Abstract
Background
Current laboratory tests fail to evaluate the hemostatic function of platelets in patients with thrombocytopenia. We investigated the use of the Total Thrombus-Formation Analysis System (T-TAS® 01 [Fujimori Kogyo Co, Tokyo, Japan]) to evaluate hemostasis under conditions of experimental thrombocytopenia, and in patients before and after platelet transfusion.
Materials and methods
Specific T-TAS 01 chips, for thrombocytopenic conditions, were used. The area under the curve (AUC) and occlusion time (OT, min) were measured in: (i) experimentally induced thrombocytopenia (183±15 to 6.3±1.2×103 platelets/μL) in blood samples from healthy donors (No.=13), and (ii) blood from oncohematological thrombocytopenic patients (No.=48), before and after platelet transfusion. The influences of hematocrit and number of transfusions were analyzed in these patients.
Results
Progressive reductions of AUC and prolongations of OT related significantly to decreasing platelet counts (p<0.05 for all) in experimental thrombocytopenia. In samples from thrombocytopenic patients, platelet counts, AUC and OT were, respectively, 10.8±0.6×103/μL, 175.2±59, and 27.2±1 min before transfusion; and 22±1.5×103/μL, 400.8±83 and 22.9±1.5 min after platelet transfusion (p<0.01 for all). A hematocrit below 25% or exposure to ten or more previous platelet transfusions had a negative impact on the T-TAS 01 performance in patients. In vitro correction of the hematocrit improved the hemostatic response in thrombocytopenic patients.
Discussion
T-TAS 01 measurements were sensitive to low platelet counts in the experimental setting. The technology was sensitive to evaluate the hemostatic capacity of platelet transfusions. Exposure to multiple medications, repeated platelet transfusions and lower hematocrits may interfere with the hemostatic performance in oncohematological patients with thrombocytopenia.
Keywords: thrombocytopenia, total thrombus-formation analysis system (T-TAS), platelet transfusion, bleeding
INTRODUCTION
Thrombocytopenia is a pathological condition defined as a platelet count below the lower limit of the normal range established by the laboratory testing of the sample. Thrombocytopenia is present when the platelet count is below 100×103 platelets/μL1. Hematological diseases and chemo-immune therapies are common causes of thrombocytopenia in adults, either through peripheral destruction of platelets or decreased platelet production in the bone marrow.
Bleeding complications in thrombocytopenic patients constitute a major problem in clinical practice. Management of thrombocytopenia may require prophylactic transfusion of platelets to stabilize hemostasis, especially in hematological patients. Studies have shown that up to 67% of platelet transfusions are received by this group of patients, and a high proportion of them are transfused prophylactically2,3. Despite the existence of published systematic reviews4, criteria for platelet transfusion are not fully standardized and may differ depending on a variety of factors5–7. The presence of bleeding symptoms justifies platelet transfusion in thrombocytopenic patients. In the absence of bleeding symptoms, platelet counts are used to trigger the presumed requirement for transfusion. Although some studies support the concept of lower platelet thresholds (5×103 platelets/μL)8, a generally suggested safer practice is to transfuse hospitalized adult patients with a platelet count of 10×103 cells/μL or less to reduce the risk of spontaneous bleeding6,7,9. In patients with additional bleeding risk factors, increasing the threshold for platelet transfusion to <20×103 platelets/ μL could be considered. However, bleeding events do not correlate significantly with platelet counts7, and severe bleeding can occur in thrombocytopenic oncohematological patients with platelet counts >10×103 platelets/μL10,11. Therefore, it would be desirable to have appropriate laboratory tests, other than routine platelet counts, which could objectively evaluate global hemostatic performance in thrombocytopenic patients in order to guide the use of platelet transfusions.
Platelets recognize areas of damaged endothelium to avoid blood loss by adhering to the subendothelium. Once exposed to injured vessels, platelets spread, release their contents, and activate other circulating platelets, thereby promoting the formation of aggregates and activating the coagulation cascade12. All these physiological processes occur under flow conditions and the rheology of blood is crucial to guarantee them13. Therefore, various different components are involved in the maintenance of adequate hemostasis, among which platelet function, platelet counts, and hematocrit are crucial.
The majority of tests used in hemostasis laboratories are designed for the diagnosis of hemorrhagic diatheses caused by platelet function defects in situations in which platelet counts are normal14. The Total Thrombus-formation Analysis System (T-TAS, Fujimori Kogyo Co, Tokyo, Japan) is a microf luidic-based technology that has contributed to the evaluation of hemostasis in other areas of medicine15,16. T-TAS 01 monitors changes in flow pressure, ref lecting the growth of platelet- and fibrin-rich thrombus in a flow chamber embedded in an analytical chip. In contrast to the lengthy, time-consuming classic perfusion systems13,17, T-TAS 01 has the potential to evaluate hemostasis rapidly and using low blood volumes. Parameters provided by this technology may provide additional information to that provided by current platelet cut-off counts.
