Abstract
Background
In the setting of mismatched-hematopoietic stem cells transplantation, the detection of antibodies directed against donor-specific HLA allele(s) or antigen(s) (DSA) represents a barrier for engraftment. It is thus necessary to plan an immunosuppressive strategy, or to select an alternative donor. This prospective study aimed at evaluating the efficacy of our strategy for testing DSAs and the efficacy of the desensitization strategy (DS) employed between November 2017 and November 2020.
Materials and methods
The anti-HLA antibody search was performed using the Luminex bead assays (Lifecode ID and LSA I/II-Immucor) and expressed as mean fluorescence intensity (MFI >1,000 positive). If the patient had DSAs and no alternative donors, a DS was employed with rituximab (day −15), 2 single volume plasmaphereses (PP; days −9 and −8), intravenous immunoglobulins (day −7) and infusion of HLA selected platelets, if persistent DSAs were directed against class I HLA. DS was scheduled with or without PP, according to the DSA MFI (>1,000 or <5,000) and FCXM (flow cytometry crossmatch).
Results
Twenty-two out of 126 patients (17.46%) showed anti-HLA antibodies, 5 of them DSAs (3.97% of total); 3 patients underwent DS obtaining engraftment. Female gender (p=0.033) and a history of previous pregnancies or miscarriages (p=0.009) showed a statistically significant impact on alloimmunization. Factors associated with a delayed neutrophil engraftment were patient’s female gender (p=0.039), stem cell source (p=0.025), and a high HSCT-specific comorbidity index (p=0.028). None of the analyzed variables, including the DSA detection, influenced engraftment.
Conclusions
Our study confirms the importance to test DSAs in mismatched-hematopoietic stem cells transplantation The DS used proved successful in removing DSAs. Prospective multicenter studies are needed to better define and validate consensus strategies on DSA management in HSCT.
Keywords: hematopoietic stem cell transplantation, anti-HLA antibodies, donor selection, engraftment, desensitization strategy
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) from a HLA-mismatched (MM) donor represents a treatment option for patients with hematologic malignancies who lack a human leukocyte antigen (HLA) matched donor. The alloreactivity related to the HLA mismatch between donor and recipient leads to the risk of both host-versus-graft (HVG), causing graft failure (GF), and graft-versus-host disease (GvHD)1. GF ranges between 4% and 20%2, with a high risk of poor outcomes. It may be due to hematopoietic stem cell rejection caused by chemo-resistant recipient T lymphocytes, NK cells against mismatched donor cells (cellular rejection) or antibody-mediated rejection (humoral rejection), involving either antibody-dependent cell-mediated cytotoxicity or complement-mediated cytotoxicity2–5. The role of anti-HLA antibodies directed against donor-specific HLA allele(s) or antigen(s) (DSA) has been well analyzed in acute and chronic graft rejection (GR) of solid organ transplantations and in transfusion medicine6–9. Recently, their role has been better recognized in the setting of HSCT6. The presence of DSAs represents a barrier for stem cell engraftment, leading to primary GF or delayed engraftment. Over the years, several methods have been developed to obtain a precise detection and characterization of anti-HLA antibodies in HSCT recipients2. In January 2018, the European Society for Blood and Marrow Transplantation (EBMT) published the Consensus Guidelines for the detection and treatment of DSAs in haploidentical hematopoietic cell transplantation10. The panel of experts suggest desensitizing patients with DSAs before HSCT, if another suitable donor is not available. However, a defined desensitizing schedule is not proposed. In fact, even if different strategies have been reported, most of them are anecdotal or referred to small series of patients, with different approaches employed10. To date, there is no standard desensitization strategy recommended. We hereby report a prospective evaluation of anti-HLA antibodies testing, monitoring and desensitization strategies employed in patients who were candidates to an allogeneic mismatched HSCT at the Hematology Center and Transfusion Medicine Unit of the “Sapienza” University between November 2017 and November 2020.
MATERIALS AND METHODS
From November 2017 to November 2020, all consecutive patients candidates at our Center in Rome for an allogeneic HSCT from mismatched related donors, mismatched unrelated donors, umbilical cord blood (UCB) and lacking an HLA identical donor, or patients for whom an HLA identical donor was still not identified, were included. All patients or their parents or legal guardians provided consent for anti-HLA antibodies testing, desensitization protocol, if necessary, and data collection. Anti-HLA antibodies analysis was performed at the time of the confirmatory test, in case of haploidentical donors, or when starting the search of an alternative donor to guide the selection. The tests were repeated at least 30 days prior to the HSCT date, as reported in our previous experience6. According to our Institutional Policy, the donor selection algorithm was first based on the HLA match, followed by donor age, patient and donor body weight ratio, gender, CMV serological status and blood group. Starting from 2020, in case of haploidentical donor selection, the EBMT consensus recommendations for donor selection in haploidentical HSCT11 were followed. In case of DSA identification, if the patient had more than one available donor, a donor without the corresponding HLA antigen was selected; if there was only one available donor, a desensitization protocol was employed.
