Abstract
Background
Recently, plant-derived exosome-like nanoparticles (PDENs) have been isolated, and active research was focusing on understanding their properties and functions. In this study, the characteristics and molecular properties of ginseng root-derived exosome-like nanoparticles (GrDENs) were examined in terms of skin protection.
Methods
HPLC-MS protocols were used to analyze the ginsenoside contents in GrDENs. To investigate the beneficial effect of GrDENs on skin, HaCaT cells were pre-treated with GrDENs (0–2 × 109 particles/mL), and followed by UVB irradiation or H2O2 exposure. In addition, the antioxidant activity of GrDENs was measured using a fluorescence microscope or flow cytometry. Finally, molecular mechanisms were examined with immunoblotting analysis.
Results
GrDENs contained detectable levels of ginsenosides (Re, Rg1, Rb1, Rf, Rg2 (S), Gyp17, Rd, C-Mc1, C–O, and F2). In UVB-irradiated HaCaT cells, GrDENs protected cells from death and reduced ROS production. GrDENs downregulated the mRNA expression of proapoptotic genes, including BAX, caspase-1, -3, -6, -7, and -8 and the ratio of cleaved caspase-8, -9, and -3 in a dose-dependent manner. In addition, GrDENs reduced the mRNA levels of aging-related genes (MMP2 and 3), proinflammatory genes (COX-2 and IL-6), and cellular senescence biomarker p21, possibly by suppressing activator protein-1 signaling.
Conclusions
This study demonstrates the protective effects of GrDENs against skin damage caused by UV and oxidative stress, providing new insights into beneficial uses of ginseng. In particular, our results suggest GrDENs as a potential active ingredient in cosmeceuticals to promote skin health.
Keywords: Ginseng-derived exosome-like nanoparticles, Ginsenosides, UV irradiation, Oxidative stress, Aging
Graphical abstract
1. Introduction
Most mammalian cells secrete extracellular vesicles, and such intracellularly derived vesicles are found in blood, urine, saliva, and cell culture media [1,2]. Extracellular vesicles (EVs) are membrane-structured organoids with a diameter of 20 nm to 5 mm. They are classified into exosomes, ectosomes, microvesicles, microparticles, and apoptotic bodies, depending on the origin, size, shape, lipid composition, and method of secretion. Exosomes are the smallest phospholipid bilayer-membrane vesicles, with a size (diameter) of 30–100 nm (< 200 nm). They are cup-shaped and originate from endosomes. Exosomes are rich in tetraspanins such as CD9, CD63, and CD81 and are often used as exosome markers. In addition, exosomes consist of cholesterol, sphingomyelin, ceramides, and phosphatidylserine. Furthermore, DNA, histone, miRNA, non-coding RNA, mRNA, and intracellular proteins can be contained within exosomes. Thus, the exosome plays an essential role in cell-to-cell interactions by mediating the exchange of substances. Even in plants and bacteria, small vesicles are released into the extracellular space [3,4]. Plant-derived vesicles are called plant-derived exosome-like nanoparticles (PDENs) or plant-derived extracellular vesicles (PDEVs) and bacteria-derived vesicles are called bacterial extracellular vesicles (BEVs) [[4], [5], [6]]. The size of PDENs is between 30 nm and 150 nm and the components of them are similar with mammalian exosomes [7]. Their main functions are defending against pathogens or adapting abiotic environmental stress [3]. In addition, the size of BEVs is 20–400 nm and they contain lipopolysaccharide (LPS), peptidoglycan, and ompA in phospholipid bilayer as well as proteins, toxins, and nucleic acids in cytoplasm [4,8]. LPS and peptidoglycan can bind to host pattern recognition receptor to promote host pathology, immune tolerance, or confer protective immunity.
Ultraviolet (UV) rays have a wavelength of 200–400 nm, causing skin lesions such as photoaging, erythema, pigmentation, and skin cancer. UV radiation is classified into UVA, UVB, and UVC according to wavelength, and these light classifications differ in their range of skin penetration and biological effects. UVC, with the shortest wavelengths (200–290 nm), is the most dangerous, but it is entirely absorbed by the ozone layer and does not reach the earth's surface. As a result, it is mainly UVA and UVB that cause skin damage. UVA with a wavelength of 320–400 nm penetrates to the dermis and causes sagging skin. In contrast, UVB with a wavelength of 290–320 nm affects the epidermis through deposition of intense energy, and repeated UVB exposure causes skin wrinkles [9].
UVB causes cell death through reactive oxygen species (ROS) generation in a range of cells [10]. Apoptosis is a form of programmed cell death triggered by stimuli, such as UV radiation and excessive ROS [11,12]. These stimuli mediate the activation of proteolytic enzyme caspases, leading to apoptosis. Caspases are expressed in pro-caspase forms, which are inactive and converted to an activated form (cleaved form) in response to a stimulus. First, extrinsic and intrinsic pathways activate caspase-8 and -9, respectively. After that, a sequential signaling cascade activates the executioner caspases (caspase-3, -6, and -7), resulting in morphological changes associated with apoptosis [13]. Caspase-1 is well known as an essential component for inflammasome activation, and its importance in the UVB-induced apoptosis of keratinocytes has recently been reported [14]. BAX, a member of the Bcl-2 gene family, is a proapoptotic effector and an essential regulator of the intrinsic apoptotic pathway [15].
UVB induces oxidative stress to temporarily or continuously upregulate the activator protein-1 (AP-1) pathway [[16], [17], [18], [19]]. AP-1 transcription factors consist of c-jun and c-fos components, which form heterogeneous or homodimer complexes. The dimer complexes move into the nucleus and bind to DNA, regulating the expression of specific AP-1 target genes. AP-1 is activated by a phosphorylation cascade mediated by the upper signaling mitogen-activated protein kinases (MAPKs: ERK, JNK, p38) [20]. In the skin, activation of AP-1 increases the expression of matrix metalloproteinases (MMPs), endopeptidases with substrate specificity. MMP2 and MMP9 break down collagen type IV, while MMP3 degrades collagen type I [21]. Therefore, the breakdown of collagen by upregulated MMPs leads to photoaging. In addition, activation of AP-1 signaling in skin induces an inflammatory response through increased expression of inflammatory proteins such as cyclooxygenease-2 (COX-2) and interleukin (IL)-6 [[22], [23], [24]]. Inflammation is a defense response that protects the body from external stimuli, but an excessive inflammatory response can cause skin damage and accelerate skin aging [25].