The present study aimed to evaluate the suitability of T-TAS 01 as a device for assessing global hemostasis under conditions of thrombocytopenia. First, we validated the ability of this device to detect alterations in hemostasis under conditions of experimental thrombocytopenia. Second, we evaluated the hemostatic response in samples from thrombocytopenic patients and improvements in their hemostatic performance after platelet transfusions. Since reductions in hematocrit levels and exposure to multiple transfusions have been suggested to have an additional impact on hemostasis and platelet recoveries, we also explored the possible influence of low hematocrits and repeated transfusion in the latest setting.
MATERIAL AND METHODS
Healthy donors, patients, and sample collection
Thirteen healthy volunteers, who had not ingested drugs affecting platelets in the preceding 10 days and 48 consecutive oncohematological thrombocytopenic patients with platelet counts less than 30×103 platelets/μL from our institution were included in this study, which was conducted between February 2021 and March 2022. Bleeding symptoms were evaluated according to the modified World Health Organization (WHO) bleeding score18.
Citrated whole blood samples were obtained from the healthy volunteers, and from 48 patients who had indications for platelet transfusion according to clinical guidelines19. In the latter, samples were drawn immediately before, and within 6 hours after major ABO-compatible transfusions had been were terminated. Blood sampling before and after transfusions was completed in 42 of the 48 patients (Table I). Platelet concentrates were obtained after pooling 4–5 units of whole blood-derived platelet intermediate products (>2.4×1011 platelets) in platelet additive solution (SPP+, Macopharma, Lille, France), using Reveos (Terumo BCT, Lakewood, CO, USA), and were not subjected to pathogen reduction technologies. Platelet concentrates were kept at 22±2°C in flatbed agitators for up to 5 days and transfused into patients at a median of 4 days of storage (95% confidence interval: 3–4 days). In no case did patients receive any other form of transfusion product (plasma or red blood cell concentrates).
Table I.
Characteristics of the patients
| Characteristic | All transfused patients (No.=48)* | Transfused patients available (No.=42, 87%) |
|---|---|---|
| Age in years, median (range) | 54 (15–82) | 54 (15–82) |
| Male sex, No. (%) | 29 (60) | 24 (57) |
| Diagnosis, No. (%) | ||
| Acute myeloid leukemia | 19 (40) | 17 (41) |
| Acute lymphoblastic leukemia | 10 (21) | 10 (24) |
| Lymphoma | 9 (19) | 6 (14) |
| Chronic myeloid syndromes | 6 (12) | 5 (12) |
| Immune disease | 3 (6) | 3 (7) |
| Other | 1 (2) | 1 (2) |
| Cause of thrombocytopenia, No. (%) | ||
| Chemo-immune therapy | 27 (56) | 26 (62) |
| HPCT | 16 (33) | 12 (29) |
| Chronic bone mar row failure | 5 (11) | 4 (9) |
| Splenomegaly, No. (%) | 17 (42) | 15 (42) |
| Portal hypertension, No. (%) | 2 (4) | 2 (5) |
| Active infection, No. (%) | 28 (60) | 25 (60) |
| Sepsis, No. (%) | 5 (10) | 4 (10) |
| Alterations of coagulation, No. (%) | ||
| PTR <1.2 | 37 (79) | 32 (78) |
| PTR 1.2–1.5 | 10 (21) | 9 (22) |
| PTR >1.5 | 2 (4) | 2 (5) |
| Previous pool transfused, No. (%) | ||
| 0 pool | 5 (11) | 5 (13) |
| 1 to 10 pools | 25 (56) | 22 (56) |
| >10 pools | 15 (33) | 12 (31) |
| Prior HPC T, No. (%) | ||
| No | 41 (85) | 36 (85) |
| Autologous | 2 (4) | 1 (3) |
| Allogenic | 5 (11) | 5 (12) |
No.: number of patients; HPCT: hematopoietic progenitor cell transplantation; PTR: prothrombin time ratio.
Includes six patients whose post-transfusion sample was not available. Six patients (12%) were transfused therapeutically because they had a WHO bleeding score of 2. None of the patients showed HLA immunization.
Experimental design and laboratory procedures
For the experimentally induced thrombocytopenia, baseline blood samples from healthy donors (mean platelet count, 183±15×103 platelets/μL) were manipulated to reach three different platelet counts categorized as low (2–14×103 platelets/μL), medium (16–36×103 platelets/μL), and high (37–62×103 platelets/μL). The initial (baseline) blood sample was always evaluated as a reference. Platelet depletion was accomplished using a filtration procedure (RC100, PAL)17,20,21. The various platelet count ranges were evaluated by a hematology analyzer (ADVIA 2120i, Siemens Healthiness, Erlangen, Germany), after mixing adequate proportions of filtered and unfiltered blood. The hemostatic capacity of each sample was measured in the T-TAS 01.
Studies in oncohematological patients
Blood samples from thrombocytopenic patients were left undisturbed and evaluated in the T-TAS 01 before and after platelet transfusion. In a small number of cases–when the volume of the sample permitted additional manipulation–the hematocrit was raised in vitro by using red blood cells separated from the same patients. For this purpose, remnant blood samples were centrifuged for 3 min at 14,000 rpm in a microcentrifuge (5417R, Eppendorf, Hamburg, Germany) and 200 μL of concentrated red cells were added to a 1 mL blood sample from the same patient. Hematocrit levels were measured in an ADVIA 2120i (Siemens Healthiness). Samples before and after the increase in hematocrit were evaluated in the T-TAS 01.