Anti-HLA antibodies and DSAs testing
The search for anti-HLA antibodies was carried out using the multianalyte bead assay, with the Luminex platform (Luminex, Austin, TX, USA), including Lifecode ID and LSA I/II (Immucor Inc., Norcross, GA, USA). The results were expressed as mean fluorescence intensity (MFI); as in the preliminary experience, a MFI >1,000 was considered as positive6. Antibody search was repeated every 7 days after HSCT for the first month, and then 60, 180 and 365 days after HSCT for all patients. In case of a mismatched donor and DSAs detection, a flow cytometric crossmatch (FCXM) test was performed.
Conditioning regimen
The conditioning regimen consisted of thiotepa, fludarabine and busulfan. The latter was infused over 3 days (9.6 mg/kg) in the myeloablative conditioning regimens (MAC) and over 2 days (6.4 mg/kg) in reduced intensity conditioning (RIC) regimens. In the case of young patients (≤30 years old), with acute lymphoblastic leukemia, total body irradiation (TBI, total dose 12 Gy) and fludarabine were employed12–14. In patients with aplastic anemia the conditioning regimen was cyclophosphamide and fludarabine with the addition or not of TBI 2 Gy on day −115.
GvHD prophylaxis
All patients received the combination of cyclosporine (CyA), short course methotrexate and anti-thymocyte globulin (ATG, Thymoglobulin, 6 or 7.5 mg/kg) in cases of an unrelated donor transplant. In patients receiving an haploidentical HSCT, mycophenolate mofetil (MMF), CyA and post-transplant cyclophosphamide (PT-Cy, 50 mg/kg on days +3 and +4) were employed. Acute (a) and chronic (c) GvHD were defined according to consensus criteria16,17.
Graft manipulation
Graft manipulation (plasma removal, erythrocyte sedimentation or both) was performed according to the ABO match between donor and recipient. Female and previously transfused male donor grafts were plasma depleted in order to avoid transfusion-related acute lung injury (TRALI)18,19. As of March 2020, according to EBMT, Italian group for blood and marrow transplantation (GITMO), and Italian Bone marrow Donor registry (IBMDR) recommendations, in case of a high risk of donor infection by community-acquired COVID-19, cryopreserved peripheral blood stem cell (PBSC) were employed20.
Definitions
Immunological events have been defined as the occurrence of previous pregnancies, miscarriages, or blood transfusions. Neutrophil recovery was defined as the achievement of an absolute neutrophil count ≥0.5×109/L for 3 consecutive days; platelet recovery was the achievement of ≥20×109/L, with no platelet transfusion requirement in the previous 7 days21. Graft failure (GF) was defined as “primary” in case of an absent initial donor cell engraftment, a peripheral neutrophil absolute count <0.5×109/L by day +28 after PBSC or bone marrow (BM) HSCT without relapse, and by day +42 after a CBU transplant. GF was defined as “secondary” if the loss of donor cells, after an initial engraftment, and a recurrent absolute neutrophil count <0.5×109/L were observed21,22. Poor graft function (PGF) was defined as severe cytopenia involving at least two cell lines and/or a transfusion requirement, in the presence of hypoplastic or aplastic bone marrow, with a full donor chimerism, in the absence of active severe GvHD or relapse21. Overall survival (OS) was defined as the time from treatment to death. Transplant related mortality (TRM) was defined as death due to any transplantation-related cause other than disease relapse.
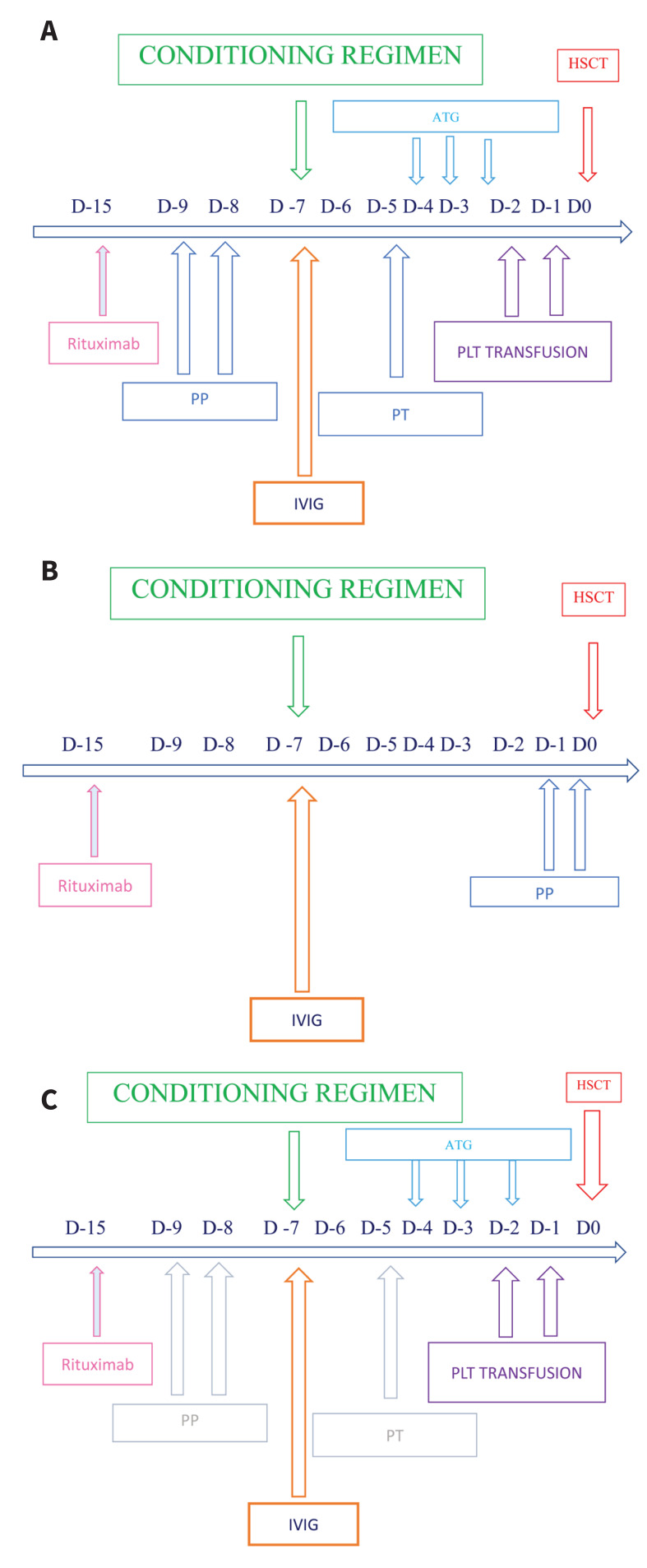
Desensitization strategy
The full desensitization strategy (Figure 1A) was scheduled with a combination of:
Figure 1.