This study evaluated the beneficial skin effects of ginseng root-derived exosome-like nanoparticles (GrDENs) isolated from ginseng root using the human keratinocytes cell line HaCaT. We demonstrated the skin-protective and anti-aging effects of GrDENs under UV exposure and ROS irritation.
2. Materials and methods
2.1. Materials and reagents
HaCaT cell lines were purchased from Antibody Research Corporation (MO, USA, catalog No: 116027) and HEK293T cell lines were purchased from the American Type Culture Collection (ATCC) (Rockville, MD, USA, catalog No: CRL-3216). Cell culture media and antibiotics (penicillin and streptomycin) were purchased from Hyclone (Logan, UT, USA). 3-(4-5-Dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) and hydrogen peroxide (H2O2) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primers for quantitative real-time polymerase chain reaction (qRT-PCR) were produced by Macrogen (Seoul, Republic of Korea). The cDNA synthesis kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies targeting cleaved-caspase-3, -8, -9, caspase-3, -8, -9, p-c-jun (Ser73), c-jun, p-c-fos (Ser32), c-fos, p-ERK (Thr202/Tyr204), ERK, p-JNK (Thr183/Tyr185), JNK, p-p38 (Thr180/Tyr182), p38, p-MEK1/2 (Ser217/221), MEK1/2, and Myc were purchased from Cell Signaling Technology (Beverly, MA, USA). In addition, β-actin antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The other chemicals used in this study were of American Chemical Society grade or higher.
2.2. Extraction and purification of GrDENs
The 4-year-old ginseng root was harvested from a farmhouse in Gyeonggi, Republic of Korea. After harvesting, the root was washed with tap water and air-dried at 45 °C for 24 h. Next, the ginseng root was finely ground using a blender and stored at 4 °C. Extraction and purification of EVs were performed by soaking, juicing and conducting serial ultra-centrifugations as previously reported [7]. 30 g of ginseng root was used to extract and to purify GrDENs, and 1.02 × 1010 particles of GrDENs were purified from the extract; the yield was 3.41 × 108 particles/g of ginseng root.
2.3. Characteristics of GrDENs
GrDENs which were used in this research were extracted and purified in the same way with previous research [7]. The shape of GrDENs was analyzed through cryo-electrom microscope and the surface was surrounded by lipid bilayer. The size of GrDENs was between 87 nm and 256 nm, and the mean size was 142 nm [7]. The lipid contents of GrDENs were analyzed with liquid chromatography-mass spectrometry [26]. They contained total 188 lipid species, belonging to 15 different classes such as triacylglycerol, phosphatidylcholine, lysophosphatidylethanolamine, phosphatidylethanolamine, and diacylglycerol [26]. Upon small RNA sequencing, it was found that GrDENs had various small RNA such as miRNA, snRNA, rRNA and tRNA.
2.4. Cell culture
HaCaT cells and HEK293T cells were grown in Dulbecco's Modified Eagle Medium supplemented with 10 % and 5 % fetal bovine serum (FBS, Gibco, Grand Island, UT, USA), respectively, and 1 % penicillin and streptomycin. The cells were incubated in a humidified incubator with 5 % CO2 at 37 °C.
2.5. Cell viability assay
HaCaT cells were seeded in a 96-well plate at 2 × 105 cells/mL and incubated for 18 h. Then the cells were treated with GrDENs at concentrations of 1.25 × 108, 2.5 × 108, 5 × 108, 1 × 109, and 2 × 109 particles/mL. After 24 h, the cell viability was determined by conventional MTT assay.
2.6. ROS generation assay
HaCaT cells were seeded in a 6-well plate at 2 × 105 cells/mL and incubated for 18 h. The cells were pre-treated with GrDENs for 30 min and treated with UVB (30 mJ/cm2) (UVB Lamp BLX-312, Vilber Lourmat, France). After 24 h, the cells were stained with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and incubated for 20 min in the dark. Then, the cells were fixed in a 4 % formaldehyde solution and stained with 4′,6-diamidino-2-phenylindole (DAPI). Photographs were captured using a Nikon Eclipse Ti fluorescence microscope (Nikon, Japan). For flow cytometry, HaCaT cells were treated with GrDENs and UVB in the same manner as mentioned above. After 24 h, cells were harvested and resuspended in 300 μL of phosphate-buffered saline. Diacetyldichlorofluorescein (DCFH-DA) was added to the cells to a concentration of 10 μM, and the cells were incubated for 20 min in the dark. The fluorescence was detected at 485/535 nm using a flow cytometer (Beckman Coulter, Brea, CA, USA).
2.7. RNA extraction and quantitative real-time PCR
HaCaT cells were seeded in a 6-well plate at a density of 2 × 105 cells/mL and incubated for 18 h in an incubator. The seeded cells were pre-treated with GrDENs for 30 min and then treated with UVB (30 mJ/cm2) or H2O2 (600 μM). After 24 h, the cells were harvested, and the total RNA was extracted with TRIzol reagent according to the manufacturer's instructions. The complementary DNA was synthesized with a cDNA synthesis kit. Finally, qRT-PCR was performed with Pcrbio's qPCRBIO SyGreen mix. The primers used in this study are listed in Supplementary Table 1.
2.8. Preparation of cell lysates and immunoblotting
HaCaT cells or HEK293T cells were lysed with lysis buffer to obtain the whole lysates. The lysis buffer consisted of 50 mM Tri-HCl (pH 7.5), 120 mM NaCl, 25 mM β-glycerol phosphate (pH 7.5), 20 mM NaF, 2 % NP-40, 2 μg/mL leupeptin, 2 μg/mL pepstatin A, 1 mM benzamide, 2 μg/mL aprotinin, 1.6 mM pervanadate, 100 μM phenylmethylsulfonyl fluoride, and 100 μM Na3VO4. Samples containing equal amounts of proteins were loaded in polyacrylamide gels and separated by size with sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by transfer of proteins to polyvinylidene fluoride (PVDF) membranes. The primary antibody was diluted 1:2500 with tris-buffered saline containing 0.1 % Tween® 20 detergent (TBST) and 3 % FBS, and the antibody was incubated on the PVDF membrane at 4 °C for 18 h. After washing three times with TBST, the secondary antibodies were incubated at a ratio of 1:2500 for 90 min in RT. After washing three times with TBST, the immunoreactive bands were detected by enhanced peroxidase with anb ELPIS-BIOTECH in a chemidoc of ATTO.