T-TAS 01
The HD chip used in these studies consists of a small capillary chamber covered with collagen and tissue factor and was specifically designed for evaluations under thrombocytopenia conditions (channel measures: width of 300 μm; depth of 50 μm)22. According to the manufacturer’s instructions, the volume of blood required is about 480 μL of citrated blood. Before performing the analysis, a solution containing corn trypsin inhibitor and calcium (Ca-CTI, 20 μL) was added to restore the coagulation potential of the citrated blood sample.
T-TAS 01 analyzes the process of hemostatic plug formation by monitoring flow pressure changes in the capillary. The T-TAS 01 device provides two basic parameters: (i) area under the curve (AUC), calculated by measuring the area under the flow pressure curve for 30 min after the start of the assay, and (ii) occlusion time (OT), defined as the time for complete capillary occlusion.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). A t-test for paired data and the Wilcoxon-Mann-Whitney test were used for comparisons. Fisher’s exact tests were used to compare categorical variables. Data were analyzed with software (IBM SPSS Statistics 23, IBM Corporation, Armonk, NY, USA). Two sub-analyses were performed in the clinical setting to evaluate the influence of concurrent hematocrits (< or ≥25%) and exposure to previous platelet transfusion (< or ≥10). The level of significance was established at p<0.05. GraphPad Prism 9.3.1 and Adobe Illustrator CC 18.0 were used for graphics.
RESULTS
T-TAS 01 evaluation of experimentally induced thrombocytopenia
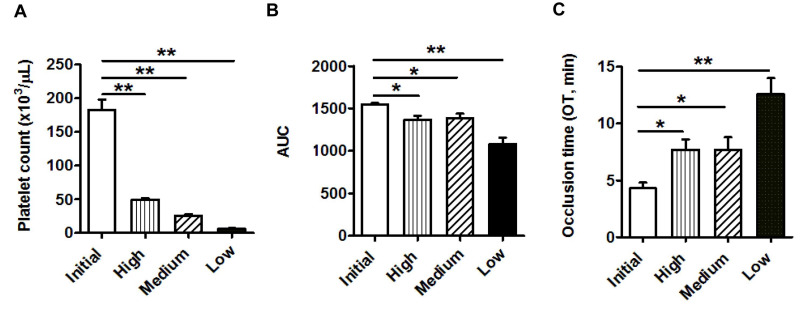
Figure 1 summarizes the results using blood samples from healthy donors (No.=13). The mean (±SEM) platelet count in the initial samples was 183±15×103 platelets/μL. After filtration, the counts were 49.5±2, 26.1±2, and 6.3±1.2×103 platelets/μL in the groups categorized as having high, medium, and low counts, respectively. All three groups showed significant differences in T-TAS 01 parameters compared with the baseline platelet counts (p<0.01) (Figure 1A).
Figure 1.
Bar diagrams showing (A) platelet count, (B) area under the curve (AUC ) and (C) occlusion time (OT, min) measured by the T-TAS 01 in samples with laboratory-induced thrombocytopenia
Initial: baseline sample (platelet count: 183±15×103/μL); high: high platelet counts (49.5±2103/μL); medium: medium platelet counts (26.1±2103/μL); low: low platelet counts (6.3±1.2103/μL). Data are mean ± standard error of mean (No.=13). *p<0.05, **p<0.01.
The AUC decreased statistically significantly after platelet depletion, with a progressive reduction from 1,547.8±16.7 for the baseline sample to 1,369.5±44.5, 1,386.8±53.9, and 1,084.4±72.5 for the high (p<0.05 vs baseline sample), medium (p<0.05 vs baseline sample), and low (p<0.01 vs baseline sample) groups, respectively (Figure 1B). The OT values measured by the T-TAS 01, were 4.3±0.5 for the baseline sample, and 7.7±0.9, 7.7±1.1, and 12.6±1.4 min for the high, medium and low platelet count groups, respectively. Differences were statistically significant between the initial and low platelet count groups (p<0.01), as well as between the initial and both the high and medium platelet count groups (both p<0.05) (Figure 1C).
T-TAS 01 evaluation of samples from thrombocytopenic patients
The baseline characteristics of the patients are shown in Table I. Information on the 42 patients from whom a post-transfusion sample was obtained is also included. Six of them (10%) received more than one pool. Of note, acute leukemia was the most frequent diagnosis, and chemo-immune therapy and hematopoietic progenitor cell transfusion (HPCT) were the most common causes of thrombocytopenia.