Desensitization strategy
ATG: anti-thymocyte globulin; HSCT: hematopoietic stem cell transplantation; IVIG: intravenous immunoglobulin; PLT: platelet PP: plasmapheresis; PT: plasma-treatment.
anti-CD20 monoclonal antibody rituximab (375 mg/m2) on day −15 to inhibit antibody production by CD20+ B lymphocytes6;
two single volume plasmapheresis (PP) procedures, to remove preformed anti-HLA antibodies6, on days −9 and −8 if ATG was employed; when ATG was not required, PP were performed on days −1 and 0 (Figure 1B);
intravenous immunoglobulins (IVIG) on day −7 (800 mg/kg), to limit antibody rebound18, followed by the infusion of HLA selected platelets for DSA absorption, if persistence of antibodies directed against class I HLA antigens.
In the case of persistent DSA levels before the administration of ATG (day −4), additional single volume PP procedures were employed with immuneadsorption techniques (plasma-treatment [PT]), using columns to selectively remove IgG antibodies, avoiding the removal of chemotherapy (Figure 1A). According to the EBMT Consensus Guidelines for the detection and treatment of DSAs10, considering the strong association between high DSA levels (MFI >5,000) and complement activation, and our preliminary experience6 we decided to differentiate the desensitization strategy employed in DSA positive patients with MFI >5,000 and/or a positive crossmatch, and those with an MFI between 1,000 and 5,000, and a negative crossmatch. In the latter cases, we employed the following schedule (Figure 1C):
Statistical analysis
Subjects’ characteristics for the whole population and stratified by anti-HLA antibodies were summarized by medians of the variables (with%) for categorical variables or by means of quantiles and mean with standard deviation (SD) for continuous variables. In univariate analysis, non-parametric tests were performed for comparisons between groups (Chi-Squared and Fisher Exact test in case of categorical variables or response rate, Mann-Whitney and Kruskal-Wallis test in case of continuous variables). Logistic regression models were used in univariate and multivariate analyses to assess whether the clinical and biological parameters were associated with response outcomes. Odds ratios (OR) and 95% confidence intervals (CI) were reported as parameter results of the logistic regression models. Survival distributions (e.g. TRM, OS) were estimated using the Kaplan-Meier Product Limit estimator. Subgroup comparisons with clinical and biological parameters were performed for descriptive purposes. Differences in terms of time to response, TRM and OS were evaluated by means of the Log-Rank test or Cox regression model, in univariate and multivariate analyses, after assessment of proportionality of hazards. Hazard ratios (HR) and 95% CI were reported as parameter results of the Cox regression models. All tests were 2-sided, accepting p<0.05 as statistically significant, and confidence intervals were calculated at 95% level. All analyses were performed using the R software.
RESULTS
From November 2017 to November 2020, 126 hematologic patients (58 females/68 males), median age 48 years (range 5–69), were prospectively enrolled in the study. Twenty-two showed anti-HLA antibodies (17.46%): 9 for class I HLA antigens, 5 for class II, and 8 for both class I and II. Seventy-nine patients (76%) had received blood transfusions, with a median of 16 units (range: 0–200) of RBCs, 13 (range: 0–61) platelet concentrates and 2 (range: 0–54) plasma units. Among female patients, 70% had had previous pregnancies or miscarriages. Female gender (p=0.033) and a history of previous pregnancies or miscarriages (p=0.009) showed a statistically significant impact on alloimmunization (Table I). In our experience, most patients had a complex immunization, directed against a median of 13 antibodies each (range 1–40). Looking at the epitope’s correspondence, we observed a median of 4 epitopes involved for each patient (range 1–11). Five patients presented with DSAs (3.97% of the total), directed against class I and II HLA antigens in 3 and 1 case, respectively. One patient had antibodies directed against both class I and class II HLA antigens. Four of the 5 patients with DSAs underwent a HSCT. One patient died before receiving the transplant, due to disease progression.