2.9. Statistical analysis
All data in this study are presented as mean ± standard deviation of at least three independent experiments. ImageJ software was used to measure the band intensity of immunoblotting analysis. The Mann-Whitney test was used to evaluate the significance of each set of data. Statistical significance was defined as p-value < 0.05.
3. Results
3.1. Identification of ginsenosides in GrDENs
Ginsenosides are the primary pharmacological components of ginseng. Therefore, we investigated ginsenoside contents inside GrDENs. The high-performance liquid chromatography-mass spectrometry (HPLC-MS) results revealed that GrDENs contained detectable ginsenosides Re, Rg1, Rb1, Rf, Rg2 (S), Gyp17, Rd, C-Mc1, C–O, and F2. Based on our previous preliminary analysis results of ginsenoside in GrDENs (data not shown), we prepared the GrDENs samples more elaborately for HPLC-MS analysis and obtained total three analyzed data. GrDENs for each analysis were from different sources. The content of each ginsenoside for these analyses is shown in Supplementary Table 2.
3.2. Protective effect of GrDENs against UV exposure-induced cell death
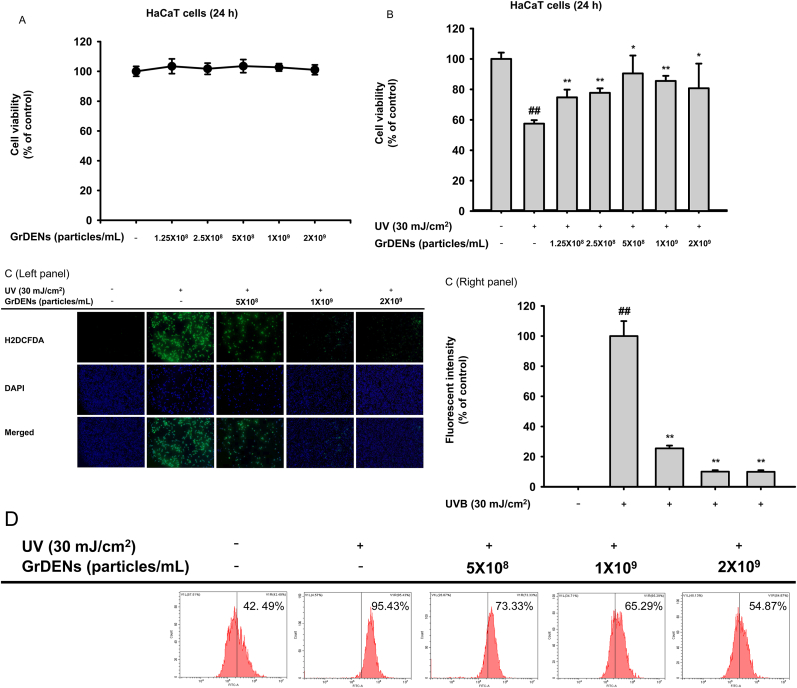
To investigate the beneficial effects of GrDENs on the skin, we evaluated UV irradiated-HaCaT cells. HaCaT cells were dose-dependently incubated with GrDENs for 24 h, and the result showed that GrDENs did not influence cell viability (Fig. 1A). Importantly, UV irradiation caused cell death, and GrDENs protected the cells from this death. Notably, GrDENs at concentrations above 5 × 108 particles/mL showed remarkable effects, recovering cell viability to 80–90 % (Fig. 1B). Excess ROS was shown to cause cell damage leading to death via activation of an apoptosis signal [12], and UV could promote the generation of ROS [27]. Thus, we tested the effect of GrDENs on ROS generation using fluorescent probes H2DCFDA and DCFH-DA as a sensor for ROS. In Fig. 1C, intracellular ROS were detected as fluorescent green, and GrDENs decreased the ROS levels induced by UV irradiation. Consistently, the proportion of DCFH-DA-positive cells was increased in the UV irradiation group from 42.40 % to 95.43 % compared to the control group. However, GrDENs limited that increase (the proportions of the DCFH-DA positive cells were 73.33 %, 65.29 %, and 54.87 % in the GrDENs groups with concentrations of 5 × 108, 1 × 109, and 2 × 109 particles/mL, respectively, Fig. 1D).
Fig. 1.
Cell viability and intracellular ROS levels of HaCaT cells treated with GrDENs. (A and B) HaCaT cells were treated alone with GrDENs (0–2 × 109 particles/mL) for 24 h in panel A. For panel B, HaCaT cells were pre-treated with GrDENs (0–2 × 109 particles/mL) for 30 min and stimulated with UVB irradiation for 24 h. Cell viability was examined by MTT assay. (C and D) HaCaT cells were pre-treated with GrDENs (0–2 × 109 particles/mL) for 30 h and irradiated by UVB for 24 h, and the cells were incubated with H2DCFDA (C) or DCFH-DA (D) for 20 min. Intracellular ROS levels were determined by fluorescent imaging (C) and flow cytometry (D). Fluorescent intensity was measured with ImageJ (C). Data in (A), (B), and (C) are presented as mean ± standard deviation of at least three independent experiments. Results in (D) are representative images from three independent experiments. ##p < 0.01 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (UV irradiation).
3.3. Inhibitory effect of GrDENs on apoptosis signaling
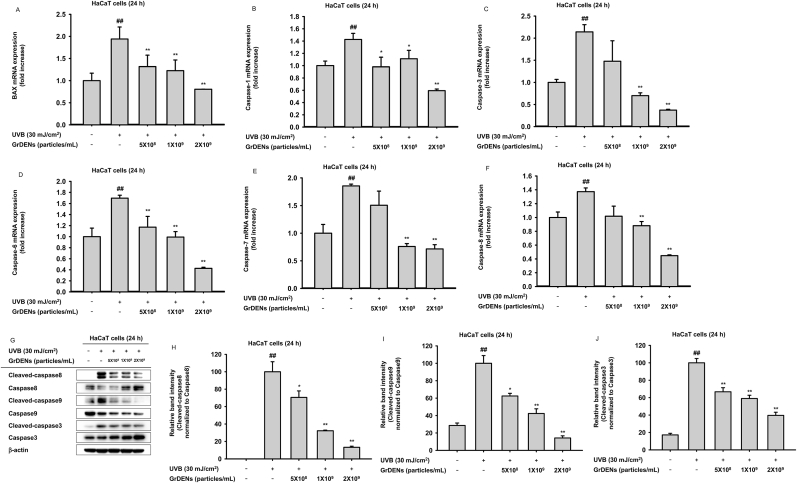
To understand the molecular mechanisms related to the GrDEN-mediated cell protection, the effect of GrDENs on proapoptotic molecules, including BAX and caspases, was examined. The mRNA expression of BAX and caspase-1, -3, -6, -7, and -8 was significantly increased by UV irradiation (Fig. 2A–F). However, GrDENs (5 × 108, 1 × 109, and 2 × 109 particles/mL) reduced the expression of BAX, caspase-1, and caspase-6 genes (Fig. 2A, B, and D). In addition, GrDENs decreased the expression of caspase-3, -7, and -8 at concentrations of 1 × 109 and 2 × 109 particles/mL (Fig. 2C–E, and F). Next, we examined the changes in the active states of caspase -8, caspase-9, and caspase-3 under UV and GrDEN treatments. Cleaved caspase levels normalized to total proteins were all elevated by UVB, while GrDENs reduced those levels (Fig. 2G–J).