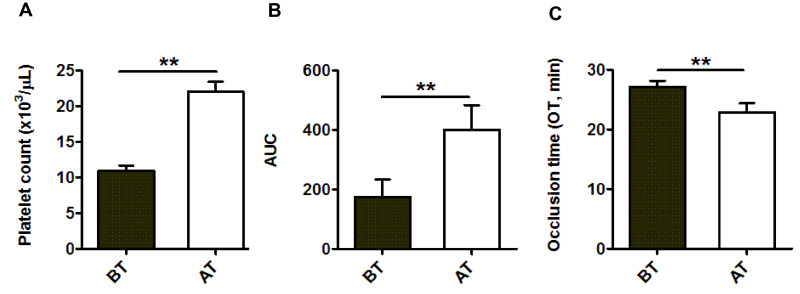
The main indication for platelet transfusion was prophylaxis, for platelet counts <10×103/μL (in 35 patients, 73%) and <20×103/μL with associated clinical conditions (in 7 patients, 15%). Six patients (12%) were given therapeutic transfusions because they had a modified WHO bleeding score of 2. As summarized in Figure 2A, platelet counts in blood samples before and after transfusion (No.=42) were 10.8±0.6 and 22±1.5×103 platelets/μL, respectively (p<0.01). No statistical differences were observed in hematocrit between samples taken before and after platelet transfusions (25.5±0.5% and 25.4±0.5%, respectively), confirming that patients had not inadvertently received additional red blood cell transfusions. The AUC value in pre-transfusion samples was 175.2±59 and improved significantly to 400.8±83 (p<0.01) after platelet transfusion (Figure 2B). The OT in the pre-transfusion samples was 27.2±1.0 min and shortened, significantly, to 22.9±1.5 min (p<0.01) in the post-transfusion samples (Figure 2C).
Figure 2.
Bar diagrams showing (A) platelet count, (B) area under the curve (AUC ) and (C) occlusion time (OT, min) measured by the T-TAS 01 in samples from thrombocytopenic patients
BT: before transfusion; AT: after transfusion. Data are mean ± standard error of mean (No.=42). **p<0.01.
Influence of hematocrit and repeated platelet transfusions on T-TAS 01 parameters in samples from thrombocytopenic patients
The good correspondence of T-TAS 01 parameters with platelet counts and hematocrit observed in the experimental setting was not fully replicated in the studies performed with samples from thrombocytopenic patients. T-TAS 01 parameters deteriorated compared to those found at the lowest ranges of platelet counts in the experimental setting.
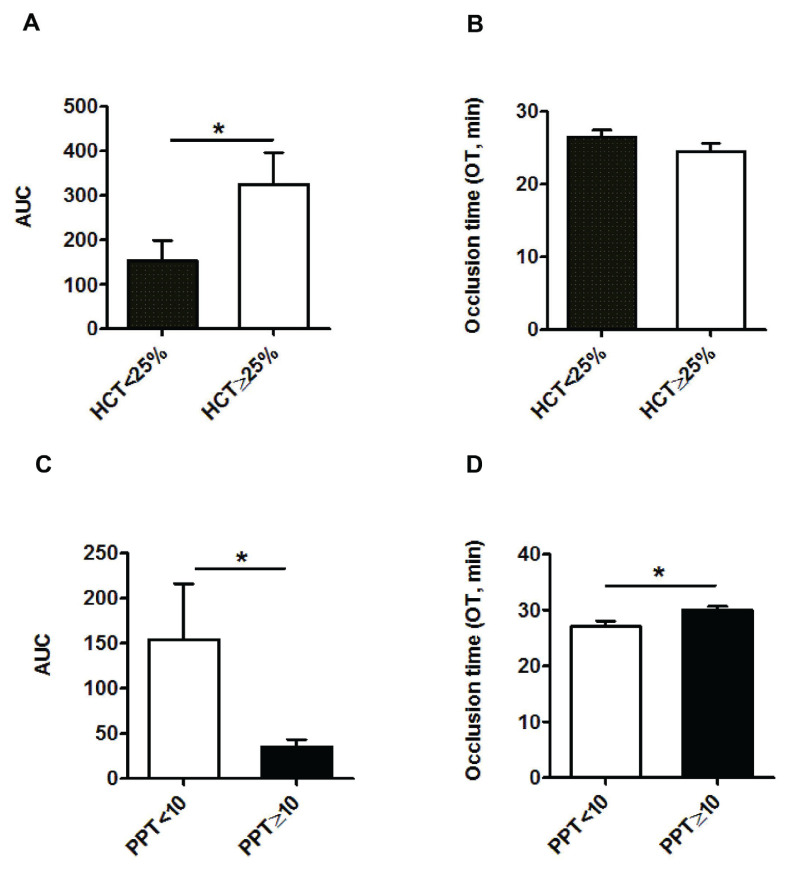
When all the results for the T-TAS 01 parameters were grouped depending on the hematocrit (<25% and ≥25%), the difference between AUC values in the two groups was statistically significant (153±46 and 325±71, respectively; p<0.05) (Figure 3A). No statistically significant difference was observed for the OT (26.5±0.9 and 24.5±1.1 min, respectively) (Figure 3B).
Figure 3.
Bar diagrams showing area under the curve (AUC ) and occlusion time (OT, min)
A and B present the results grouped depending on whether the hematocrit (HCT) was <25% or ≥25%. (No.=48 before platelet transfusion and No.=42 after platelet transfusion). C and D present the results grouped depending on whether the number of previous platelet transfusions (PPT) was <10 or ≥10 (Information available for 45 of 48 patients). Data are expressed as mean ± standard error of mean. *p<0.05.
T-TAS 01 results with samples before platelet transfusion were also analyzed considering the number of platelet transfusions previously received by the patients during the same oncohematological episode. Two subgroups were considered: patients who had previously received <10 and ≥10 platelet transfusions. AUC values for the first and second groups were 154±62 and 35±8.5, respectively (p<0.05) (Figure 3C), while those for the OT were 27±1.1 and 29.9±0.7 min (p<0.05) (Figure 3D).