Table I.
General characteristichs (patients undergoing antibodies’ screening)
| Characteristics | Overall No.=126 | Anti-HLA antibodies | p-value1 | |
|---|---|---|---|---|
|
| ||||
| No No.=104 |
Yes No.=22 |
|||
|
| ||||
| Age, median (range) | 48 (5, 69) | 48 (8, 69) | 55 (5, 61) | 0.56 |
|
| ||||
| Gender, No. (%) | ||||
| Female | 58 (46%) | 43 (41%) | 15 (68%) | 0.033 |
| Male | 68 (54%) | 61 (59%) | 7 (32%) | |
|
| ||||
| Pregnancies, No. (%) | 33 (70%)* | 21 (60%) | 12 (100%) | 0.009 |
|
| ||||
| Pre-HSC T blood transfusions, No. (%) | 79 (76%)** | 62 (74%) | 17 (85%) | 0.39 |
|
| ||||
| RBC transfusions, median (range) | 16 (0, 200) | 16 (0, 200) | 15 (0–71) | 0.69 |
|
| ||||
| PLT transfusions, median (range) | 13 (0, 61) | 14 (0, 61) | 10 (1–45) | 0.87 |
|
| ||||
| Plasma transfusions, median (range) | 2 (0, 54) | 2 (0, 54) | 5 (0–31) | 0.72 |
1Wilcoxon rank sum test; Fisher’s exact test. All percentages are evaluated on available data.
Data available on 47 patients;
Data available on 104 patients.
HSCT characteristics
Eighty-five out of the 126 patients underwent a HSCT (Online Supplementary Content, Figure S1). The donor was unrelated in 55 patients (65%) (10/10 HLA matched in 34, 9/10 in 21), and a haploidentical donor in 26 (31%). Four patients (4.7%), who underwent a transplant from an identical HLA related donor, were also included in the observation, since the anti-HLA antibody research was performed together with the family HLA typing. Thirty-two donors (38%) were female, fifty-three were males (62%), with a median age of 33 years (range 19–67) (Table II).
Table II.
General characteristics of patients undergoing HSCT
| Characteristics | Overall, No.= 85 |
|---|---|
|
| |
| Age, median (range) | 48 (5, 69) |
|
| |
| Gender, No. (%) | |
| Female | 36 (42%) |
| Male | 49 (58%) |
|
| |
| Diagnosis, No. (%) | |
| Lymphoproliferative disorder | 25 (29%) |
| Myeloproliferative disorder | 59 (69%) |
| Aplastic anemia | 1 (1.2%) |
|
| |
| Donor, No. (%) | |
| Haplo | 26 (31%) |
| MUD- MMUD | 55 (65%) |
| Sibling | 4 (4.7%) |
|
| |
| HLA match, No. (%) | |
| MUD 10/10 | 34 (40%) |
| MMUD 9/10 | 21 (24.7%) |
| Haplo | 26 (30.6%) |
| Sibling | 4 (4.7%) |
|
| |
| ABO, No. (%) | |
| Matched, No. (%) | 42 (49%) |
| Major MM, No. (%) | 15 (18%) |
| Minor MM, No. (%) | 23 (27%) |
| Bidirectional MM, No. (%) | 5 (5.9%) |
|
| |
| HSC source, No. (%) | |
| BM | 21 (25%) |
| PBSC | 59 (69%) |
| PBSC cryopreserved | 5 (5.9%) |
|
| |
| Sorror, No. (%) | |
| 0–2 | 67.2% |
| 3–4 | 26.4% |
| 5–6 | 6% |
|
| |
| Conditioning regimen, No. (%) | |
| MAC | 56 (66%) |
| RIC | 29 (34%) |
|
| |
| Graft manipulation, No. (%) | |
| No manipulation | 38 (45%) |
| Plasma-depletion | 40 (47%) |
| Plasma-depletion + HES | 2 (2.4%) |
| Plasma-depletion + cryopreservation | 2 (2.4%) |
| Cryopreservation | 3 (3.5%) |
|
| |
| Donor gender, No. (%) | |
| Female | 32 (38%) |
| Male | 53 (62%) |
|
| |
| Donor age, median (range) | 33 (19–67) |
|
| |
| GvHD prophylaxis, No. (%) | |
| ATG+CyA+MTX | 57 (67%) |
| CyA+MMF+PTCy | 26 (31%) |
| CyA+MTX | 2 (2.4%) |
Anti-HLA antibodies, DSA in patients who underwent a HSCT and desensitization strategy
Nine of the 85 patients who underwent a HSCT showed anti-HLA antibodies (10.59%): 5 for class I HLA antigens, 1 for class II, and 3 for both class I and II. Before starting the conditioning regimen 4 of the 9 patients with anti-HLA antibodies showed DSA (4.70% of the total) directed against class I HLA (Cw6-MFI 1,667; A2-MFI 1,109; A2-MFI 2,263) and class II HLA antigens (DR7-MFI 1,200) in 3 and 1 patient, respectively (Online Supplementary Content, Table SI). In 1 of the 4 patients with DSAs (A2-MFI 1,109), both the antibody detection and the FCXM, repeated before starting the conditioning regimen, were negative and a desensitization strategy was thus not necessary. Donor neutrophil and platelet engraftments were reached on days +18 and +27, respectively. Seven months after the HSCT, the patient is in good clinical conditions, with a full donor chimerism. The three remaining patients underwent the desensitization strategy. Since all of them showed an MFI >1,000 and <5,000 (cut-off values, according to our internal protocol), and a negative FCXM, we employed the second desensitization strategy option. DSA levels, monitored during and after the desensitization treatment (every 7 days after HSCT for the first month, and then 60, 180 and 365 days) in all treated patients, showed a decreasing profile before stem cell infusion, without antibody rebound. All patients obtained a donor neutrophil engraftment within 20 days after the transplant and a platelet engraftment was reached at a median of 27 days (range 25–64) (Online Supplementary Content, Table SI and SII).
