Abstract
Background
Extracorporeal photopheresis (ECP) is a well-established but lengthy and burdensome cell-based therapy for various diseases such as cutaneous T-cell lymphoma, graft-versus-host disease and organ rejection after transplantation. The number of mononuclear cells (MNCs) that needs to be collected to obtain a clinical response to ECP is still under debate. The purpose of this retrospective study was to determine the number of lymphocytes, monocytes and neutrophils in mononuclear cell products (MCP) by flow cytometry and the collection efficiency in the offline ECP setting.
Materials and methods
We collected data from 10 different patients undergoing 162 ECP procedures using the Spectra Optia device for MNC collection. White blood cell (WBC) count of MCP was determined using a hematology analyzer. MNCs were analyzed for CD45 and CD14 expression by flow cytometry to exactly determine the collected lymphocyte and monocyte fractions.
Results
Collected MCP showed high cell yields with 55.3×106/kg MNCs and 41.1×106/kg lymphocytes. MCP were characterized by high MNC (81.3%) and low neutrophils (18.7%) percentage. Mean collection efficiency for WBCs and for MNCs was 23.9% and 62.0%, respectively. The MNC fraction showed a moderate to high correlation between peripheral blood cell count of patients and MCP count.
Discussion
This study is one of a few reports showing the monocyte-to-lymphocyte relation in MCP for ECP determined by flow cytometry. In comparison to historical data from inline ECP, the offline ECP processing one total blood volume results in considerably higher cell yields. For this reason, and to reduce the burden on patients, we propose that the offline ECP processing time can be substantially reduced.
Keywords: photopheresis, mononuclear cells, collection efficiency, graft vs host disease, flow cytometry
INTRODUCTION
Extracorporeal photopheresis (ECP) has proved to be an effective treatment for a wide range of diseases. Initially used in the field of dermatology in the late 1980s for the treatment of cutaneous T-cell lymphoma, it is nowadays an established therapeutic option for graft-versus-host disease (GvHD), rejection after solid organ transplantation and a variety of autoimmune diseases1–3. In ECP, the patient’s own leukocytes are collected and treated with 8-methoxy-psoralen (8-MOP), inducing UV-A light sensitivity in collected cells. These cells are either irradiated directly in the apheresis device, which is called “inline ECP”, or using an external device, called “off line ECP”. The UV-A light induces apoptosis of collected and 8-MOP-treated leukocytes. After this treatment, the apoptotic cells are returned to the patient. Although dendritic cell initiation, modification of the cytokine profile and stimulation of regulatory T-cells can be observed, the mechanisms of action of this therapeutic approach are still not fully understood4–7.
There are ambivalent opinions regarding the number of leukocytes that need to be collected during ECP. While some experts define a rather low number as a cut-off value (13.9×106/kg body weight MNCs)8,9, there are also others recommending comparatively high values (>100×106/kg body weight MNCs)10. By collecting mononuclear cells (MNCs) with a conventional off line apheresis device, a significantly higher amount of cells can be obtained than with the inline system. Nevertheless, in a direct comparison of both ECP systems, a clear relationship between cell dose and clinical response could not be found11.
Furthermore, most ECP studies determine the ratio of the different leukocyte types by conventional blood cell counting11–16. Depending on the hematology analyzer used, it is not always possible to distinguish accurately between different cell types, leading to rather unclear results. Flow cytometry is state-of-the-art for analyzing the expression of cell surface molecules and determining various cell types in a heterogeneous cell population. Therefore, we used the pan-leucocyte and monocyte markers CD45 and CD14, respectively, in evaluating collection efficiency (CE) of the off line apheresis system.
We hypothesize that ECP optimization may help to reduce time-consuming treatment related burden for patients as well as citrate anticoagulation associated side effects. Addressing the CE of various ECP systems, as well as the correlation between patient’s peripheral blood counts and cell counts of the apheresis products, will help to optimize ECP procedures and may become essential parameters for cell-dose-response correlations in future prospective clinical ECP studies.