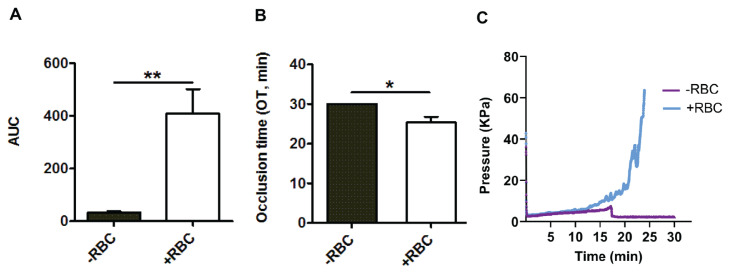
Additional blood samples from ten thrombocytopenic patients of our series were reevaluated after moderately raising the hematocrit in vitro. Hematocrit values were 24.3±0.9% and 35.2±0.8% before and after in vitro addition of red cells (p<0.01). The calculated AUC values were 33±4.6 and 408±93 before and after the increase in hematocrit (p<0.01) (Figure 4A). As shown in Figure 4B, the OT also improved statistically significantly from 30±0 to 25.3±1.5 min, respectively (p<0.05). Figure 4C shows graphics obtained by the T-TAS 01 using a sample from one patient with an initial hematocrit of 24% that was corrected to 35%.
Figure 4.
Bar diagrams showing (A) area under the curve (AUC ), (B) occlusion time (OT, min); and (C) representative tracing diagram, illustrating the hematocrit modification in thrombocytopenic samples
RBC: red blood cells. Data are expressed as mean ± standard error of mean (No.=10). *p<0.05, **p<0.01.
DISCUSSION
Evaluation of the hemostatic function of platelets in thrombocytopenic samples is challenging with the currently available tests. The present study was not designed to assess whether the parameters provided by the T-TAS 01 could represent an improvement over standard platelet counts used to trigger platelet transfusions. It was, in fact, carried out to explore the ability of the T-TAS 01 device to measure hemostasis in thrombocytopenic samples, using newly designed chips (HD) and different strategies. We tested blood samples with normal platelet counts and hematocrit in which thrombocytopenia was then induced in the laboratory and in samples from thrombocytopenic patients before and after platelet transfusion. We also evaluated the effect of in vitro correction of the hematocrit in patients’ samples. The major findings of this study indicate that: (i) the results provided by the T-TAS 01-HD in the experimental setting were associated with the platelet counts in samples with a normal hematocrit; (ii) in samples from oncohematological thrombocytopenic patients before transfusion, results were quantitatively lower than those observed in the experimental studies; (iii) T-TAS 01 HD chips detected the recovery of hemostatic function following platelet transfusion, and (iv) results of the T-TAS 01 are highly dependent on the hematocrit. Interestingly, the T-TAS 01 results demonstrate that the number of platelet transfusions previously received has a significant impact on the hemostatic performance in oncohematological patients.
Thrombocytopenic patients with bleeding complications require platelet transfusions. In addition, oncohematological patients are usually given prophylactic platelet transfusions when platelet counts are <10×103/μL, or <20×103/μL with additional bleeding risk factors6,9,19. However, the role of prophylactic platelet transfusions remains controversial. In principle, clinicians rely upon threshold values of platelet numbers to determine the timing of platelet transfusions. This strategy has been questioned because of the relatively short half-life of transfused platelets, the risk of accelerating alloimmunization or refractoriness against future platelet transfusions23, and its elevated cost24.
There are no tests available that can reliably assess global hemostasis under conditions of thrombocytopenia, although there are some technical approaches that can provide relevant information on the clinical efficacy of platelet concentrates. Studies under flow conditions using classic annular chambers have contributed greatly to a better understanding of the mechanisms of hemostasis and have provided relevant information on the clinical efficacy of platelet concentrates17,21. The main advantage of these perfusion devices is that they allow the evaluation of adhesive, aggregating and procoagulant activities of platelets interacting with damaged vessels under physiological flow conditions. Perfusion-related methodologies have contributed to the assessment of quality and mechanisms of action of standard and new blood derivatives13,25,26. Although microf luidic versions of perfusion devices have facilitated the performance of perfusion studies27, these technologies are mainly investigational, remain time-consuming, and require sophisticated equipment not widely available to many hospital transfusion facilities.
Earlier versions of the T-TAS 01 equipment proved useful for predicting bleeding events in patients with atrial fibrillation exposed to anticoagulants28 and for reflecting the hemostatic consequences of diminished red cell numbers under blood flow29. In our hands, the T-TAS 01-HD performed as a promising device for assessing hemostatic competence in thrombocytopenic patients and for evaluating their response to platelet transfusion, therefore, providing reliable therapeutic guidance. The results of our study of patients using the T-TAS 01-HD, mainly in the prophylactic setting, are concordant with those previously reported in a small number of bleeding-thrombocytopenic patients requiring platelet transfusions22. In that study, the authors reported the recovery of hemostatic potential after platelet transfusion was confirmed with the T-TAS 01-HD and correlated with the function, rather than the count, of transfused platelets. The range of platelet counts in the mentioned study was well above the prophylactic range used in our study.