Donor engraftment
Neutrophil engraftment occurred at a median of 20 days (range 10–37) after HSCT. Factors associated with a delayed neutrophil engraftment were patient’s female gender (p=0.039), bone marrow as stem cell source (p=0.025), and a high HSCT specific comorbidity index (p=0.028)23. No variable was statistically correlated with platelet engraftment, observed at a median of 24 days (range 11–178) after HSCT. A full donor chimerism was observed in 69 patients (81.18%) within 20 days after HSCT; the employment of a MAC conditioning regimen showed a statistically significant association with the probability of obtaining a full chimerism at 20 days after HSCT (p=0.046%). Three patients died within 20 days after HSCT, without showing trilinear engraftment. Only 2 patients (2.35%), who did not present anti-HLA antibodies, experienced a GF. Eleven patients (12.94%) had a PGF. None of the variables analyzed, including the detection of anti-HLA antibodies or DSAs, influenced the risk of developing a GF or a PGF (Table III).
Table III.
Statistical correlations
| Characteristics | p-value1 |
|---|---|
|
| |
| Neutrophil engraftment | |
| Gender | 0.039 |
| HSC source | 0.025 |
| Sorror | 0.028 |
|
| |
| PLT engraftment | No statistical correlation among the evaluated variables |
|
| |
| Day +20 chimerism | |
| MAC conditioning regimen | 0.046 |
|
| |
| Poor graft function No statistical correlation among the evaluated variables | |
1Wilcoxon rank sum test; Fisher’s exact test.
All variables analyzed: age, median (range); aGvHD, No. (%); anti-HLA Ab, No. (%); bacterial infections, No. (%); CD3+, median (range); CD34+, median (range); conditioning regimen, No. (%); diagnosis, No. (%), donor, No. (%); donor age, median (range); donor gender, No. (%); DSA, No. (%); fungal infections, No. (%); graft manipulation, No. (%); GvHD prophylaxis, No. (%); HLA match, No. (%); ABO match, No. (%); HSC source, No. (%); pregnancies, No. (%); pre-HSCT blood transfusions, No. (%); Sorror, No. (%); viral infections, No. (%).
HSCT and overall outcomes
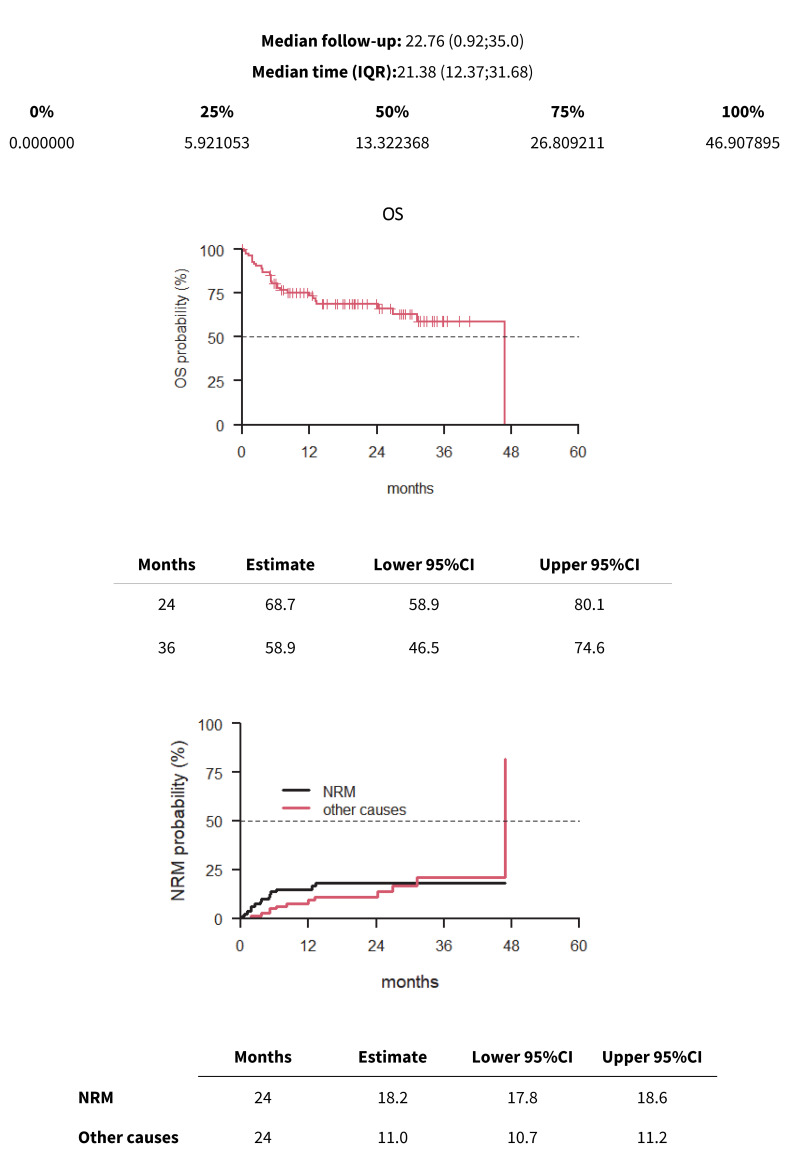
The median follow-up was 22.76 months (range 0.92–35.0). The 2-year and 3-year OS were 68.7% and 58.9%, respectively (Figure 2). Patients’ age (p=0.027), a PBSC stem cell source (p=0.009), the Sorror HCT-specific comorbidity index23 (p=0.003), and the GvHD prophylaxis platform (p=0.018) showed a statistical correlation with OS. Multivariate analysis confirmed that the HSCT-specific comorbidity index (p=0.022) and the stem cell source (p=0.027) had the most significant impact on OS (Table IV). The 2-year NRM was 18.2% (Figure 2). The TRM rate at 100 days was 8.2%. In our experience, the overall OS and TRM did not differ between patients with or without anti-HLA antibodies and DSAs.