MATERIAL AND METHODS
Sample collection and study design
In this retrospective study, we analyzed 162 ECP treatments of 10 different patients. All participants signed informed consent. The study was approved by the Institutional Ethics Committee (Ethikkommission für das Bundesland Salzburg) (approval number: 1022/2022). For a better distribution of peripheral blood counts, we included patients with different diseases. Patients were diagnosed with chronic GvHD after allogeneic stem cell transplantation, bronchitis-obliterans-syndrome (BOS) due to lung transplantation and Crohn’s disease, respectively. Patient’s characteristics are summarized in Table I. Before ECP was conducted, a complete cell blood count (CBC) (white blood cells (WBCs), hemoglobin, hematocrit, platelets and percentages of neutrophils, lymphocytes and monocytes) had been done (Table II). In addition, following data related to ECP procedures were obtained: processed total blood volume (TBV), MNC collection runtime, Anticoagulant Citrate Dextrose Solution (ACD-A) used and infused, MCP volume, the ratio of processed blood volume to total blood volume and photoactivation time (Table II).
Table I.
Patient characteristics
| Patients (No.) | 10 |
|
| |
| Age in years (mean ± SEM) | 55±3.31 |
|
| |
| Sex M/F (n) | 2/8 |
|
| |
| Patients disease (No.) | |
| GvHD | 7 |
| BOS | 2 |
| Mb. Crohn | 1 |
|
| |
| Weight in kg (mean ± SEM) | 59.52±0.85 |
|
| |
| Total blood volume in mL (mean ± SEM) | 3,883.35±55.44 |
GvHD: graft versus host disease; BOS: bronchitis-obliterans-syndrome; SEM: standard error of the mean.
Table II.
Pre procedure peripheral blood cell count and procedure data
| Pre-procedure peripheral blood cell count (mean ± SEM) | |
| WBCs (×10 9 /L) | 5.06±0.18 |
| Hb (g/dL) | 11.62±0.12 |
| Hct (%) | 34.68±0.36 |
| Plts (×10 9 /L) | 271.85±4.88 |
| Neutrophils % | 66.20±1.17 |
| Lymphocytes % | 22.07±0.94 |
| Monocytes % | 9.80±0.36 |
| MNC % | 31.87±1.16 |
| ECP (mean ± SEM) | |
| Processed blood volume | 3,809.12±52.79 |
| MNC collection runtime (min) | 112.15±0.31 |
| ACD -A used (mL) | 346.67±4.86 |
| ACD -A infused (mL) | 338.68±4.74 |
| MCP volume (mL) | 99.35±0.58 |
| MCP concentrate Hct (%) | 0.81±0.02 |
| Processed blood volume/TBV | 1.00±0.00 |
| Photoactivation time (min) | 10.04±0.05 |
WBCs: white blood cells; Hb: hemoglobin; Hct: hematocrit; Plts: platelets; MNC: mononuclear cells; ACD-A: anticoagulant citrate dextrose solution; MCP: mononuclear cell products; SEM: standard error of the mean.
Offline ECP treatment
The ECP treatments analyzed in this study took place from 04/2019 to 12/2021 and were performed based on published guidelines1–3. Before each treatment, a medical evaluation of the patient’s health status was done to exclude contraindications for ECP treatment. Furthermore, blood pressure, pulse, temperature and the patient’s weight were measured. ACD-A was used as a coagulation agent in a ratio of 1:12 during ECP. Collection of cells was performed using the continuous MNC program (cMNC) of the Spectra Optia apheresis device (Terumo BCT, Lakewood, Colorado, USA) by processing one TBV. The hematocrit target value (<2%) was monitored visually throughout the procedure by following the color to the corresponding required layer of buffy coat on a color scale (colorgram). After collecting the MNCs, 8-MOP was injected and cells were photoactivated with an UVA illuminator (wavelength 365 nm, 2.0 J/cm2; Macogenic G2, Macopharma, Mouvaux, France). The photoactivated mononucleated cell product (MCP) was then reinfused to the patient.