Data obtained in the present study indicate that in blood samples from healthy volunteers, with hematocrits in the normal range, T-TAS 01 parameters showed a good correspondence with decreasing platelet counts. The HD chips were sensitive to evaluate hemostasis even when platelet counts were significantly below normal ranges. Our present results also confirm that the hemostatic effect of platelet transfusions could be detected by the T-TAS 01 in samples from patients after being transfused. However, in the samples from thrombocytopenic oncohematological patients, T-TAS 01 parameters were found to be more severely affected than anticipated from the experimental thrombocytopenia setting. Mechanisms of hemostasis are known to be already deteriorated in oncohematological patients30. The complexity of such patients, their variable platelet functional status, the diverse degrees of endothelial damage, and exposure to multiple medications may result in the development of a subset of dysfunctional platelets that could explain the more deteriorated results of the T-TAS 01 in thrombocytopenic patients, independently of their platelet counts.
The hypothesis of dysfunctional platelets in the setting of thrombocytopenic oncohematological patients would be difficult to demonstrate. On the one hand, hemostatic alterations related to impaired platelet function have been found in patients with acute myeloid leukemia, even in those with moderate thrombocytopenia and nearnormal platelet counts31,32. In this regard, there were 19 patients in our cohort with acute myeloid leukemia, a condition in which a platelet dysfunction of clonal origin has been demonstrated33. On the other hand, current tests for the evaluation of platelet function, such as platelet aggregation, are impractical with platelet counts below 50×103 platelets/μL and unfeasible in samples with platelet counts in the range of 10 and 20×103 platelets/μL34.
In addition to the possible existence of a certain level of platelet dysfunction in oncohematological patients with thrombocytopenia, low hematocrits and repeated exposure to previous platelet transfusions were identified in our present study as two variables that could have a negative impact on T-TAS 01 parameters. Regarding the role of hematocrit, we observed a significant difference in T-TAS 01 parameters between groups of patients with hematocrits above or below 25%. Moreover, experiments correcting the hematocrit in patients’ samples in which both platelet counts and hematocrit were low, confirmed that the ability of the T-TAS 01 to measure hemostasis improves after increasing the hematocrit in vitro. Overall, these results indicate that, even under extreme conditions, the T-TAS was still useful for providing qualitative information on the hemostatic performance of transfused platelets that goes beyond a simple increment in platelet counts.
It has been speculated that anemia can impair hemostasis in thrombocytopenic patients35. Red blood cells play a critical role in the maintenance of rheological conditions that facilitate the interaction between platelets and subendothelial structures. Our group previously demonstrated in studies under flow conditions that the formation of platelet aggregates on denuded vascular surfaces is markedly impaired at lower hematocrits, independently of the final platelet count36. Another classic study demonstrated a significant decrease in the bleeding time when anemia in patients with kidney failure was corrected with red blood cell transfusion37. It has been suggested that bleeding risk and/or hemostatic performance evaluated based only on the platelet count may be underestimated. It is, therefore, possible that hematocrit levels that ensure adequate levels of hemoglobin to maintain correct oxygen transport, may not be sufficient to preserve optimal rheological conditions necessary for the correct interaction of platelets with injured surfaces. This implies that, in clinical practice, raising the hematocrit in thrombocytopenic oncohematological patients could improve hemostasis without making platelet transfusions necessary in some cases of low platelet counts38.
Previous studies had also documented a lower efficacy of platelet transfusions in patients who had received more than 10 transfusions39,40. Mechanisms causing refractoriness to platelets or reduced count increments after platelet transfusion are mainly of unknown, non-immune origin41. AUC and OT showed sub-optimal results in the group of patients who had received ≥10 platelet transfusions indicating that independently of the responses to transfusion these patients may have already impaired hemostatic competence. These results warrant further investigations and a rapid, easy methodology, such as the T-TAS 01 automated flow chamber system with HD microchips, may be useful for this purpose.
The present study has several limitations. First, the time from sample extraction to processing varied from minutes to a few hours. Although this approach may be a better approximation to practice in real life, the time variation may have affected the T-TAS 01 parameters. Second, AUC and OT were analyzed in a single laboratory and, therefore, our present results should be confirmed in a larger cohort of patients including not only oncohematological patients but also those with different diagnoses with and without bleeding symptoms.
CONCLUSIONS
The newly designed T-TAS 01, using a modified microchip-based flow chamber, can measure global hemostatic performance in samples with thrombocytopenia and normal hematocrit. Although the T-TAS 01 cannot substitute the platelet count, it proved useful in evaluating qualitative aspects of the hemostatic performance of transfused platelets. Importantly, T-TAS 01 results confirm that hematocrit is crucial for better hemostatic responses in cases of thrombocytopenia. Moreover, our data indicate that the number of previously received platelet transfusions has a significant impact on the hemostatic performance of transfused platelets. The present findings may have implications for the evaluation of bleeding risk and the transfusional management of patients with thrombocytopenia and anemia.
ACKNOWLEDGMENTS
The Authors thank Prof. J McCullough for his advice on the manuscript. We are grateful to the nurses of the Hematology unit for their excellent work that made possible the accomplishment of this study.
