blood circulation, regulate lipid profile and ameliorate vasomotor tone in physiological and pathologic conditions related to cardiovascular diseases such as myocardial ischemia, cardiac inflammation. Therefore, we review ginseng and ginsenosides could effectively attenuate the these diseases by COVID-19. Therefore, ginseng or ginsenosides could likely regulate these pathways and mitigate the severe complications such as heart and lung. But, there is no precise report of ginseng could be effective in the treatment for diseases caused by COVID-19. Nevertheless, ginseng and ginsenosides could provide a basis for prevention of cardiac and pulmonary complications by COVID-19.
Declaration of competing interest
The authors have declared no conflict of interest
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(2020R1l1A3072212). This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(2019R1A6A1A03033084).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2023.10.002.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Su W.L., Lu K.C., Chan C.Y., Chao Y.C. COVID-19 and the lungs: a review. J Infect Public Health. 2021;14(11):1708–1714. doi: 10.1016/j.jiph.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., von der Thusen J., Timofeev S., Gorkiewicz G., Lunardi F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477(3):359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N., Soni D., Das S., Hasan M., Patel M., et al. Diagnostics for SARS-CoV-2 infections. Nat Mater. 2021;20(5):593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., Zhang Y., Wang F., Liu B., Li H., Tang G., Chang Z., Liu A., Fu C., Lv Y., et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID-19) BMC Cardiovasc Disor. 2020;20(1):1–7. doi: 10.1186/s12872-020-01758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J., Zhu X., Jian J., Chen X., Yin K. Cardiovascular disease in patients with COVID-19: evidence from cardiovascular pathology to treatment. Acta Biochim Biophys Sin(Shangai). 2021;53(3):273–282. doi: 10.1093/abbs/gmaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi Y.-S. Potential benefits of ginseng against COVID-19 by targeting inflammasomes. J Ginseng Res. 2022;46(6):722–730. doi: 10.1016/j.jgr.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.Y., Quah Y., Shin J.H., Kwon H.W., Lee D.H., Han J.E., Park J.K., Kim S.D., Kwak D., Park S.C., et al. COVID-19 and Panax ginseng: targeting platelet aggregation, thrombosis and the coagulation pathway. J Ginseng Res. 2022;46(2):175–182. doi: 10.1016/j.jgr.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H., Wang C., Yu H., Liu Y., Tan L., He S., Li Z., Wang C., Wang F., Li P., et al. Protective effect of total Saponins from American ginseng against cigarette smoke-induced COPD in mice based on integrated metabolomics and network pharmacology. Biomed Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112823. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Lu K., Sun F., Tan S., Zhang X., Sheng W., Hao W., Liu M., Lv W., Han W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J Transl Med. 2021;19(1):1–14. doi: 10.1186/s12967-021-02745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan S., Xin Y., Ding Y., Zhang Q., Han W. Ginsenoside Rg1 protects against cardiac remodeling in heart failure via SIRT1/PINK1/parkin‐mediated mitophagy. Chem Biodivers. 2023;20(2) doi: 10.1002/cbdv.202200730. [DOI] [PubMed] [Google Scholar]
- 13.Yu T., Xu X., Wei J., Xu J., Luo W., Li A., Liang G., Wang M. Ginsenoside Rg5 alleviates Ang II-induced cardiac inflammation and remodeling by inhibiting the JNK/AP-1 pathway. Int Immunopharmacol. 2023;120 doi: 10.1016/j.intimp.2023.110408. [DOI] [PubMed] [Google Scholar]