Determination of cell blood counts and CE
The complete cell blood count including neutrophils, lymphocytes and monocytes of the venous blood as well as the WBC count of the MCP were determined using a Sysmex XN-9000 hematology analyzer (Sysmex Corporation, Kobe, Japan). CE was assessed using the CE 2 method (%) = (WBCs [or lymphocytes, or monocytes, or MNCs, or neutrophils] collected [×106/mL] × product volume [mL]) / (preapheresis WBCs [or lymphocytes, or monocytes, or MNCs, or neutrophils] [×106/mL] × [processed volume {mL} − volume ACD {mL}]) × 10011,13,15.
Flow cytometry
To calculate absolute lymphocyte and monocyte counts in the MCP, CD45 and CD14 expression was measured by flow cytometry. 100 μL of the apheresis product, which was diluted with phosphate buffered saline (PBS, phosphate buffered saline powder, pH 7.4 for preparing 1 L solutions, Sigma-Aldrich Co, #P3813) to obtain a WBC count of maximum 10,000/μL, was incubated with 10 μL CD45 FITC/CD14 PE (BD Simultest™ Leucogate™, BD Biosciences, San Jose, CA, USA, #342408) for 15 minutes at room temperature in the dark. Then, 1 mL of lysing solution (BD Pharm Lyse™, BD Biosciences, #555899, diluted with distilled water 1:10) was added. After a further incubation period of 5 minutes at room temperature in the dark, the sample was measured by the BD FACSLyric™ flow cytometer using BD FACSuite™ software (BD Biosciences) using the acquisition criteria of 300 seconds or 15,000 leukocytes. After removing cell debris, leukocytes were gated on a CD45 vs side scatter dot plot. Lymphocytes and monocytes were identified on a CD45 vs CD14 dot plot. Granulocytes (neutrophils) were either gated due to their properties in the CD45 vs CD14 or in the CD45 vs side scatter dot plot. Lymphocytes, monocytes and granulocytes were reported as percentages of leukocytes.
Statistical analysis
Data are presented as arithmetic mean and standard error of the mean (SEM). To analyze the data, at first correlations between WBCs, MNCs, neutrophils, lymphocytes and monocytes in the patient’s blood and MCP cell count were calculated using Pearson’s correlation coefficient. A correlation <0.2 was defined as very weak, between 0.2 and 0.4 as weak, between 0.4 and 0.6 as moderate, between 0.6 and 0.8 as strong, and >0.8 as very strong. In addition, linear regression models were considered to more accurately determine the linear relationship between peripheral and MCP cell counts of the cell types. The two-sided significance level α=0.05 was used for all hypothesis tests. All calculations were done with the statistical software R (version 4.1.3, The R Foundation, Vienna, Austria)17.
RESULTS
Study cohort and MNC collection
As summarized in Table I, the study cohort consisted of 8 female and 2 male patients with a mean age of 55±3.31 years. The mean body weight of patients was 59.52±0.85 kg with a mean total body volume of 3,883.35±55.44 mL. Seven patients were diagnosed with chronic GvHD after allogeneic stem cell transplantation. Two patients suffered from BOS due to lung transplantation and one patient from Crohn’s disease. As shown in Table II, the peripheral blood count of patients showed a mean WBC count of 5.06±0.18×109/L with a mean percentage of 22.07±0.94 lymphocytes and 9.80±0.36 monocytes. A mean volume of 3,809.12±52.79 mL was processed within 112.15±0.31 minutes using 346.67±4.86 mL of ACD-A. The final MCP volume was 99.35±0.58 mL. After addition of 8-MOP, the MCP was irradiated for 10.04±0.05 minutes. All procedures were carried out without any technical issues and none of the patients showed significant adverse events (Table II).