Fig. 2.
Effect of GrDENs on the expression of apoptotic genes and activities of caspases. HaCaT cells were pre-treated with GrDENs for 30 min and irradiated by UVB for 24 h. (A–F) The mRNA levels of BAX (A), caspase-1 (B), caspase-3 (C), caspase-6 (D), caspase-7 (E), and caspase-8 (F) were determined by real-time PCR. (G–J) To examine the alterations of caspase activities when treated with GrDENs, cleaved caspase levels were analyzed by immunoblotting (G). Band intensity was measured with ImageJ, and the relative band intensities of cleaved caspase-8 (H), cleaved caspase-9 (I), and cleaved caspase-3 (J) were normalized to the corresponding total caspases. Data in (A), (B), (C), (D), (E), (F), (H), (I), and (J) are presented as mean ± standard deviation of three independent experiments. Results in (G) are representative images from three independent experiments. ##p < 0.01 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (UV irradiation).
3.4. The beneficial effect of GrDENs on skin aging
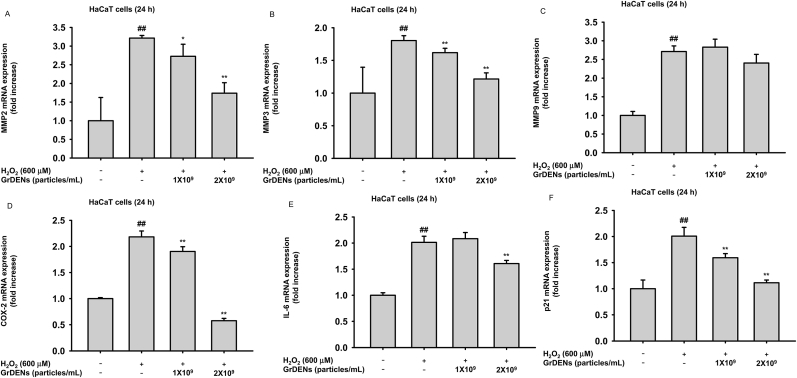
Excessive oxidative stress accelerates skin aging [28], so we hypothesized the anti-aging activity of GrDENs based on the observations that GrDENs have ROS scavenging ability. The gene expression of MMP-2, -3, and -9, the main contributors to skin wrinkling, was increased in H2O2-exposed HaCaT cells (Fig. 3A–C). Additionally, GrDENs reduced the mRNA expression of MMP-2 and MMP-9 but not that of MMP-3 (Fig. 3A–C). Because enhanced inflammatory responses are also considered as a pathogenesis of skin aging, we identified changes in the expression of proinflammatory genes, such as COX-2 and IL-6, under GrDEN treatment. As expected, H2O2 exposure elevated the COX-2 and IL-6 gene expression, and GrDENs reduced their expression at 1 × 109 or 2 × 109 particles/mL doses (Fig. 3D and E). We further assessed the level of p21, a well-established cellular senescence biomarker [29]. The mRNA level of p21 was increased by H2O2 but decreased in GrDEN (1 × 109 and 2 × 109 particles/mL) groups (Fig. 3F).
Fig. 3.
Effect of GrDENs in expression of skin aging- and inflammation-related genes. HaCaT cells were pre-treated with GrDENs (0–2 × 109 particles/mL) for 30 min and exposed by H2O2 for 24 h. (A–C) The expression of the aging-related genes, such as MMP-2 (A), MMP-3 (B), and MMP-9 (C), was determined by real-time PCR. (D and E) The mRNA levels of the inflammation-associated genes, such as COX-2 (D) and IL-6 (E), were examined with real-time PCR. (F) The gene expression of p21, a cellular senescence biomarker, was determined with real-time PCR. All data are presented as mean ± standard deviation of three independent experiments. ##p < 0.01 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (H2O2 exposure group).
3.5. Effect of GrDENs on AP-1 signaling
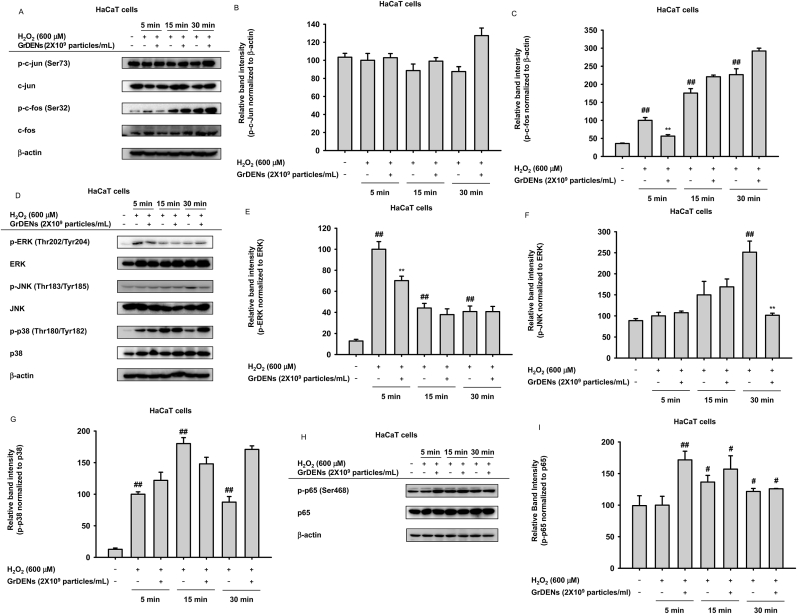
Because H2O2-induced AP-1 activation can contribute to skin aging by modulating the expression of MMPs, inflammatory proteins, and p21 [[30], [31], [32]], we assessed the effects of GrDENs on AP-1 signaling. H2O2 augmented the phosphorylation of c-Fos at exposure times of 5, 10, and 15 min, and GrDENs (2 × 109 particles/mL) curbed the p-c-Fos levels at 5 min (Fig. 4A and C), which supported our hypothesis. However, H2O2 and GrDENs did not affect p-c-Jun level (Fig. 4A and B). Next, we evaluated the effect of GrDENs on the downstream molecules of c-Fos, such as ERK, JNK, and p38. H2O2 exposure increased p-ERK and p-p38 levels after 15 min and that of JNK after 30 min (Fig. 4D–G). GrDENs inhibited H2O2-induced ERK and JNK phosphorylation at 5 and 30 min but did not inhibit p-p38 levels (Fig. 4D–G). For checking the possibility of the involvement of NF-κB pathway, we assessed the phosphorylation of p65 in same condition. The phosphorylation level of p65 was increased by H2O2 treatment but GrDENs did not affect that level (Fig. 4H and I).