- 14.Hama Amin B.J., Kakamad F.H., Ahmed G.S., Ahmed S.F., Abdulla B.A., Mohammed S.H., Mikael T.M., Salih R.Q., Ali R.K., Salh A.M., et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond). 2022;77 doi: 10.1016/j.amsu.2022.103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An W., Kang J.S., Wang Q., Kim T.E. Cardiac biomarkers and COVID-19: a systematic review and meta-analysis. J Infect Public Health. 2021;14(9):1191–1197. doi: 10.1016/j.jiph.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim K.H., Kang C.W., Choi J.Y., Kim J.H. Korea red ginseng induced cardioprotection against myocardial ischemia in Guinea pig. Korean J Physiol Pharmacol. 2013;17(4):283. doi: 10.4196/kjpp.2013.17.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C.H., Lin Y.A., Chen S.L., Hsu M.C., Hsu C.C. American ginseng attenuates eccentric exercise-induced muscle damage via the modulation of lipid peroxidation and inflammatory adaptation in males. Nutrients. 2021;14(1):78. doi: 10.3390/nu14010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim K.H., Cho J.Y., Kim B., Bae B.S., Kim J.H. Red ginseng (panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J Med Food. 2014;17(1):111–118. doi: 10.1089/jmf.2013.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang Y.J., Lee D., Hossain M.A., Aravinthan A., Kang C.W., Kim N.S., Kim J.H. Korean Red Ginseng enhances cardiac hemodynamics on doxorubicin-induced toxicity in rats. J Ginseng Res. 2020;44(3):483–489. doi: 10.1016/j.jgr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong-Minh K., van der Does Y., Engelen S., de Jong E., Ramakers C., Gommers D., van Gorp E., Endeman H. High procalcitonin levels associated with increased intensive care unit admission and mortality in patients with a COVID-19 infection in the emergency department. BMC Infect Dis. 2022;22(1):1–9. doi: 10.1186/s12879-022-07144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazal M. C-reactive protein a promising biomarker of COVID-19 severity. The Korean J Clin Lab Sci. 2021;53(3):201–207. [Google Scholar]
- 23.Saboori S., Falahi E., Yousefi Rad E., Asbaghi O., Khosroshahi M.Z. Effects of ginseng on C-reactive protein level: a systematic review and meta-analysis of clinical trials. Complement Ther Med. 2019;45:98–103. doi: 10.1016/j.ctim.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Jung S.-J., Hwang J.-H., Park S.-H., Choi E.-K., Ha K.-C., Baek H.-I., Shin D.-G., Seo J.-H., Chae S.-W. A 12-week, randomized, double-blind study to evaluate the efficacy and safety of liver function after using fermented ginseng powder (GBCK25) Food Nutr Res. 2020;64(0) doi: 10.29219/fnr.v64.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutanto Y.S., Reviono R., Aphridasari J., Ramlie A., Kurniawan H. The effect of ginsenoside 4% on inflammation, bacteremia and clinical improvement in community acquired pneumonia patients. SRP. 2021;12(1):686–691. [Google Scholar]
- 26.Estaki M., Noble E.G. North American ginseng protects against muscle damage and reduces neutrophil infiltration after an acute bout of downhill running in rats. Appl Physiol Nutr Metab. 2015;40(2):116–121. doi: 10.1139/apnm-2014-0331. [DOI] [PubMed] [Google Scholar]
- 27.Lin H.F., Tung K., Chou C.C., Lin C.C., Lin J.G., Tanaka H. Panax ginseng and salvia miltiorrhiza supplementation abolishes eccentric exercise-induced vascular stiffening: a double-blind randomized control trial. BMC Complement Altern Med. 2016;16(1):1–10. doi: 10.1186/s12906-016-1139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L., Fu W., Xu H., Li S., Yang X., Yang W., Sui D., Wang Q. Ginsenoside Rc attenuates myocardial ischaemic injury through antioxidative and anti-inflammatory effects. Pharm Biol. 2022;60(1):1038–1046. doi: 10.1080/13880209.2022.2072518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Ma J., Liu S., Chen C., Li Q., Qin M., Ren L. Ginsenoside F1 attenuates pirarubicin-induced cardiotoxicity by modulating Nrf2 and AKT/Bcl-2 signaling pathways. J Ginseng Res. 2023;47(1):106–116. doi: 10.1016/j.jgr.