MCP characteristics and CE
For the present study, 162 ECP procedures were analyzed. Table III shows the number of leukocytes collected and the ratio of the different leukocytes determined by flow cytometry. Our data revealed MNCs as the largest cell population, with a lymphocyte value of 61.60±1.69% and a monocytes value of 19.69±0.79%. The smallest cell population were neutrophils with a value of 18.72±1.85%. The values obtained were also converted to value per kg body weight and resulted in 71.76×106/kg WBCs, 41.12×106/kg lymphocytes, 14.20×106/kg monocytes and 55.32×106/kg MNCs, respectively (Table III). Table IV reveals the CE of WBCs with a value of 23.85±0.63%. The majority of the cells collected were lymphocytes (CE of 69.27±2.01%). Monocytes had a CE of 47.65±1.63% and neutrophils of 6.46±0.74%.
Table III.
MCP concentrate characteristics (mean ± SEM) as determined by flow cytometry
| MCP volume, mL | 99.35±0.58 |
| WBCs (×10 9 /L) | 41.89±1.25 |
| WBCs (×10 6 /kg) | 71.76±2.35 |
| WBCs (×10 9 ) | 4.15±0.12 |
| MNCs (×10 9 ) | 3.25±0.10 |
| MNCs (×10 6 /kg) | 55.32±1.74 |
| MNCs (%) | 81.29±1.85 |
| Neutrophils (%) | 18.72±1.85 |
| Lymphocytes (×10 6 /kg) | 41.12±1.35 |
| Lymphocytes (%) | 61.60±1.69 |
| Monocytes (×10 6 /kg) | 14.20±0.80 |
| Monocytes (%) | 19.69±0.79 |
MCP: mononuclear cell products; WBCs: white blood cells; MNC: mononuclear cells; SEM: standard error of the mean.
Table IV.
Cell collection efficiency assessment (mean ± SEM)
| CE 2% | |
|---|---|
| WBC s | 23.85±0.63 |
| MNC s | 61.96±1.54 |
| Lymphocytes | 69.27±2.01 |
| Monocytes | 47.65±1.63 |
| Neutrophils | 6.46±0.74 |
WBCs: white blood cells; MNC: mononuclear cells; SEM: standard error of the mean.
Correlation of cell counts in MCP and patients’ peripheral blood
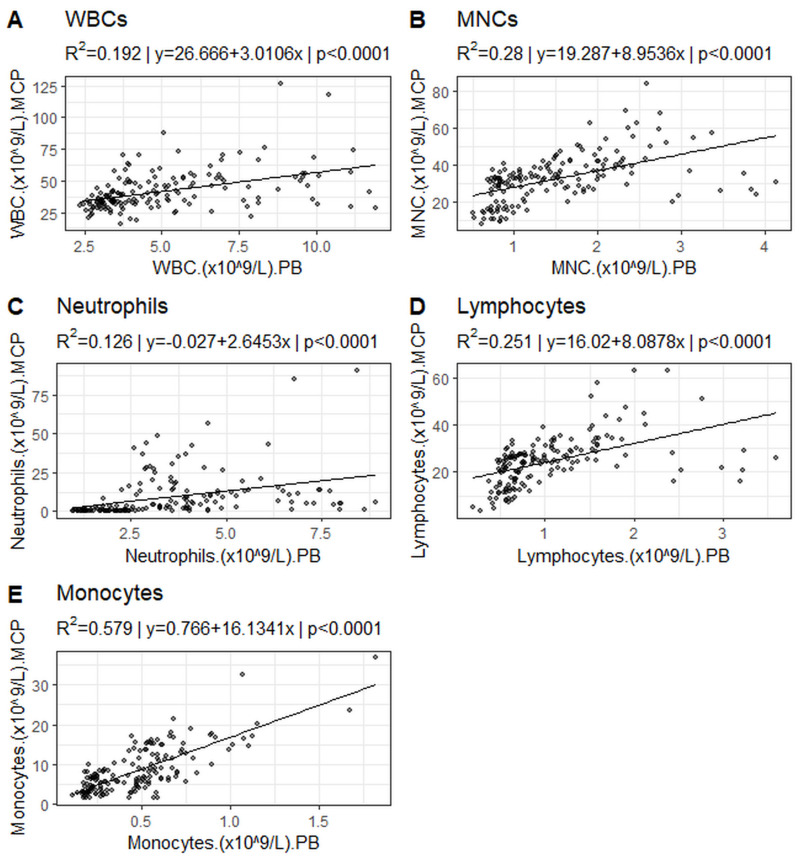
Pearson’s correlation analysis and linear regression models revealed a moderate correlation between WBCs, lymphocytes and MNCs in the MCP cell count and the peripheral blood count of patients (WBCs R2=0.19, p<0.0001; lymphocytes R2=0.25, p<0.0001; MNC R2=0.28, p<0.0001;) (Figure 1A, B, and D, Table V). Monocytes showed a strong correlation between MCP cell count and periphery (R2=0.58, p<0.0001) (Figure 1E, Table V). Concerning neutrophils the expected weak correlations were observed (neutrophils R2=0.13, p<0.0001) (Figure 1C, Table V).