Fig. 4.
Effect of GrDENs in AP-1 signaling. HaCaT cells were pre-treated with GrDENs (2 × 109 particles/mL) for 30 min, and the cells were then exposed to H2O2 for the indicated time in the figures. (A–C) Phosphorescence and total levels of AP-1 subunits, including c-jun (A and B) and c-fos (A and C), were determined by immunoblotting. (D–G) Phosphorescence and total levels of AP-1 pathway-related molecules such as ERK (D and E), JNK (D and F), and p38 (D and G) were detected with immunoblotting analysis. (H and I) Phosphorescence and total levels of NF-kB pathway-related molecule, p65 were detected with immunoblotting analysis. ImageJ was used to measure the band intensity, and the relative band intensity of phospho-proteins was normalized to the corresponding total proteins. Data in (B), (C), (E), (F), (G), and (I) are shown as mean ± standard deviation of three independent experiments, and representative images are presented in (A), (D), and (H). ##p < 0.01, #p < 0.05 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (H2O2 exposure group).
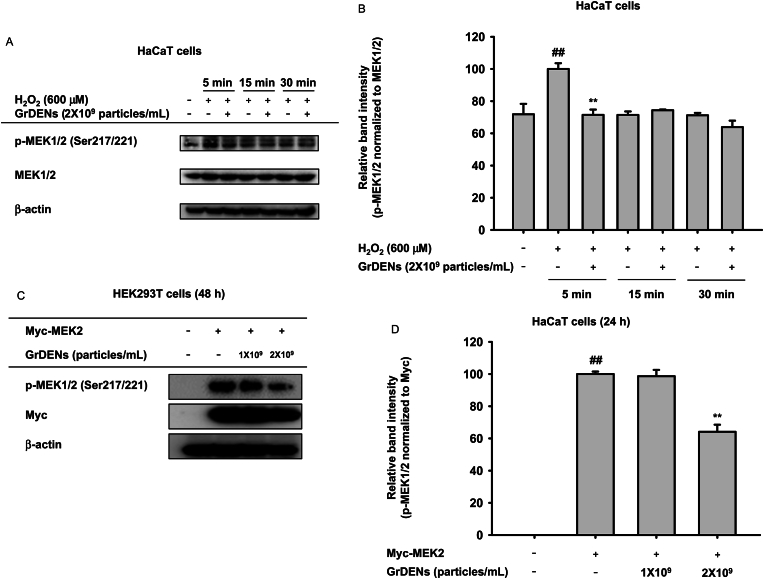
3.6. Suppressive effect of GrDENs on MEK1/2
To determine the target molecule of GrDENs, we further assessed the fluctuations of MEK1/2, upstream molecules of MAPKs, in GrDEN-treated HaCaT cells. In Fig. 5A and B, H2O2 enhanced the phosphorylation of MEK1/2 5 min after exposure, and GrDENs down-regulated the p-MEK1/2 levels at 5 min (Fig. 5A and B). To confirm MEK2 as a target of GrDENs, we overexpressed Myc-MEK2 in HEK293T cells and then examined the effect of GrDENs. Interestingly, GrDENs (2 × 109 particles/mL) significantly suppressed the p-MEK1/2 levels, indicating that GrDENs target MEK2 and not other upstream proteins (Fig. 5C and D).
Fig. 5.
Effect of GrDENs on MEK1/2. (A and B) HaCaT cells were pre-treated with GrDENs (2 × 109 particles/mL) for 30 min, and the cells were exposed to H2O2 for 5, 15, or 30 min. (C and D) The HaCaT cells were transfected with Myc-MEK2 for 24 h, and the cells were treated with GrDENs (0–2 × 109 particles/mL) for an additional 24 h. Phosphorescence and total MEK1/2 levels were determined with immunoblotting, and β-actin was used as a loading control. ImageJ was used to measure the band intensity, and the relative band intensity of phospho-proteins was normalized to the corresponding total proteins. Data in (B) and (D) are presented as mean ± standard deviation of three independent experiments, and representative images are presented in (A) and (C). ##p < 0.01 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (H2O2 exposure or Myc-MEK2 overexpression group).
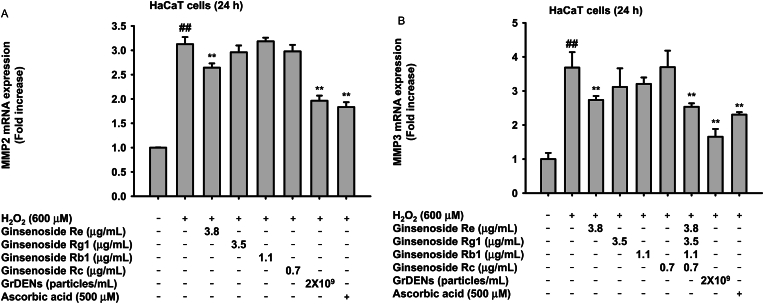
3.7. The effect of each ginsenoside component upon H2O2 exposure
To confirm that the protective effects of GrDENs upon H2O2 exposure came from the ginsenoside components in GrDENs or from their own abilities of GrDENs, we checked the mRNA expressions of MMP2 and MMP3 in H2O2-treated cells under ginsenoside-treated conditions (Fig. 6A and B). The 4 most abundant ginsenosides, Re, Rg1, Rb1, and Rc were selected and treatment concentration of each ginsenoside was decided by calculating that how much ginsenoside was determined in 2 × 109 particles/mL. As shown in Fig. 6A and B, ginsenoside Re alone showed significant inhibitory effects on mRNA expression of MMP2 and MMP3. Mixture of ginsenosides (Re, Rg1, Rb1, and Rc) showed slightly increased inhibition level of MMP3 expression (Fig. 6B). However, the inhibitory effects of GrDENs were higher than ginsenoside individual groups, implying that other minor ginsenosides or components might be involved. Ascorbic acid was used as positive control and showed strong suppressive activity (Fig. 6A and B).