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing J.J., Hou J.G., Liu Y., Zhang R.B., Jiang S., Ren S., Wang Y.P., Shen Q., Li W., Li X.D., et al. Supplementation of saponins from leaves of panax quinquefolius mitigates cisplatin-evoked cardiotoxicity via inhibiting oxidative stress-associated inflammation and apoptosis in mice. Antioxidants (Basel) 2019;8(9):347. doi: 10.3390/antiox8090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q.W., Yu X.F., Xu H.L., Jiang Y.C., Zhao X.Z., Sui D.Y. Ginsenoside Re attenuates isoproterenol-induced myocardial injury in rats. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/8637134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue Y., Fu W., Yu P., Li Y., Yu X., Xu H., Sui D. Ginsenoside Rc alleviates myocardial ischemia-reperfusion injury by reducing mitochondrial oxidative stress and apoptosis: role of SIRT1 activation. J Agric Food Chem. 2023;71(3):1547–1561. doi: 10.1021/acs.jafc.2c06926. [DOI] [PubMed] [Google Scholar]
- 33.Han X., Li M., Zhao Z., Zhang Y., Zhang J., Zhang X., Zhang Y., Guan S., Chu L. Mechanisms underlying the cardio-protection of total ginsenosides against myocardial ischemia in rats in vivo and in vitro: possible involvement of L-type Ca(2+) channels, contractility and Ca(2+) homeostasis. J Pharmacol Sci. 2019;139(3):240–248. doi: 10.1016/j.jphs.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Xue Y., Yu X., Zhang X., Yu P., Li Y., Fu W., Yu J., Sui D. Protective effects of ginsenoside Rc against acute cold exposure-induced myocardial injury in rats. J Food Sci. 2021;86(7):3252–3264. doi: 10.1111/1750-3841.15757. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Jiang Y., Fu W., Yu X., Sui D. Combination of the ginsenosides Rb3 and Rb2 exerts protective effects against myocardial ischemia reperfusion injury in rats. Int J Mol Med. 2020;45(2):519–531. doi: 10.3892/ijmm.2019.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramlie A., Reviono R., Aphridasari J. Effect of ginseng extract supplementation on procalcitonin level, neutrophil, and length of stay in patients with community acquired pneumonia. Respiratory Science. 2022;3(1):25–37. [Google Scholar]
- 37.Oh J., Lee H., Park D., Ahn J., Shin S.S., Yoon M. Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med. 2012;2012:1–14. doi: 10.1155/2012/265023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh S.J., Kim K., Lim C.J. Suppressive properties of ginsenoside Rb2, a protopanaxadiol-type ginseng saponin, on reactive oxygen species and matrix metalloproteinase-2 in UV-B-irradiated human dermal keratinocytes. Biosci Biotechnol Biochem. 2015;79(7):1075–1081. doi: 10.1080/09168451.2015.1020752. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z., Pan H., Zhang Y., Zheng Z., Xiao W., Hong X., Chen F., Peng X., Pei Y., Rong J., et al. Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3beta signaling pathway. J Biochem Mol Toxicol. 2022;36(1) doi: 10.1002/jbt.22885. [DOI] [PubMed] [Google Scholar]
- 40.Yang L., Chen P.P., Luo M., Shi W.L., Hou D.S., Gao Y., Xu S.F., Deng J. Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed Pharmacother. 2019;114 doi: 10.1016/j.biopha.2019.108851. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Zhang Y., Song W., Zhang Y., Dong X., Tan M. Ginsenoside Rh2 inhibits migration of lung cancer cells under hypoxia via mir-491. Anticancer Agents Med Chem. 2019;19(13):1633–1641. doi: 10.2174/1871520619666190704165205. [DOI] [PubMed] [Google Scholar]