Figure 1.
Linear regression between cell counts in peripheral blood and in MCP
A) white blood cells (WBCs), B) mononuclear cells (MNCs), C) neutrophils, D) lymphocytes and E) monocytes.
Table V.
Correlation and linear regression of peripheral and MCP cell count
| Cell type | Correlation (coefficient, r) | Correlation (strength) | Linear (Regression, R2) | Slope |
|---|---|---|---|---|
| WBC s | 0.44 | moderate | 0.19 | 3.01 |
| MNC s | 0.53 | moderate | 0.28 | 8.95 |
| Neutrophils | 0.35 | weak | 0.13 | 2.65 |
| Lymphocytes | 0.50 | moderate | 0.25 | 8.09 |
| Monocytes | 0.76 | strong | 0.58 | 16.13 |
WBCs: white blood cells; MNC: mononuclear cells.
DISCUSSION
Most centers applying the off line system for ECP treatment are using protocols with average treatment times of more than 140 minutes including collection, irradiation and reinfusion of cells11,12,16. The processing time of inline ECP has been reported to be rather short between 75 and 133 minutes11,13,15. Aim of the present retrospective study was to evaluate an off line ECP system by focusing on monocyte and lymphocyte enrichment in MCP with the hypothesis that the processing time can be reduced in view of high collection efficiency.
As expected, our MCP contained high amounts of lymphocytes and monocytes. CE of MNC was over 60%, confirming previously reported data for the inline system by Piccirillo et al. However, our results are considerably higher compared to 35% CE in the reported off line system11. These differences may be explained by the fact that Piccirillo et al. used the MNC program of the Spectra Optia with a dual-stage separation. In this report, we evaluated the cMNC program with continuous collection of cells and a buffy coat collection rate of 1 mL/min and could confirm previous results showing a higher CE for the cMNC protocol18. Del Fante et al. obtained a similar MNC collection efficiency of 58.7% for the cMNC protocol and 42.1% for the MNC protocol, respectively.
In the present study, the collection of lymphocytes and MNCs per kg body weight (BW) resulted in values considerably higher than MNC counts described for the inline ECP system11. In the study by Piccirillo et al., the inline system resulted in 25×106 MNCs per kg BW, while the offline system showed a yield of 48×106 MNCs per kg BW. Worel et al. defined a lymphocyte threshold of 8.4×106/kg and a MNC threshold of 13.9×106/kg treated per single procedure associated with clinical response to ECP after 1 month8. These thresholds are more than 70% lower than collection yields resulted from the off line system with the Spectra Optia device in our study. Therefore, we conclude that the TBV in the off line setting can be substantially reduced in order to shorten the entire ECP processing time.
ECP guidelines recommend two consecutive treatments as one treatment cycle1–3. However, a treatment schedule of lengthy off line ECP procedures on two consecutive days with processing two TBV in each therapy session should be put into question. Performing a one-day off line ECP schedule processing one TBV might have a similar clinical response, as indicated in the retrospective study of Cid et al., which showed that this new ECP schedule is efficacious and safe for GvHD patients19. An additional reduction of the processing time could further increase the comfort for patients in need of ECP while maintaining the intended therapeutic effects. Further prospective clinical studies are needed to corroborate these speculations.