Fig. 6.
The effect of each ginsenoside component upon H2O2 exposure. (A and B) HaCaT cells were pre-treated with ginsenoside Re (3.8 μg/mL), Rg1 (3.5 μg/mL), Rb1 (1.1 μg/mL), Rc (0.7 μg/mL), GrDENs (2 × 109 particles/mL), or Ascorbic acid (500 μM) for 30 min and exposed by H2O2 for 24 h. The expression of the aging-related genes, such as MMP-2 (A), MMP-3 (B) was determined by real-time PCR. All data are presented as mean ± standard deviation of three independent experiments. ##p < 0.01 compared to the normal group (non-treatment), and *p < 0.05, **p < 0.01 compared to the control group (H2O2 exposure group).
4. Discussion
Ginseng possesses a variety of pharmacological effects, such as improving cognitive function [33] as well as anti-cancer [34], anti-inflammatory [35], anti-stress [36], antifatigue [37], antioxidant [38], anti-aging [39], and anti-diabetic [40] effects. Therefore, ginseng is consumed in an array of forms as a functional component of health supplements and cosmetics. The different pharmacological efficacies of ginseng are derived from physiologically active substances, including ginsenoside, phenolic compounds, and acidic polysaccharides, that are in ginseng. Many studies have been conducted on the active effects of ginsenosides, which are ginseng saponins. Interestingly, it has recently been reported that PDENs have health-beneficial functions. For example, blueberry-derived EVs increase cell viability based on their anti-inflammatory and antioxidant properties [41]. Similarly, strawberry-derived EVs have been reported to prevent oxidative stress [42]. In addition, it has been confirmed that PDENs derived from grapes, grapefruit, ginger, and carrot contribute to intestinal homeostasis through anti-inflammatory action [43]. These reports motivated us to explore the possibility of pharmacological effects of ginseng-derived EVs. In this study, we demonstrated the biological activity of GrDENs in improving skin health.
We analyzed the gene expression of factors associated with apoptosis, aging, and inflammation in skin cells. In addition, we examined the activity of signaling proteins to understand the molecular mechanisms underlying the effect of GrDENs. We observed that GrDENs protect cells through antioxidant efficacy when irradiated with UVB light and exhibit anti-inflammatory and anti-aging activity through the suppression of AP-1 signaling under the oxidative stress caused by H2O2 exposure.
The efficacy of exosomes has been reported to vary depending on plant origin. Therefore, the composition of exosomes is expected to differ from plant species. Moreover, strawberry-derived EVs contain vitamin C as the active ingredient [42]. Interestingly, the components of GrDENs contain ginsenosides, such as Re, Rg1, Rb1, Rf, Rg2, Gyp17, Rd, C-Mc1, C–O, and F2. The antioxidant potency of ginsenosides is well-documented in vitro, in vivo, and in clinical studies [38]. For example, ginsenoside Rg1 enhances the antioxidant system in muscles and the liver [44,45]. In addition, ginsenosides Re and Rg1 have been reported to reduce p-ERK level increased by lipopolysaccharides in N9 microglia [46], and ginsenoside Re reduces intimal hyperplasia through MEK1/2 inhibition [47]. Furthermore, ginsenosides Re, Rg1, and Rb1 showed ROS scavenging activity in rat liver and brain by direct antioxidative [48,49] and indirect MAPK/AP-1 pathway-inhibitory activities [50,51]. Moreover, it showed that the mixture of ginsenosides in GrDENs had slightly higher reducing effects on MMP3 expression upon H2O2 exposure than that on Re activity (Fig. 6B), implying that Re might be a major component to contribute to antioxidative activity of GrDENs. Nonetheless, the antioxidative activity level was largely found in GrDENs-treated group (Fig. 6A and B). The previous reports mentioned that EVs can augment the uptake of their own internal components into cells. [52]. So, GrDENs seem to facilitate the uptake of ginsenoside or other chemical components such as syringaresinol into cells than only ginsenosides treatment, leading to increased pharmacological activities of GrDEN (Fig. 6). Based on our results, therefore, it is expected that GrDENs do strongly give pharmacologically beneficial activity not only to ginsenosides, but also to other minor compounds. Although GrDENs showed promising pharmacological activity, the procedure of extracting and purifying EV is more difficult and unusual in comparison to preparation of individual or mixture of ginsenosides. Meanwhile, as GrDENs may include genetic and epigenetic materials found in other plants [53], further research on the possible mode of actions affecting AP-1 signaling are needed on this hypothesis. Previous works suggest that PDEVs contain DNA, mRNA, miRNA, or plant immune-associated molecules for reducing the power of pathogens by silencing virulence-related genes [[54], [55], [56]].
PDENs are expected to have cross-kingdom activity because they have a similar composition and structure to mammalian-derived exosomes. As expected, several PDENs, including lemon- or blueberry-derived EVs, exert interspecies regulation on human cells [41,57]. Similarly, GrDENs have shown biological effects in human keratinocytes, suggesting them as a novel resource with the potential to improve health. In particular, this study demonstrates that GrDENs can be used as active ingredients in cosmetics to improve skin health. However, further study is necessary on the conditions for maintaining PDENs stability or mass production methods for industrial applications.
