Abstract
Polo‐like kinase 1 (PLK1; also known as serine/threonine‐protein kinase PLK1) serves as a central player in cell proliferation, exerting critical regulatory roles in mitotic processes and cell survival. We conducted an analysis of PLK1 protein expression in a large cohort of samples from papillary thyroid carcinoma (PTC) patients and examined its functional significance in PTC cell lines, both in vitro and in vivo. PLK1 overexpression was noted in 54.2% of all PTC and was significantly associated with aggressive clinicopathological parameters; it was also found to be an independent prognostic marker for shorter recurrence‐free survival. Given the significant association between PLK1 and forkhead box protein M1 (FoxM1), and their concomitant overexpression in a large proportion of PTC samples, we explored their correlation and their combined inhibitions in PTC in vitro and in vivo. Inhibition of PLK1 expression indeed suppressed cell proliferation, leading to cell cycle arrest and apoptosis in PTC cell lines. Significantly, the downregulation of PLK1 reduced the self‐renewal capability of spheroids formed from PTC cells. Immunoprecipitation analysis shows that PLK1 binds to FoxM1 and vice versa in vitro. Mechanistically, PLK1 knockdown suppresses FoxM1 expression, whereas inhibition of FoxM1 does not affect PLK1 expression, which suggests that PLK1 acts through the FoxM1 pathway. The combined treatment of a PLK1 inhibitor (volasertib) and a FoxM1 inhibitor (thiostrepton) demonstrated a synergistic effect in reducing PTC cell growth in vitro and delaying tumor growth in vivo. This study highlights the important role of PLK1 in PTC tumorigenesis and prognosis. It also highlights the synergistic therapeutic potential of dual‐targeting PLK1 and FoxM1 in PTC, unveiling a potential innovative therapeutic strategy for managing aggressive forms of PTC.
Keywords: apoptosis, FoxM1, papillary thyroid cancer, PLK1, stemness
The authors examined the prognostic relevance of PLK1 in papillary thyroid carcinoma (PTC) patients, studying its role in PTC cell lines in vitro and in vivo. PLK1 overexpression in PTC correlates significantly with aggressive clinicopathological features. Interestingly, PLK1 shows a physical association with FoxM1, and the combined inhibition of these proteins demonstrates a synergistic anti‐tumor effect in both in vitro and in vivo settings.
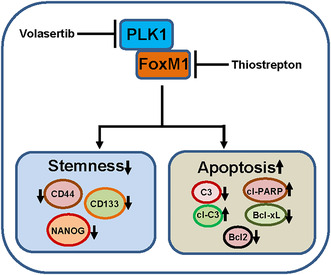
Abbreviations
- ATCC
American‐Type Culture Collection
- Bcl‐2
B‐cell lymphoma 2
- Bcl‐xL
B‐cell lymphoma‐extra large
- CDK1
cyclin‐dependent kinase 1
- DMEM
Dulbecco's Modified Eagle Medium
- DSMZ
Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH
- EMT
epithelial to mesenchymal transition
- FoxM1
forkhead box protein M1
- IHC
immunohistochemistry
- PARP
poly (ADP‐ribose) polymerase
- PLK1
Polo‐like kinase 1
- PTC
papillary thyroid carcinoma
- TERT
telomerase reverse transcriptase
- TMA
tissue microarray
1. Introduction
Papillary thyroid carcinoma accounts for more than 90% of endocrine malignancies [1]. Despite favorable outcomes following standard treatment with surgery and radioiodine (RAI) therapy, a small proportion may develop recurrence and metastases [2]. Predictive biomarkers are needed to be identified and develop effective combinatorial treatment approaches. Mammalian Polo‐like kinase 1 (PLK1) is a serine/threonine kinase with significant roles in regulating cell proliferation and serving as a crucial regulator for the progression through the G2‐M phase of the cell cycle [3, 4]. PLK1 is also known for regulating centrosome mutation [5]. PLK1 is overexpressed in several cancers, including anaplastic thyroid carcinoma [6], gastric [7], breast [8], neuroblastoma [9], and ovarian cancer [10], where high PLK1 expression is correlated with unfavorable prognostic outcomes [9, 11]. These investigations indicate that targeting PLK1 holds great promise as a valuable therapeutic strategy for cancer treatment.