Footnotes
AUTHORSHIP CONTRIBUTIONS: SS performed studies, collected data, conducted the formal analysis, and wrote the original draft. JAP coordinated the study at the Department of Hematology, organized the patients’ availability and blood sampling, validated data, and wrote the original draft. ABMC coordinated laboratory tests, validated methodologies, collected data, and performed the statistical analysis. MP, STM and JMS performed laboratory tests, validated results and participated in data collection and analysis. ML and CS participated in the interpretation of the results, provided concepts and bibliography related to the study, and reviewed, discussed and edited the text. GE and MDR designed the study, and participated in its conceptualization, data analysis and writing the original draft. All Authors approved the final version of the manuscript.
ETHICAL CONSIDERATION: This study was approved by the Institutional Ethics Committee at the Hospital Clínic of Barcelona (HCB/2021/0513). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
FUNDING: This study was partially supported by Fujimori Kogyo Co., Instituto de Salud Carlos III (FIS PI19/00888), Fundació Marató de TV3 (202026-10), and Generalitat de Catalunya (2017-SGR675, CERCA).
DISCLOSURE OF CONFLICTS OF INTEREST: MDR has received honoraria from Jazz and research funding from Zacros (Fujimori Kogyo Co., [Japan], Cellphire Therapeutics [USA], CSL Behring [Spain], and Sysmex Europe GmbH [Germany]). No company participated in the content of the manuscript in any way. The remaining Authors have no conflicts of interest directly related to this work.
REFERENCES
- 1.Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155–159. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- 2.Greeno E, McCullough J, Weisdorf D. Platelet utilization and the transfusion trigger: a prospective analysis. Transfusion. 2007;47:201–205. doi: 10.1111/j.1537-2995.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 3.Cameron B, Rock G, Olberg B, Neurath D. Evaluation of platelet transfusion triggers in a tertiary-care hospital. Transfusion. 2007;47:206–211. doi: 10.1111/j.1537-2995.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Mhaskar R, Grossman BJ, Kaufman RM, Tobian AA, Kleinman S, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion. 2015;55:1116–1127. doi: 10.1111/trf.12943. quiz 1115. [DOI] [PubMed] [Google Scholar]
- 5.Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. Platelet transfusions in haematology patients: are we using them appropriately? Vox Sang. 2012;103:284–293. doi: 10.1111/j.1423-0410.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–213. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 7.Stanworth SJ, Shah A. How I use platelet transfusions. Blood. 2022;140:1925–1936. doi: 10.1182/blood.2022016558. [DOI] [PubMed] [Google Scholar]
- 8.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380:1309–1316. doi: 10.1016/S0140-6736(12)60689-8. [DOI] [PubMed] [Google Scholar]
- 10.Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev. 2002;16:34–45. doi: 10.1053/tmrv.2002.29403. [DOI] [PubMed] [Google Scholar]
- 11.Stanworth SJ, Estcourt LJ, Llewelyn CA, Murphy MF, Wood EM TOPPS Study Investigators. Impact of prophylactic platelet transfusions on bleeding events in patients with hematologic malignancies: a subgroup analysis of a randomized trial. Transfusion. 2014;54:2385–2393. doi: 10.1111/trf.12646. [DOI] [PubMed] [Google Scholar]
- 12.Escolar G, Galan A, Mazzara R, Castillo R, Ordinas A. Measurement of platetet interactions with subendothelial substrata: Relevance to transfusion medicine. Transfus Med Rev. 2001;15:144–156. [PubMed] [Google Scholar]
- 13.Escolar G, McCullough J. Platelet in vitro assays: their correspondence with their in vivo hemostatic potential. Transfusion. 2019;59:3783–3793. doi: 10.1111/trf.15559. [DOI] [PubMed] [Google Scholar]
- 14.Gresele P Subcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13:314–322. doi: 10.1111/jth.12792. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, et al. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132:263–270. doi: 10.1016/j.thromres.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Ito T, Nagasato T, Shinnakasu A, Kurano M, Arimura A, et al. Effects of glycemic control and hypoglycemia on thrombus formation assessed using automated microchip flow chamber system: an exploratory observational study. Thromb J. 2019;17:17. doi: 10.1186/s12959-019-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escolar G, Mazzara R, Castillo R, Ordinas A. The role of the Baumgartner technique in transfusion medicine: research and clinical applications. Transfusion. 1994;34:542–549. doi: 10.1046/j.1537-2995.1994.34694295074.x. [DOI] [PubMed] [Google Scholar]
- 18.Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771–1780. doi: 10.1056/NEJMoa1212772. [DOI] [PubMed] [Google Scholar]
- 19.Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–394. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 20.Galan AM, Bozzo J, Hernandez MR, Pino M, Reverter JC, Mazzara R, et al. Infusible platelet membranes improve hemostasis in thrombocytopenic blood: experimental studies under flow conditions. Transfusion. 2000;40:1074–1080. doi: 10.1046/j.1537-2995.2000.40091074.x. [DOI] [PubMed] [Google Scholar]