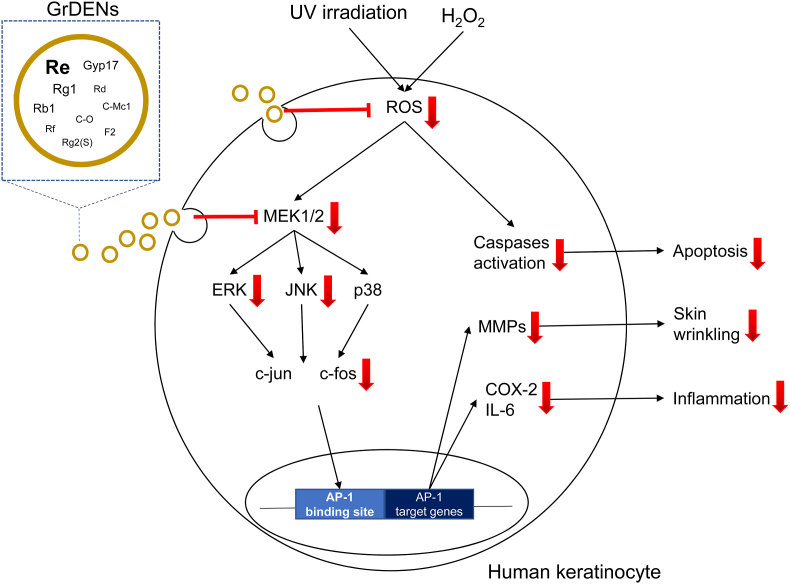
Conclusively, this study can prove the protective effects of GrDENs against skin damage caused by UV and oxidative stress as summarized in Fig. 7, implicating new insights into beneficial uses of ginseng. In particular, our results suggest GrDENs as a potential active ingredient in cosmeceuticals to promote skin health.
Fig. 7.
Summary of protective effect of GrDENs against UV irradiation and oxidative stress.
Declaration of competing interest
The authors have declared that no competing interest exists.
Acknowledgements
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), the Ministry of Science and ICT, Republic of Korea (2017R1A6A1A03015642) and by AmorePacific Co., Republic of Korea (2022).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2024.01.001.
Contributor Information
Wooram Choi, Email: chwoo1028@naver.com.
Jeong Hun Cho, Email: huny841215@amorepacific.com.
Sang Hee Park, Email: 84701@naver.com.
Dong Seon Kim, Email: wetdry20@hanmail.net.
Hwa Pyoung Lee, Email: leehwapyoung57@gmail.com.
Donghyun Kim, Email: dhkim417@amorepacific.com.
Hyun Soo Kim, Email: hsookim@amorepacific.com.
Ji Hye Kim, Email: kjhkjhmlml@skku.edu.
Jae Youl Cho, Email: jaecho@skku.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Loyer X., Vion A.-C., Tedgui A., Boulanger C.M. Microvesicles as cell–cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 2.Ohno S., Ishikawa A., Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398–401. doi: 10.1016/j.addr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Niu G., Jian T., Gai Y., Chen J. Microbiota and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Adv Drug Deliv Rev. 2023;196 doi: 10.1016/j.addr.2023.114774. [DOI] [PubMed] [Google Scholar]
- 4.Chronopoulos A., Kalluri R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene. 2020;39:6951–6960. doi: 10.1038/s41388-020-01509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An Q., Hückelhoven R., Kogel K.H., van Bel A.J. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006;8:1009–1019. doi: 10.1111/j.1462-5822.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 6.Regente M., Pinedo M., Elizalde M., de la Canal L. Apoplastic exosome-like vesicles: a new way of protein secretion in plants? Plant Signal Behav. 2012;7:544–546. doi: 10.4161/psb.19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J.H., Hong Y.D., Kim D., Park S.J., Kim J.S., Kim H.-M., et al. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl Biol Chem. 2022;65:8. [Google Scholar]
- 8.Liu H., Zhang Q., Wang S., Weng W., Jing Y., Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169–181. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imokawa G., Ishida K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int J Mol Sci. 2015;16:7753–7775. doi: 10.3390/ijms16047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salucci S., Burattini S., Battistelli M., Baldassarri V., Maltarello M.C., Falcieri E. Ultraviolet B (UVB) irradiation-induced apoptosis in various cell lineages in vitro. Int J Mol Sci. 2012;14:532–546. doi: 10.3390/ijms14010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee G., Gupta N., Kapoor A., Raman G. UV induced bystander signaling leading to apoptosis. Cancer Lett. 2005;223:275–284. doi: 10.1016/j.canlet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 13.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sollberger G., Strittmatter G.E., Grossi S., Garstkiewicz M. Auf dem Keller U, French LE et al. Caspase-1 activity is required for UVB-induced apoptosis of human keratinocytes. J Invest Dermatol. 2015;135:1395–1404. doi: 10.1038/jid.2014.551. [DOI] [PubMed] [Google Scholar]
- 15.Pena-Blanco A., Garcia-Saez A.J. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 16.Perluigi M., Di Domenico F., Blarzino C., Foppoli C., Cini C., Giorgi A., et al. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: a proteomic approach. Proteome Sci. 2010;8:13. doi: 10.1186/1477-5956-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs D., Raffa S., Flori E., Aspite N., Briganti S., Cardinali G., et al. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J Dermatol Sci. 2009;54:106–113. doi: 10.1016/j.jdermsci.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Amstad P., Crawford D., Muehlematter D., Zbinden I., Larsson R., Cerutti P. Oxidants stress induces the proto-oncogenes, C-fos and C-myc in mouse epidermal cells. Bull Cancer. 1990;77:501–502. [PubMed] [Google Scholar]
- 19.Devary Y., Gottlieb R.A., Lau L.F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmarsh A.J., Davis R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 21.Pittayapruek P., Meephansan J., Prapapan O., Komine M., Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:686 doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckman S.Y., Gresham A., Hale P., Hruza G., Anast J., Masferrer J., et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 23.Oh J., Kim J.H., Park J.G., Yi Y.S., Park K.W., Rho H.S., et al. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediat Inflamm. 2013;2013 doi: 10.1155/2013/787042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uluckan O., Guinea-Viniegra J., Jimenez M., Wagner E.F. Signalling in inflammatory skin disease by AP-1 (Fos/Jun) Clin Exp Rheumatol. 2015;33:S44–S49. [PubMed] [Google Scholar]
- 25.Salminen A., Kaarniranta K., Kauppinen A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm Res. 2022;71:817–831. doi: 10.1007/s00011-022-01598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho E.G., Choi S.Y., Kim H., Choi E.J., Lee E.J., Park P.J., et al. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: an eco-friendly and sustainable way to use ginseng substances. Cells. 2021:10:486. doi: 10.3390/cells10030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jager T.L., Cockrell A.E., Du Plessis S.S. Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol. 2017;996:15–23. doi: 10.1007/978-3-319-56017-5_2. [DOI] [PubMed] [Google Scholar]
- 28.Tu Y., Quan T. Oxidative stress and human skin connective tissue aging. Cosmetics. 2016;3:28. [Google Scholar]
- 29.Ho C.Y., Dreesen O. Faces of cellular senescence in skin aging. Mech Ageing Dev. 2021;198 doi: 10.1016/j.mad.2021.111525. [DOI] [PubMed] [Google Scholar]