A recent study indicates that PLK1 is involved in differentiated thyroid cancer (DTC) [12], where PLK1 was shown to be up‐regulated in DTC and pharmacological inhibition has reduced cells in the s‐phase and increased cells in G2/M phase, and induced apoptosis in DTC cell lines [12]. FoxM1, a member of the Fox transcription factor family, plays a crucial role in the regulation of cell cycle progression via activating various genes pivotal for G1 to S and G2 to M phase transition including PLK1 [13, 14]. Interestingly, PLK1 interacts directly with FoxM1 and phosphorylates it, leading to the complete transcription activation of FoxM1 and the formation of a feedback loop [14, 15, 16].
Several studies have highlighted the important role of FoxM1 in tumor pathogenesis, tumor progression, invasion, and metastasis in a variety of cancers including thyroid cancer, breast cancer, ovarian cancer, and others [17, 18, 19]. Moreover, overexpression of FoxM1 is correlated with aggressive behavior, drug resistance, cancer phenotype, and poor outcome [19, 20, 21, 22, 23, 24]. The aforementioned information lays the scientific premise for this study, where our hypothesis posits a potential correlation between PLK1 and FoxM1 in a large Middle Eastern PTC cohort. Moreover, exploring the simultaneous inhibition of PLK1 and FoxM1, both in vitro and in vivo, may establish a rationale for considering the combined inhibition of these two factors as a potential therapeutic approach for managing PTC.
2. Materials and methods
2.1. Patient selection and clinico‐pathological data
One thousand seven‐hundred and sixteen PTC patients diagnosed between 1988 and 2020 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. Diagnosis of PTC was based on clinical history and confirmed by fine needle aspiration cytology. Table 1 summarizes the baseline clinico‐pathological data, collected from patient medical records. Eighth edition of American Joint Committee on Cancer (AJCC) staging system was used for PTC staging [25]. Recurrence was defined as any newly detected tumor (local or distant) or metastatic lymph node based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment.
Table 1.
Clinico‐pathological characteristics of papillary thyroid carcinoma cohort.
| No. | % | |
|---|---|---|
| No. of patients | 1716 | |
| Age (years) | ||
| Median (range) | 38.5 (6.0–87.6) | |
| ≤ 55 | 319 | 18.6 |
| > 55 | 1397 | 81.4 |
| Sex | ||
| Female | 1300 | 75.7 |
| Male | 416 | 24.3 |
| Histology type | ||
| Classical variant | 1080 | 63.0 |
| Follicular variant | 301 | 17.5 |
| Tall‐cell variant | 180 | 10.5 |
| Other variants | 155 | 9.0 |
| Tumor laterality | ||
| Unilateral | 1158 | 67.5 |
| Bilateral | 558 | 32.5 |
| Tumor focality | ||
| Unifocal | 851 | 49.6 |
| Multifocal | 865 | 50.4 |
| Extrathyroidal extension | ||
| Absent | 1006 | 58.6 |
| Present | 710 | 41.4 |
| Lymphovascular invasion | ||
| Absent | 1246 | 72.6 |
| Present | 470 | 27.4 |
| pT | ||
| T1 | 688 | 40.1 |
| T2 | 555 | 32.3 |
| T3 | 344 | 20.0 |
| T4 | 125 | 7.3 |
| Unknown | 4 | 0.3 |
| Lymph node metastasis | ||
| Absent | 716 | 41.7 |
| Present | 831 | 48.4 |
| Unknown | 169 | 9.9 |
| Distant metastasis | ||
| Absent | 1568 | 91.4 |
| Present | 148 | 8.6 |
| Stage | ||
| I | 1450 | 84.5 |
| II | 182 | 10.6 |
| III | 24 | 1.4 |
| IV | 53 | 3.1 |
| Unknown | 7 | 0.4 |
| BRAF mutation | ||
| Present | 934 | 54.4 |
| Absent | 744 | 43.4 |
| Unknown | 38 | 2.2 |
| TERT mutation | ||
| Present | 207 | 12.1 |
| Absent | 1393 | 81.2 |
| Unknown | 116 | 6.7 |
| Recurrence | ||
| Yes | 307 | 17.9 |
| No | 1409 | 82.1 |
2.2. Ethics declarations
Institutional Review Board of King Faisal Specialist Hospital and Research Centre provided ethical approval for the current study (RAC# 2110 031, RAC# 2220 002 and 2211 168). Research Advisory Council (RAC) granted waiver of informed consent for use of retrospective patient case data. All the methods were carried out in accordance with the Declaration of Helsinki.