Figure 2.
Median follow-up, OS and TRM
OS: overall survival; TRM: transplant related mortality.
Table IV.
Overall survival
| OS univariate | |||
|---|---|---|---|
| Characteristics | HR1 | 95% CI 1 | p-value |
| Age | 1.02 | 1.00, 1.04 | 0.027 |
| PBSC | 6.80 | 1.60, 28.9 | 0.009 |
| Sorror as continuous | 1.44 | 1.13, 1.83 | 0.003 |
| CyA+MTX | 6.01 | 1.36, 26.5 | 0.018 |
| OS multivariate | |||
| Characteristics | HR 1 | 95% CI 1 | p-value |
| Sorror_n | 1.33 | 1.04, 1.70 | 0.022 |
| HSC source (PBSC) | 5.17 | 1.20, 22.3 | 0.027 |
1HR: hazard ratio; CI: confidence interval.
All variables analyzed: age, median (range); aGvHD, No. (%); anti-HLA Ab, No. (%); bacterial infections, No. (%); CD3+, median (range); CD34+, median (range); conditioning regimen, No. (%); diagnosis, No. (%), donor, No. (%); donor age, median (range); donor gender, No. (%); DSA, No. (%); fungal infections, No. (%); graft manipulation, No. (%); GvHD prophylaxis, No. (%); HLA match, No. (%); ABO match, No. (%); HSC source, No. (%); pregnancies, No. (%); pre-HSCT blood transfusions, No. (%); Sorror, No. (%); viral infections, No. (%).
DISCUSSION
Considering the increasing use of partially HLA-matched donors, DSAs represent a new hurdle in HSCT, being a risk factor for GF, PGF, and GR10,24–30. Many aspects should be investigated to define risk factors for developing anti-HLA antibodies. The main causes are pregnancies, blood transfusions or previous allogeneic transplantation2, but the impact of each one is not well clarified. The effect of pregnancies and transfusion on alloimmunization have been analyzed in studies on the prevalence of anti-HLA antibodies in blood donors31–35. A study on 7920 volunteer donors showed a similar prevalence of anti-HLA antibodies in non-transfused (1%) and transfused male donors (1.7%)36. This low prevalence of HLA immunization in transfused males is probably related to the long interval (median 22 years) between a previous transfusion and blood donation36–38. Anti-HLA antibodies were detected in 17.3% of all female donors and in 24.4% of those with a history of pregnancies, increasing progressively with the number of pregnancies, up to 32.2% if more than 4 pregnancies were reported in the personal history. Our study confirms that the female gender (p=0.033) and a history of previous pregnancies or miscarriages (p=0.009) had a statistically significant impact on alloimmunization. In a large retrospective analysis on 617 patients with hematologic diseases, Seftel et al.37 reported an alloimmunization in 19% and 7% of patients receiving post-storage or pre-and post-storage leukoreduced blood products, respectively. Thanks to the introduction of online universal leukoreduction in 2008, we observed no statistically significant differences in alloimmunization between transfused and non-transfused patients (p=0.39). However, it is possible to detect “natural antibodies” also in non-transfused males, due to cross reactivity with bacterial antigens, foods, and inhaled allergens31–33. Moreover, HLA antibodies appear to be dynamic, with reactivation of DSA without re-exposure39. In our analysis, the number of patients without immunizing events is too limited to analyze this aspect. Further studies are required to understand better the factors capable of inf luencing HLA immunogenicity and antigenicity. Besides, it is still unknown whether antibodies directed against class I or class II HLA antigens are more implicated in the pathogenesis of engraftment failure. Many aspects are involved, including their expression and density on the different cellular surfaces24,39. Another unmet need is to establish the prohibitive levels of DSA. MFI levels generally considered positive are those >500–1,0002, even if some groups have recently reported that the presence of low DSA levels in the absence of desensitization did not correlate with the risk of developing a GF or a PGF40. Regarding necessary monitoring activities, considering the persistent negative results without antibody rebound obtained after desensitization in all treated cases in both of our experiences, we consider as sufficient to repeat DSA detection within the first two months without retesting at 180 and 365 days after HSCT. Antibody detection should be considered in case of disease relapse. The expansion of anti-HLA antibody testing methods has allowed to obtain a higher sensitivity. Nowadays, solid-phase assays are the most frequently employed methods, providing semi-quantitative results. The main limitations are related to the lot-to-lot variability in the amounts of coated proteins and the inter-assay variabilities. These aspects limit the possibility of establishing stringent cut-off levels, thus highlighting the role of local laboratories’ experience and standardization39. Overall, solid phase assays require an advanced understanding of molecular polymorphism and serologic patterns of cross-reactivity39. The epitope’s specificity identification could represent an important tool to be evaluated in patients with a complex immunization. Moreover, many studies have demonstrated the critical role of complement-binding DSA in the development of GR in haploidentical HSCT recipients and solid organ transplantation10,29,30. In