- 30.Bergman M.R., Cheng S., Honbo N., Piacentini L., Karliner J.S., Lovett D.H. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J. 2003;369:485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hungness E.S., Pritts T.A., Luo G-j, Sun X., Penner G.C., Hasselgren P.-O. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1β-stimulated human enterocytes. Shock. 2000;14:386–391. doi: 10.1097/00024382-200014030-00025. [DOI] [PubMed] [Google Scholar]
- 32.Chung Y.W., Jeong D.W., Won J.Y., Choi E.J., Choi Y.H., Kim I.Y. H2O2-induced AP-1 activation and its effect on p21WAF1/CIP1-mediated G2/M arrest in a p53-deficient human lung cancer cell. Biochem Biophys Res Commun. 2002;293:1248–1253. doi: 10.1016/S0006-291X(02)00360-1. [DOI] [PubMed] [Google Scholar]
- 33.Jakaria M., Haque M.E., Kim J., Cho D.Y., Kim I.S., Choi D.K. Active ginseng components in cognitive impairment: therapeutic potential and prospects for delivery and clinical study. Oncotarget. 2018;9:33601–33620. doi: 10.18632/oncotarget.26035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahuja A., Kim J.H., Kim J.H., Yi Y.S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42:248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.K., Shin K.K., Kim H., Hong Y.H., Choi W., Kwak Y.S., et al. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J Ginseng Res. 2021;45:717–725. doi: 10.1016/j.jgr.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S., Rhee D.K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res. 2017;41:589–594. doi: 10.1016/j.jgr.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y., Wang H., Zhang M., Shi M., Yang C., Ni Q., et al. Safety and antifatigue effect of Korean Red Ginseng capsule: a randomized, double-blind and placebo-controlled clinical trial. J Ginseng Res. 2022;46:543–549. doi: 10.1016/j.jgr.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S.K., Hyun S.H., G Park C.K., Kwak Y.S., Jang Y.J., et al. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: a systemic review through in vivo and clinical trials. J Ginseng Res. 2021;45:41–47. doi: 10.1016/j.jgr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin K.K., Yi Y.S., Kim J.K., Kim H., Hossain M.A., Kim J.H., et al. Korean red ginseng plays an anti-aging role by modulating expression of aging-related genes and immune cell subsets. Molecules. 2020;25:1492 doi: 10.3390/molecules25071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S.J., Nam J., Ahn C.W., Kim Y. Anti-diabetic properties of different fractions of Korean red ginseng. J Ethnopharmacol. 2019;236:220–230. doi: 10.1016/j.jep.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 41.De Robertis M., Sarra A., D’Oria V., Mura F., Bordi F., Postorino P., et al. Blueberry-derived exosome-like nanoparticles counter the response to TNF-alpha-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10:742 doi: 10.3390/biom10050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perut F., Roncuzzi L., Avnet S., Massa A., Zini N., Sabbadini S., et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021:11:87. doi: 10.3390/biom11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mu J., Zhuang X., Wang Q., Jiang H., Deng Z.B., Wang B., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome‐like nanoparticles. Mol Nutr Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu S.H., Huang H.Y., Korivi M., Hsu M.F., Huang C.Y., Hou C.W., et al. Oral Rg1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J Int Soc Sports Nutr. 2012;9:23. doi: 10.1186/1550-2783-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y., Chu S., Shao Q., Zhang M., Xia C., Wang Y., et al. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. 2017;51:1–13. doi: 10.1080/10715762.2016.1234710. [DOI] [PubMed] [Google Scholar]
- 46.Wu C.F., Bi X.L., Yang J.Y., Zhan J.Y., Dong Y.X., Wang J.H., et al. Differential effects of ginsenosides on NO and TNF-α production by LPS-activated N9 microglia. Int Immunopharm. 2007;7:313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Gao C., Zhang K., Liang F., Ma W., Jiang X., Wang H., et al. Inhibition of the Ras/ERK1/2 pathway contributes to the protective effect of ginsenoside Re against intimal hyperplasia. Food Funct. 2021;12:6755–6765. doi: 10.1039/d1fo00015b. [DOI] [PubMed] [Google Scholar]
- 48.Deng H.L., Zhang J.T. Anti-lipid peroxilative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl) 1991;104:395–398. [PubMed] [Google Scholar]
- 49.Cong L., Ma J., Zhang Y., Zhou Y., Cong X., Hao M. Effect of anti-skin disorders of ginsenosides- A Systematic Review. J Ginseng Res. 2023;47:605–614. doi: 10.1016/j.jgr.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B., Cui X., Jin H.H., Hong L., Liu X., Li X., et al. Ginsenoside Re prevents angiotensin II-induced gap-junction remodeling by activation of PPARgamma in isolated beating rat atria. Life Sci. 2017;190:36–45. doi: 10.1016/j.lfs.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Cho J.S., Moon Y.M., Um J.Y., Moon J.H., Park I.H., Lee H.M. Inhibitory effect of ginsenoside Rg1 on extracellular matrix production via extracellular signal-regulated protein kinase/activator protein 1 pathway in nasal polyp-derived fibroblasts. Exp Biol Med (Maywood) 2012;237:663–669. doi: 10.1258/ebm.2012.011342. [DOI] [PubMed] [Google Scholar]
- 52.Kanchanapally R., Khan M.A., Deshmukh S.K., Srivastava S.K., Khushman M., Singh S., et al. Exosomal formulation escalates cellular uptake of honokiol leading to the enhancement of its antitumor efficacy. ACS Omega. 2020;5:23299–23307. doi: 10.1021/acsomega.0c03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao J., Feng S., Wang X., Long K., Luo Y., Wang Y., et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6 doi: 10.7717/peerj.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen L.L., Nielsen M.E. Plant exosomes: using an unconventional exit to prevent pathogen entry? J Exp Bot. 2017;69:59–68. doi: 10.1093/jxb/erx319. [DOI] [PubMed] [Google Scholar]
- 55.Regente M., Pinedo M., San Clemente H., Balliau T., Jamet E., de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 56.Cai Q., Qiao L., Wang M., He B., Lin F.M., Palmquist J., et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baldini N., Torreggiani E., Roncuzzi L., Perut F., Zini N., Avnet S. Exosome-like nanovesicles isolated from Citrus limon L. exert antioxidative effect. Curr Pharm Biotechnol. 2018;19:877–885. doi: 10.2174/1389201019666181017115755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.