- 42.Wang D., Wu C., Liu D., Zhang L., Long G., Hu G., Sun W. Ginsenoside Rg3 inhibits migration and invasion of nasopharyngeal carcinoma cells and suppresses epithelial mesenchymal transition. Biomed Res Int. 2019;2019 doi: 10.1155/2019/8407683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramezani S., Ezzatifar F., Hojjatipour T., Hemmatzadeh M., Shabgah A.G., Navashenaq J.G., Aslani S., Shomali N., Arabi M., Babaie F., et al. Association of the matrix metalloproteinases (MMPs) family gene polymorphisms and the risk of coronavirus disease 2019 (COVID-19); implications of contribution for development of neurological symptoms in the COVID-19 patients. Mol Biol Rep. 2022;50(1):173–183. doi: 10.1007/s11033-022-07907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi S., Su M., Shen G., Hu Y., Yi F., Zeng Z., Zhu P., Yang G., Zhou H., Li Q., et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J Med Virol. 2021;93(1):528–532. doi: 10.1002/jmv.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohammadhosayni M., Sadat Mohammadi F., Ezzatifar F., Mahdavi Gorabi A., Khosrojerdi A., Aslani S., Hemmatzadeh M., Yazdani S., Arabi M., Marofi F., et al. Matrix metalloproteinases are involved in the development of neurological complications in patients with Coronavirus disease 2019. Int Immunopharmacol. 2021;100 doi: 10.1016/j.intimp.2021.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho W.H., Kim Y.H., Heo H.J., Kim D., Kwak T.W., Kim K.H., Yeo H.J. Ginsenoside ameliorated ventilator-induced lung injury in rats. J Intensive Care. 2020;8(1):1–9. doi: 10.1186/s40560-020-00509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitra A., Rahmawati L., Lee H.P., Kim S.A., Han C.-K., Hyun S.H., Cho J.Y. Korean Red Ginseng water extract inhibits cadmium-induced lung injury via suppressing MAPK/ERK1/2/AP-1 pathway. J Ginseng Res. 2022;46(5):690–699. doi: 10.1016/j.jgr.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y., Yang D.U., Huo Y., Pu J., Lee S.J., Yang D.C., Kang S.C. In vitro evaluation of anti-lung cancer and anti-COVID-19 effects using fermented black color ginseng extract. Nat Prod Commun. 2021;16(9) [Google Scholar]
- 49.Seo S.H. Ginseng protects ACE2-transgenic mice from SARS-CoV-2 infection. Frontiers in Biosci(Landmark Ed) 2022;27(6):180. doi: 10.31083/j.fbl2706180. [DOI] [PubMed] [Google Scholar]
- 50.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N., et al. Preventive effect of KRG for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci. 2012;27(12):1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.H., Min D.S., Lee C.W., Song K.H., Kim Y.S., Kim H.P. Ginsenosides from KRG ameliorate lung inflammatory responses: inhibition of the MAPKs/NF-κB/c-Fos pathways. J Ginseng Res. 2018;42(4):476–484. doi: 10.1016/j.jgr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lev-ari S., Starr A.N., Vexler A., Kalich-Philosoph L., Yoo H.S., Kwon K.R., Yadgar M., Bondar E., Bar-shai A., Volovitz I., et al. Rh2-enriched Korean ginseng (Ginseng Rh2+) inhibits tumor growth and development of metastasis of non-small cell lung cancer. Food Funct. 2021;12(17):8068–8077. doi: 10.1039/d1fo00643f. [DOI] [PubMed] [Google Scholar]
- 53.Kim H., Choi P., Kim T., Kim Y., Song B.G., Park Y.T., Choi S.J., Yoon C.H., Lim W.C., Ko H., et al. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J Ginseng Res. 2021;45(1):134–148. doi: 10.1016/j.jgr.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D., Lv L., Xu Y., Jiang K., Chen F., Qian J., Chen M., Liu G., Xiang Y. Cardioprotection of Panax Notoginseng saponins against acute MI and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111287. [DOI] [PubMed] [Google Scholar]