our experience, using solid-phase assays in conjunction with FCXM allowed to better evaluate DSA’s sensitivity, specificity, and strength. Moreover, the DSA testing and monitoring strategy during donor selection allowed to identify patients at a high risk of developing GF. Hence, we had time to select alternative donors, as first option, or to start our desensitization protocol, avoiding any interference with the pharmacokinetics of the conditioning regimen and ATG administration. This strategy was tuned thanks to our previous experience on 65 consecutive patients screened for anti-HLA antibodies, candidates for a mismatched HSCT: the desensitization strategy was effective and safe in 2 treated patients6. Our strategy permits us to overcome DSA obstacles: GF was observed only in 2 patients (2.35%) who did not present anti-HLA antibodies nor DSAs. PGF, observed in 12% of patients, did not correlate with DSAs. Factors associated with a delayed neutrophil engraftment were the patient’s female gender (p=0.039), the use of bone marrow as stem cell source (p=0.025), and a high HSCT–specific comorbidity index (p=0.028). We did not observe any statistical correlation among the evaluated variables on platelet engraftment. OS and TRM did not differ between patients with or without anti-HLA antibodies and DSAs.
Conclusions
Our analysis shows that anti-HLA antibodies are frequent in hematologic patients (17.46%), with a high probability of identifying DSAs. We confirm the importance of routine testing and monitoring for anti-HLA antibodies and DSAs in mismatched HSCT procedures and the need to plan desensitization strategies if other donors are unavailable. To date, there is no recommended standard desensitization strategy. The desensitization schedule employed at our Center appears safe and effective in obtaining a stable engraftment. Future prospective studies are needed to standardize anti-HLA antibodies and DSA management and to test our tailored strategy on larger series of patients, taking into account HLA match, conditioning regimen, and MFI. To make this possible, a strong collaboration between transplant physicians, transfusion and immunogenetics specialists is required.
Supplementary Information
Footnotes
Commented by doi 10.2450/BloodTransfus.630
This publication uses data collected within the framework of the PhD thesis “Donor specific anti-HLA antibodies in Hematopoietic Stem Cell Transplantation. A stepwise project to establish a better definition of their role and a desensitization strategy” of Ursula La Rocca, published in 2021 at Ph.D. School “Innovation in Immuno-mediated and Hematological disorders” Cycle XXXIII, Sapienza, University of Rome.
AUTHORS’ CONTRIBUTIONS: API and ULR conceived the idea of the study and the protocol and wrote the manuscript; ULR collected the data; AP performed the statistical analysis. MPP, ULR, PG, LL, PC, GiG and CC performed and analyzed tests and results. API, WB and RR managed HSCT and follow up. DC and NC performed the hematopoietic stem cell manipulation. MG and MSB performed the desensitization strategies. RR, GG, RF, MM, SC, and API revised the data, tables, figures and manuscript. All the Authors approved the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Juric MK, Ghimire S, Ogonek J, Weissinger EM, Holler E, van Rood JJ, et al. Milestones of hematopoietic stem cell transplantation - From first human studies to current developments. Front Immunol. 2016;477:470. doi: 10.3389/fimmu.2016.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kongtim P, Cao K, Ciurea SO. Anti-HLA antibodies: assessment and mitigating strategies. In: Ciurea S, Handgretinger R, editors. Haploidentical transplantation Advances and controversies in hematopoietic transplantation and cell therapy. Springer; Cham: 2018. pp. 127–143. [DOI] [Google Scholar]
- 3.Warren RP, Storb R, Weiden PL, Su PJ, Thomas ED. Lymphocyte-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity in patients with aplastic anemia: distinguishing transfusion-induced sensitization from possible immune-mediated aplastic anemia. Transplant Proc. 1981;13:245–247. [PubMed] [Google Scholar]
- 4.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108:3611–3619. doi: 10.1182/blood-2006-04-017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattsson J, Ringdén O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:165–170. doi: 10.1016/j.bbmt.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Rocca U, Perrone MP, Piciocchi A, Cinti P, Barberi W, Gozzer M, et al. Anti-HLA donor-specific antibodies in allogeneic stem cell transplantation: management and desensitization protocol. Bone Marrow Transplant. 2019;54:1717–1720. doi: 10.1038/s41409-019-0497-1. [DOI] [PubMed] [Google Scholar]
- 7.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 8.Zachary AA, Leffell MS. Barriers to successful transplantation of the sensitized patient. Expert Rev Clin Immunol. 2010;6:449–460. doi: 10.1586/eci.10.14. [DOI] [PubMed] [Google Scholar]
- 9.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–360. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:521–534. doi: 10.1038/s41409-017-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciurea SO, Al Malki MM, Kongtim P, Fuchs EJ, Luznik L, Huang XJ, et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55:12–24. doi: 10.1038/s41409-019-0499-z. [DOI] [PubMed] [Google Scholar]