- 21.Escolar G, Galan AM, Mazzara R, Castillo R, Ordinas A. Measurement of platelet interactions with subendothelial substrata: relevance to transfusion medicine. Transfus Med Rev. 2001;15:144–156. [PubMed] [Google Scholar]
- 22.Atari B, Ito T, Nagasato T, Ohnishi T, Hosokawa K, Yasuda T, et al. A modified microchip-based flow chamber system for evaluating thrombogenicity in patients with thrombocytopenia. Thromb J. 2020;18:31. doi: 10.1186/s12959-020-00244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 24.Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:283–299. doi: 10.1200/JCO.2017.76.1734. [DOI] [PubMed] [Google Scholar]
- 25.Keuren JF, Cauwenberghs S, Heeremans J, de Kort W, Heemskerk JW, Curvers J. Platelet ADP response deteriorates in synthetic storage media. Transfusion. 2006;46:204–212. doi: 10.1111/j.1537-2995.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 26.Cid J, Escolar G, Galan A, Lopez-Vilchez I, Molina P, Diaz-Ricart M, et al. In vitro evaluation of the hemostatic effectiveness of cryopreserved platelets. Transfusion. 2016;56:580–586. doi: 10.1111/trf.13371. [DOI] [PubMed] [Google Scholar]
- 27.Van Aelst B, Feys HB, Devloo R, Vandekerckhove P, Compernolle V. Microfluidic flow chambers using reconstituted blood to model hemostasis and platelet transfusion in vitro. J Vis Exp. 2016;19:53823. doi: 10.3791/53823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito M, Kaikita K, Sueta D, Ishii M, Oimatsu Y, Arima Y, et al. Total Thrombus-Formation Analysis System (T-TAS) can predict periprocedural bleeding events in patients undergoing catheter ablation for atrial fibrillation. J Am Heart Assoc. 2016;5:e002744. doi: 10.1161/JAHA.115.002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaoi H, Shida Y, Ogiwara K, Hosokawa K, Shima M, Nogami K. Role of red blood cells in the anemia-associated bleeding under high shear conditions. Haemophilia. 2017;23:750–758. doi: 10.1111/hae.13252. [DOI] [PubMed] [Google Scholar]
- 30.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 31.Glembotsky AC, Sliwa D, Bluteau D, Balayn N, Marin Oyarzun CP, Raimbault A, et al. Downregulation of TREM-like transcript-1 and collagen receptor alpha2 subunit, two novel RUNX1-targets, contributes to platelet dysfunction in familial platelet disorder with predisposition to acute myelogenous leukemia. Haematologica. 2019;104:1244–1255. doi: 10.3324/haematol.2018.188904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bumbea H, Vladareanu AM, Dumitru I, Popov VM, Ciufu C, Nicolescu A, et al. Platelet defects in acute myeloid leukemia-potential for hemorrhagic events. J Clin Med. 2021;11:118. doi: 10.3390/jcm11010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma-Barqueros V, Bastida JM, Lopez Andreo MJ, Zamora-Canovas A, Zaninetti C, Ruiz-Pividal JF, et al. Platelet transcriptome analysis in patients with germline RUNX1 mutations. J Thromb Haemost. 2023;21:1352–1365. doi: 10.1016/j.jtha.2023.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Cattaneo M, Cerletti C, Harrison P, Hayward CP, Kenny D, Nugent D, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost. 2013 doi: 10.1111/jth.12231. [Ahead of print.] [DOI] [PubMed] [Google Scholar]
- 35.Valeri CR, Cassidy G, Pivacek LE, Ragno G, Lieberthal W, Crowley JP, et al. Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion. 2001;41:977–983. doi: 10.1046/j.1537-2995.2001.41080977.x. [DOI] [PubMed] [Google Scholar]
- 36.Escolar G, Garrido M, Mazzara R, Castillo R, Ordinas A. Experimental basis for the use of red cell transfusion in the management of anemic-thrombocytopenic patients. Transfusion. 1988;28:406–411. doi: 10.1046/j.1537-2995.1988.28588337325.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez F, Goudable C, Sie P, Ton-That H, Durand D, Suc JM, et al. Low haematocrit and prolonged bleeding time in uraemic patients: effect of red cell transfusions. Br J Haematol. 1985;59:139–148. doi: 10.1111/j.1365-2141.1985.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 38.Valeri CR, Khuri S, Ragno G. Role of t he Hct in the treatment of thrombocytopenic patients. Transfusion. 2003;43:1761–1763. doi: 10.1111/j.0041-1132.2003.00595.x. [DOI] [PubMed] [Google Scholar]
- 39.Song T, Zhang Y, Huang J, Liu Z. Transfusion-induced platelet antibodies and regulatory T cells in multiply transfused patients. J Clin Lab Anal. 2021;35:e23864. doi: 10.1002/jcla.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sintnicolaas K, Vriesendorp HM, Sizoo W, Stenfert Kroese WF, Haije WG, Hop WC, et al. Delayed alloimmunisation by random single donor platelet transfusions. A randomised study to compare single donor and multiple donor platelet transfusions in cancer patients with severe thrombocytopenia. Lancet. 1981;1:750–754. doi: 10.1016/s0140-6736(81)92626-x. [DOI] [PubMed] [Google Scholar]
- 41.Rebulla P. A mini-review on platelet refractoriness. Haematologica. 2005;90:247–253. doi: 10.3324/%x. [DOI] [PubMed] [Google Scholar]