In the present study, the MNC fraction within collected cells was high and a moderate to high correlation between peripheral blood cell counts of patients and cell doses in the MCP was observed. This finding is comparable to data of MNCs collected with the Optia device in other studies11–15. The relative amount of MNC in the off line as well as in the inline MCP of recently published data show a high range of variation. This might be attributed to patient variability but might also be a result of inaccurate quantification of cell types by means of conventional hematology analyzers. In our study, the Sysmex XN-9000 device detected variable results for the differentiation of WBCs, therefore additional flow cytometry analyses were applied.
Our retrospective study has some limitations: Even though 162 ECP procedures were included, the number of patients (No.=10) is rather small. However, we included patients with different diseases to have a better distribution of peripheral blood counts. Furthermore, this retrospective study does not directly compare MCP data from an off line with an inline system. In addition, our study does not include data regarding the clinical response of patients to ECP treatment due to the retrospective study design. So far, we compared our results with published data regarding the application of inline collection procedures of other apheresis centers11,13,15, but a direct prospective and randomized comparison of both procedures is currently planned.
CONCLUSIONS
This is one of the few reports of ECP collection data focusing on the main leukocyte types in the MNC fraction by flow cytometry and therefore these data show a more precise composition of MCP in off line ECP compared to conventional blood cell counting. In addition, we corroborate published data that revealed a more efficient collection of MNCs in the off line setting with high yields of lymphocytes and monocytes when compared to the inline ECP system. These differences are due to the fact that the off line system uses a different protocol with a higher processed blood volume than the inline system (1 TBV vs 1,500 mL). Comparing our results with published data of the inline system, we conclude that the collection of MNCs can be substantially shortened and that there is no need to process two TBV per single treatment on two consecutive days as recommended by official guidelines1–3. This change of schedule would lead to a reduced treatment related burden for patients, not only because of a reduced processing time but also due to a reduction of ACD volume during off line ECP. Further randomized prospective studies focusing on the clinical response to the different ECP systems are planned to define the minimum cell dose needed.
ACKNOWLEDGEMENTS
The Authors thank all employees of the Institute of Transfusion Medicine of the University Hospital of Salzburg, particularly, Agne Binderyte, BScN, MarthaRoth, Christine Rönnestedt, Gabriele Spitzeneder, Rüdiger Steiner and Katharina Siller for excellent technical assistance.
Footnotes
ETHICAL CONSIDERATION: This study was approved by the Institutional Ethics Committee (Ethikkommission für das Bundesland Salzburg) (approval number/protocol number: 1022/2022). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
AUTHORS’ CONTRIBUTIONS: Conceptualization: OK and CG; methodology: CG, CM and GZ; validation: CG, WL, GZ; formal analysis: OK, CG, CM; investigation: OK, NL, FF, LO and CG; data curation: OK, CG, NL, OK; writing, original draft preparation: OK, WL and CG; writing, review and editing: CG, ER and SL-P; visualization: OK, CG and WL; project administration: CG. All Authors have read and agreed to the published version of the manuscript.
The Authors declare no conflicts of interest.
FUNDING: GZ gratefully acknowledges the support of the WISS 2025 project "IDA-Lab Salzburg" (20204-WISS/225/197-2019 and 20102-F1901166-KZP).