- 12.Sanz J, Boluda JC, Martín C, Gonzales M, Ferrà C, Serrano D, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47:1287–1293. doi: 10.1038/bmt.2012.13. [DOI] [PubMed] [Google Scholar]
- 13.Saraceni F, Labopin M, Hamladji RM, Mufti G, Sociè G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2017;9:3379–3393. doi: 10.18632/oncotarget.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):44–353. doi: 10.1182/blood-2014-02-514778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Socie’ G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Severe aplastic anemia working party of the european group for blood and marrow transplantation. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filipovich AH. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:251–257. doi: 10.1016/j.beha.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 pathology working group report. Biol Blood Marrow Transplant. 2015;21:589–603. doi: 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters AL, Van Stein D, Vlaar AP. Antibody-mediated transfusion-related acute lung injury; from discovery to prevention. Br J Haematol. 2015;170:597–614. doi: 10.1111/bjh.13459. [DOI] [PubMed] [Google Scholar]
- 19.Reesink HW, Lee J, Keller A, Dennington P, Pink J, Holdsworth R, et al. Measures to prevent transfusion-related acute lung injury (TRALI) Vox Sang. 2012;103:231–259. doi: 10.1111/j.1423-0410.2012.01596.x. [DOI] [PubMed] [Google Scholar]
- 20.Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55:2071–2076. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozdemir ZN, Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57:163–167. doi: 10.1016/j.transci.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opin Pharmacother. 2014;15:23–36. doi: 10.1517/14656566.2014.852537. [DOI] [PubMed] [Google Scholar]
- 23.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladstone DE, Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: challenges and opportunities. Hematology Am Soc Hematol Educ Program. 2017;8:645–650. doi: 10.1182/asheducation-2017.1.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47:508–515. doi: 10.1038/bmt.2011.131. [DOI] [PubMed] [Google Scholar]
- 26.Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118:6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115:2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647–652. doi: 10.1016/j.bbmt.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maślanka K, Michur H, Zupańska B, Uhrynowska M, Nowak J. Leucocyte antibodies in blood donors and a look back on recipients of their blood components. Vox Sang. 2007;92:247–249. doi: 10.1111/j.1423-0410.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 32.Powers A, Stowell CP, Dzik WH, Saidman SL, Lee H, Makar RS. Testing only donors with a prior history of pregnancy or transfusion is a logical and cost-effective transfusion-related acute lung injury prevention strategy. Transfusion. 2008;48:2549–2558. doi: 10.1111/j.1537-2995.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 33.El-Awar N, Terasaki PI, Nguyen A, Sasaki N, Morales-Buenrostro LE, Saji H, Maruya E, et al. Epitopes of HLA antibodies found in sera of normal healthy males and cord blood. Hum Immunol. 2009;70:844–853. doi: 10.1016/j.humimm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Hickey MJ, Valenzuela NM, Reed EF. Alloantibody generation and effector function following sensitization to human leukocyte antigen. Front Immunol. 2016;7:30. doi: 10.3389/fimmu.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duquesnoy RJ. Human leukocyte antigen epitope antigenicity and immunogenicity. Curr Opin Organ Transplant. 2014;19:428–435. doi: 10.1097/MOT.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 36.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, et al. The effect of previous pregnancy and transfusion on hla alloimmunization in blood donors: implications for a transfusion related acute lung injury (TRALI) risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seftel MD, Growe GH, Petraszko T, Benny WB, Le A, Lee CY, et al. Universal prestorage leukoreduction in Canada decreases platelet alloimmunization and refractoriness. Blood. 2004;103:333–339. doi: 10.1182/blood-2003-03-0940. [DOI] [PubMed] [Google Scholar]
- 38.Yoshihara S, Taniguchi K, Ogawa H, Saji H. The role of HLA antibodies in allogeneic SCT: is the ‘type-and-screen’ strategy necessary not only for blood type but also for HLA? Bone Marrow Transplant. 2012;47:1499–1506. doi: 10.1038/bmt.2011.249. [DOI] [PubMed] [Google Scholar]
- 39.Bettinotti MP, Zachary AA, Leffell MS. Clinically relevant interpretation of solid phase assays for HLA antibody. Curr Opin Organ Transplant. 2016;21:453–458. doi: 10.1097/MOT.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bramanti S, Nocco A, Mauro E, Milone G, Morabito L, Sarina B, et al. Desensitization with plasma exchange in a patient with human leukocyte antigen donor-specific antibodies before T-cell-replete haploidentical transplantation. Transfusion. 2016;56:1096–1100. doi: 10.1111/trf.13523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.