- 55.Wan S., Cui Z., Wu L., Zhang F., Liu T., Hu J., Tian J., Yu B., Liu F., Kou J., et al. Ginsenoside Rd promotes omentin secretion in adipose through TBK1-AMPK to improve mitochondrial biogenesis via WNT5A/Ca(2+) pathways in heart failure. Redox Biol. 2023;60 doi: 10.1016/j.redox.2023.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Y., Li Q., Yang Y., Ke Z., Chen S., Li M., Fan W., Wu H., Yuan J., Wang Z., et al. Cardioprotective effect of stem-leaf saponins from panax notoginseng on mice with sleep deprivation by inhibiting abnormal autophagy through PI3K/Akt/mTOR pathway. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.832174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C.Y., Yang P., Jiang Y.L., Lin Z., Pu Y.W., Xie L.Q., Sun L., Lu D. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomed Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109913. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q., Wang R., Shi Y., Li W., Li M., Chen P., Pan B., Wang Q., Li C., Wang J., et al. Synthesis and biological evaluation of panaxatriol derivatives against myocardial ischemia/reperfusion injury in the rat. Eur J Med Chem. 2020;185 doi: 10.1016/j.ejmech.2019.111729. [DOI] [PubMed] [Google Scholar]
- 59.Sato K., White N., Fanning J.P., Obonyo N., Yamashita M.H., Appadurai V., Ciullo A., May M., Worku E.T., Helms L., et al. Impact of renin–angiotensin–aldosterone system inhibition on mortality in critically ill COVID-19 patients with pre-existing hypertension: a prospective cohort study. BMC Cardiovasc Disord. 2022;22(1):1–12. doi: 10.1186/s12872-022-02565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rysz S., Al-Saadi J., Sjöström A., Farm M., Campoccia Jalde F., Plattén M., Eriksson H., Klein M., Vargas-Paris R., Nyrén S., et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat Commun. 2021;12(1):2417. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee K.H., Bae I.Y., Park S.I., Park J.-D., Lee H.G. Antihypertensive effect of KRG by enrichment of ginsenoside Rg3 and arginine–fructose. J Ginseng Res. 2016;40(3):237–244. doi: 10.1016/j.jgr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Q., Su J., Xu J., Yu H., Jin X., Wang Y., Yan M., Yu J., Chen S., Wang Y., et al. Beneficial effects of Panax notoginseng (Burkill) F. H. Chen flower saponins in rats with metabolic hypertension by inhibiting the activation of the renin–angiotensin–aldosterone system through complement 3. BMC Complement Med Ther. 2023;23(1):13. doi: 10.1186/s12906-022-03828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salama A., Mansour D., Hegazy R. The cardio and renoprotective role of ginseng against epinephrine-induced MI in rats: involvement of angiotensin II type 1 receptor/protein kinase C. Toxicol Reps. 2021;8:908–919. doi: 10.1016/j.toxrep.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J., Liu Y., Lin H., Zhou B., Yu H., Li L., Wang C., Li X., Li P., Liu J., et al. The effect of ginsenoside Rg5, isolated from black ginseng, on heart failure in zebrafish based on untargeted metabolomics. J Funct Foods. 2021;76 [Google Scholar]
- 65.Cusato J., Manca A., Palermiti A., Mula J., Costanzo M., Antonucci M., Trunfio M., Corcione S., Chiara F., De Vivo E.D., et al. COVID-19: a possible contribution of the MAPK pathway. Biomedicines. 2023;11(5):1459. doi: 10.3390/biomedicines11051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimes J.M., Grimes K.V. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goel S., Saheb Sharif-Askari F., Saheb Sharif Askari N., Madkhana B., Alwaa A.M., Mahboub B., Zakeri A.M., Ratemi E., Hamoudi R., Hamid Q., et al. SARS-CoV-2 switches ‘on’ MAPK and NFκB signaling via the reduction of nuclear DUSP1 and DUSP5 expression. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang S., Li F., Lu S., Ren L., Bian S., Liu M., Zhao D., Wang S., Wang J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J Ethnopharmacol. 2022;283 doi: 10.1016/j.jep.2021.114739. [DOI] [PubMed] [Google Scholar]