REFERENCES
- 1.Knobler R, Arenberger P, Arun A, Assaf C, Bagot M, Berlin G, et al. European dermatology forum - updated guidelines on the use of extracorporeal photopheresis 2020 - part 1. J Eur Acad Dermatol Venereol. 2020;34:693–2716. doi: 10.1111/jdv.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knobler R, Arenberger P, Arun A, Assaf C, Bagot M, Berlin G, et al. European dermatology forum: Updated guidelines on the use of extracorporeal photopheresis 2020 - Part 2. J Eur Acad Dermatol Venereol. 2021;35:27–49. doi: 10.1111/jdv.16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth Special Issue. J Clin Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 4.Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 5.Cho A, Jantschitsch C, Knobler R. Extracorporeal photopheresis-an overview. Front Med (Lausanne) 2018;5:236. doi: 10.3389/fmed.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda A, Schwarz A, Kernebeck K, Gross N, Aragane Y, Peritt D, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 8.Worel N, Lehner E, Fuhrer H, Kalhs P, Rabitsch W, Mitterbauer M, et al. Extracorporeal photopheresis as second-line therapy for patients with acute graft-versus-host disease: does the number of cells treated matter? Transfusion. 2018;58:1045–1053. doi: 10.1111/trf.14506. [DOI] [PubMed] [Google Scholar]
- 9.Hackstein H, Misterek J, Nockher A, Reiter A, Bein G, Woessmann W. Mini buffy coat photopheresis for children and critically ill patients with extracorporeal photopheresis contraindications. Transfusion. 2009;49:2366–2373. doi: 10.1111/j.1537-2995.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 10.Perseghin P, Galimberti S, Balduzzi A, Bonanomi S, Baldini V, Rovelli A, et al. Extracorporeal photochemotherapy for the treatment of chronic graft-versus-host disease: trend for a possible cell dose-related effect? Ther Apher Dial. 2007;11:85–93. doi: 10.1111/j.1744-9987.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo N, Putzulu R, Massini G, Di Giovanni A, Giammarco S, Metafuni E, et al. Inline and offline extracorporeal photopheresis: device performance, cell yields and clinical response. J Clin Apher. 2021;36:118–126. doi: 10.1002/jca.21851. [DOI] [PubMed] [Google Scholar]
- 12.Pascual C, Gonzalez-Arias E, Perez-Corral AM, Bailen R, Gayoso J, Besson N, et al. Mononuclear cell collection for extracorporeal photopheresis by using the „off-line“ system: a comparative study between COBE Spectra and Spectra Optia devices. J Clin Apher. 2019;34:359–366. doi: 10.1002/jca.21679. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo N, Putzulu R, Massini G, Di Giovanni A, Chiusolo P, Sica S, et al. Inline extracorporeal photopheresis: evaluation of cell collection efficiency. Transfusion. 2019;59:3714–3720. doi: 10.1111/trf.15570. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo N, Putzulu R, Massini G, Fiore AG, Chiusolo P, Sica S, et al. Mononuclear cell collection for extracorporeal photopheresis: Concentrate characteristics for off-line UV-A irradiation procedure. J Clin Apher. 2018;33:217–221. doi: 10.1002/jca.21574. [DOI] [PubMed] [Google Scholar]
- 15.Mayer W, Kontekakis A, Maas C, Kuchenbecker U, Behlke S, Schennach H. Comparison of procedure times and collection efficiencies using integrated and multistep nonintegrated procedures for extracorporeal photopheresis. J Clin Apher. 2022;37:332–339. doi: 10.1002/jca.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bueno JL, Alonso R, Gonzalez-Santillana C, Naya D, Romera I, Alarcon A, et al. A paired trial comparing mononuclear cell collection in two machines for further inactivation through an inline or offline extracorporeal photopheresis procedure. Transfusion. 2019;59:340–346. doi: 10.1111/trf.14975. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Accessed on 03.03.2023]. Availavle at: https://www.R-project.org/ [Google Scholar]
- 18.Del Fante C, Scudeller L, Viarengo G, Cervio M, Perotti C. Mononuclear cell collection for extracorporeal photochemotherapy: a study comparing an automatic and a semiautomatic apheresis device. Transfusion. 2013;53:2027–2033. doi: 10.1111/trf.12065. [DOI] [PubMed] [Google Scholar]
- 19.Cid J, Carbasse G, Suarez-Lledo M, Moreno DF, Martinez C, Gutierrez-Garcia G, et al. Efficacy and safety of one-day offline extracorporeal photopheresis schedule processing one total blood volume for treating patients with graft-versus-host disease. Transfusion. 2019;59:2636–2642. doi: 10.1111/trf.15384. [DOI] [PubMed] [Google Scholar]