2.3. BRAF and TERT mutation analysis
BRAF and TERT mutation data were assessed in our laboratory by Sanger sequencing and has been published by us previously [26, 27, 28]. BRAF mutation analysis was performed in 1678 cases and TERT mutation analysis was done in 1600 cases.
2.4. Tissue microarray construction and immunohistochemistry analysis
Tissue microarray (TMA) format was utilized for immunohistochemical (IHC) analysis of the PTC samples. TMA was constructed as previously described [29]. Briefly, modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI, USA) was used to punch tissue cylinders with a diameter of 0.6 mm from representative tumor area of the donor tissue block and brought into the recipient paraffin block. Two cores of PTC were arrayed from each case.
Tissue microarray slides were processed and stained manually as described previously [30]. Primary antibodies against PLK1 (mouse monoclonal, ab‐17 056, 1 : 500, pH 9.0; Abcam, Cambridge, UK) and FoxM1 (clone K‐19, 1 : 2500, pH 9.0; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The Dako Envision Plus System kit (Glostrup, Denmark) was used as the secondary detection system with 3, 30‐diaminobenzidine as chromogen. All slides were counter stained with hematoxylin, dehydrated, cleared, and mounted. Negative controls included omission of the primary antibody. Normal tissues of different organ system were also included in the TMA to serve as control. Only fresh‐cut slides were stained simultaneously to minimize the influence of slide aging and maximize reproducibility of the experiment.
PLK1 staining was scored using immunoreactivity score (IRS), as described previously [31]. Briefly, staining intensity was scored as 0: negative; 1: weak; 2: moderate; or 3: strong, and staining proportion was scored as 0, 0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; or 4, more than 80% positive cells. The final IRS score was calculated by multiplying the intensity and proportion scores. Low expression of PLK1 was defined as IRS 0–6 and high expression of PLK1 was defined as IRS more than 6. FoxM1 staining was scored using H score, as described previously [18]. x‐tile software [32] was used to define the optimal cut‐off score. Based on x‐tile plots, PTC cases with complete absence (H score = 0) was defined as no expression and the other group (H score ≥ 1) was defined as overexpression for FoxM1.
2.5. Cell culture
Nthy‐ori 3–1 (RRID:CVCL_2659), normal human primary thyroid follicular epithelial cell line was obtained from Sigma‐Aldrich (St. Louis, MO, USA). The PTC cell line, BCPAP (RRID:CVCL_0153), was purchased from DSMZ (Braunschweig, Germany), and TPC‐1 (RRID:CVCL_6298) was kindly provided by B. McIver (Department of Endocrinology, Mayo Clinic, Rochester, MN, USA). K1 cell line (RRID:CVCL_2537) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cell lines were cultured using RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), along with 100 Units·mL−1 of penicillin/streptomycin and 100 Units·mL−1 of Glutamine, following previously established protocols [18]. These cell lines were tested negative for mycoplasma and their authentication was conducted internally using short tandem repeats PCR, and the obtained results were consistent with previously published data [26, 33].