- 69.Xin C., Quan H., Kim J.M., Hur Y.H., Shin J.Y., Bae H.B., Choi J.I. Ginsenoside Rb1 increases macrophage phagocytosis through p38 mitogen-activated protein kinase/Akt pathway. J Ginseng Res. 2019;43(3):394–401. doi: 10.1016/j.jgr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kwak D., Kim S., Roh S.S., Kim S.D., Nah S.Y., et al. A novel KRG compound gintonin inhibited inflammation by MAPK and NF-kappaB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/624132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fattahi S., Khalifehzadeh-Esfahani Z., Mohammad-Rezaei M., Mafi S., Jafarinia M. PI3K/Akt/mTOR pathway: a potential target for anti-SARS-CoV-2 therapy. Immunol Res. 2022;70(3):269–275. doi: 10.1007/s12026-022-09268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Qahtani A.A., Pantazi I., Alhamlan F.S., Alothaid H., Matou-Nasri S., Sourvinos G., Vergadi E., Tsatsanis C. SARS-CoV-2 modulates inflammatory responses of alveolar epithelial type II cells via PI3K/AKT pathway. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1020624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pelzl L., Singh A., Funk J., Witzemann A., Zlamal J., Marini I., Weich K., Abou-Khalel W., Hammer S., Uzun G., et al. Platelet activation via PI3K/AKT signaling pathway in COVID-19 [abstract] Res Pract Thromb Haemost. 2021;5 [Google Scholar]
- 74.Han S.Y., Kim J., Kim E., Kim S.H., Seo D.B., Kim J.-H., Shin S.S., Cho J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J Ginseng Res. 2018;42(4):496–503. doi: 10.1016/j.jgr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byun B.H., Cho T.H., Park K.M. Inhibition of mTOR signaling pathway by aqueous extract of Siberian ginseng. J Korean Med. 2017;38(2):7–14. [Google Scholar]
- 76.Kwon H.W., Shin J.H., Cho H.J., Rhee M.H., Park H.J. Total saponin from Korean Red Ginseng inhibits binding of adhesive proteins to glycoprotein IIb/IIIa via phosphorylation of VASP (Ser(157)) and dephosphorylation of PI3K and Akt. J Ginseng Res. 2016;40(1):76–85. doi: 10.1016/j.jgr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xing Jj, Hou Jg, Ma Zn, Wang Z., Ren S., Wang Yp, Liu Wc, Chen C., Li W. Ginsenoside Rb3 provides protective effects against cisplatin‐induced nephrotoxicity via regulation of AMPK‐/mTOR‐mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52(4) doi: 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng X., Wang S., Zou X., Jing Y., Yang R., Li S., Wang F. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp Anim. 2017 Aug 5;66(3):217–228. doi: 10.1538/expanim.16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang Y., Li M., Lu Z., Wang Y., Yu X., Sui D., Fu L. Ginsenoside Rg3 induces ginsenoside Rb1-comparable cardioprotective effects independent of reducing blood pressure in spontaneously hypertensive rats. Exp Ther Med. 2017;14(5):4977–4985. doi: 10.3892/etm.2017.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H., Cui L., Liu Q., Dou S., Wang W., Xie M., Xu X., Zheng C., Li T., Huang S., et al. Ginsenoside Rb3 alleviates CSE-induced TROP2 upregulation through p38 MAPK and NF-kappaB pathways in basal cells. Am J Respir Cell Mol Biol. 2021;64(6):747–759. doi: 10.1165/rcmb.2020-0208OC. [DOI] [PubMed] [Google Scholar]
- 81.Li H., Zhu J., Xu Y.W., Mou F.F., Shan X.L., Wang Q.L., Liu B.N., Ning K., Liu J.J., Wang Y.C., et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after MI. Redox Biol. 2022;54 doi: 10.1016/j.redox.2022.102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Wu W., Wang G., Xu W., Zhang F., Wu B., Tian Y. Protective effect of ginsenoside Rg3 on lung injury in diabetic rats. J Cell Biochem. 2019;120(3):3323–3330. doi: 10.1002/jcb.27601. [DOI] [PubMed] [Google Scholar]
- 83.Oh J.M., Chun S. Ginsenoside CK inhibits the early stage of adipogenesis via the AMPK, MAPK, and AKT signaling pathways. Antioxidants (Basel) 2022;11(10):1890. doi: 10.3390/antiox11101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaukat A., Guo Y.F., Jiang K., Zhao G., Wu H., Zhang T., Yang Y., Guo S., Yang C., Zahoor A., et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced Acute Lung Injury through attenuating NF-kappaB and MAPK activation. Microb Pathog. 2019;132:302–312. doi: 10.1016/j.micpath.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Jin Y., Tangchang W., Kwon O.S., Lee J.Y., Heo K.S., Son H.Y. Ginsenoside Rh1 ameliorates the asthma and allergic inflammation via inhibiting Akt, MAPK, and NF-kappaB signaling pathways in vitro and in vivo. Life Sci. 2023;321 doi: 10.1016/j.lfs.2023.121607. [DOI] [PubMed] [Google Scholar]
- 86.Qin G.W., Lu P., Peng L., Jiang W. Ginsenoside Rb1 inhibits cardiomyocyte autophagy via PI3K/Akt/mTOR signaling pathway and reduces myocardial ischemia/reperfusion injury. Am J Chin Med. 2021;49(8):1913–1927. doi: 10.1142/S0192415X21500907. [DOI] [PubMed] [Google Scholar]
- 87.Chen P., Li X., Yu X., Yang M. Ginsenoside Rg1 suppresses non-small-cell lung cancer via MicroRNA-126-PI3K-AKT-mTOR pathway. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/1244836. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Zhao T., Wang X., Liu Q., Yang T., Qu H., Zhou H. Ginsenoside Rd promotes cardiac repair after MI by modulating monocytes/macrophages subsets conversion. Drug Des Devel Ther. 2022;16:2767–2782. doi: 10.2147/DDDT.S377624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian G., Li J., Zhou L. Ginsenoside Rg1 regulates autophagy and endoplasmic reticulum stress via the AMPK/mTOR and PERK/ATF4/CHOP pathways to alleviate alcohol-induced myocardial injury. Int J Mol Med. 2023;52(1):1–11. doi: 10.3892/ijmm.2023.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fachrurrodji F., Sidharta B.R.A., Ariningrum D., Suparyatmo J., Pramudianti M.D. The effect of ginseng extract on serum interleukin-6 levels in patients with community-acquired pneumonia. Indonesian J. Clin. Pathol. Med. Lab. 2022 Sep. 19;28(3):278–284. [Internet] [cited 2023 Jul. 4] [Google Scholar]
- 91.Lu S., Zhang Y., Li H., Zhang J., Ci Y., Han M. Ginsenoside Rb1 can ameliorate the key inflammatory cytokines TNF-alpha and IL-6 in a cancer cachexia mouse model. BMC Complement Med Ther. 2020;20(1):1–9. doi: 10.1186/s12906-019-2797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jung J.H., Kang T.K., Oh J.H., Jeong J.U., Ko K.P., Kim S.T. The effect of KRG on symptoms and inflammation in patients with allergic rhinitis. Ear Nose Throat J. 2021;100(5_suppl) doi: 10.1177/0145561320907172. 712S-9S. [DOI] [PubMed] [Google Scholar]
- 93.Kim J.K., Shin K.K., Kim H., Hong Y.H., Choi W., Kwak Y.-S., Han C.-K., Hyun S.H., Cho J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J Ginseng Res. 2021;45(6):717–725. doi: 10.1016/j.jgr.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee J.S., Ko E.J., Hwang H.S., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 2014;34(1):183–190. doi: 10.3892/ijmm.2014.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paik S., Choe J.H., Choi G.-E., Kim J.-E., Kim J.-M., Song G.Y., Jo E.-K. Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci Rep. 2019;9(1):4342. doi: 10.1038/s41598-019-40690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen T., Xiao L., Zhu L., Ma S., Yan T., Ji H. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38(5):1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 97.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yin J.X., Agbana Y.L., Sun Z.S., Fei S.W., Zhao H.Q., Zhou X.N., Chen J.H., Kassegne K. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023;12(1):43. doi: 10.1186/s40249-023-01086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabaka P., Koščálová A., Straka I., Hodosy J., Lipták R., Kmotorková B., Kachlíková M., Kušnírová A. Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect Dis. 2021;21(1):1–8. doi: 10.1186/s12879-021-05945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.