2.6. Reagents and antibodies
The PLK1 inhibitor, volasertib, was purchased from Selleck Chemicals (Houston, TX, USA). Antibodies against PLK1 (4513), Bcl‐2 (2876), Bcl‐xl (2762), cleaved caspase‐3 (9664), PARP (9542), CD44 (3570), CD133 (64326), NANOG (4903), Cyclin B1 (4135), phospho‐Bad (9295), Bad (9292) and GAPDH (5174) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against FoxM1 (sc‐376 471) and caspase‐3 (sc‐56 053) were purchased from Santa Cruz Biotechnology, Inc. Cyclin D1 (ab139260) was obtained from Abcam (Cambridge, MA, USA). Phospho‐CDK1/CDC2 (orb127843) was purchased from Biorbyt Ltd (Cambridge, UK).
2.7. Gene silencing using siRNA
FoxM1 siRNA and scrambled control siRNA were purchased from Qiagen (Valencia, CA, USA). Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for a duration of 6 h, after which the lipid‐siRNA complex was removed, and fresh growth medium supplemented with 20% fetal bovine serum was added. Following a 48‐h post‐transfection period, cells were harvested and utilized for various experiments.
2.8. Plasmid and transfection
Plasmid DNA encoding human PLK1 and shRNA targeting human PLK1 were purchased from Origene (Rockville, MD, USA). The forced expression and knockdown of PLK1 in Nthy‐ori 3–1 cell line and PTC cell lines, respectively, were performed using Lipofectamine™2000 (Invitrogen) according to the manufacturer's protocol. In brief, cells were initially seeded in 6‐well culture plates. When they reached approximately 50% confluence, transfection was carried out using 4 μg of plasmid. After a 48‐h post‐transfection period, stable clones with overexpressed PLK1, resistant to G418, and stable knockdown clones, resistant to puromycin, were isolated. The confirmation of successful overexpression and knockdown of PLK1 protein production was validated through immunoblotting.
2.9. Sphere forming assay
Cells, at a density of 500 cells per well, were seeded in Corning 24‐well ultra‐low attachment plates (Sigma‐Aldrich). These cells were cultured in serum‐free DMEM‐F12 (ATCC) medium, which was supplemented with B27 (Thermo Fisher Scientific, Grand Island, NY, USA), 20 ng·mL−1 EGF (Sigma‐Aldrich), 0.4% BSA (Sigma‐Aldrich), and 4 μg·mL−1 insulin (Sigma‐Aldrich). Fresh medium was added every 2 days. Spheroids were counted and photographed on day 14. To assess secondary spheroid formation, the primary spheroids were dissociated into single cells and subsequently cultured in 24‐well ultra‐low attachment plates, using spheroid culture medium, for an additional 10 days.
2.10. Animals and xenografts study
Six‐week‐old female nude mice (NU/J) were procured from Jackson Laboratories (Bar Harbor, ME, USA) and were housed in a sterile and pathogen‐free environment (12 h light/12 h darkness) with food and water ad libitum at least 1 week before use. All animal experiments were conducted in strict adherence to institutional guidelines and were approved by the Animal Care and Use Committee (ACUC) of King Faisal Specialist Hospital and Research Center on 24 October 2022 (RAC#2220002). For the xenograft study, TPC‐1 cells (4 × 106 cells per mouse) were resuspended in serum‐free medium combined with matrigel basement membrane matrix at a 1 : 1 ratio. These cell mixtures were subsequently subcutaneously injected into the flanks of NU/J mice (n = 5). Once the tumors reached a size of approximately 100 mm3, the mice received intraperitoneal treatments, which included vehicle (0.1% DMSO), volasertib (20 mg·kg−1), thiostrepton (20 mg·kg−1), and a combination of volasertib and thiostrepton. These treatments were administered twice a week for 30 days. Throughout the study, the body weight and tumor volume of each mouse were closely monitored on a weekly basis. Following 5 weeks of treatment, the mice were euthanized, and individual tumors were weighed before being rapidly snap‐frozen in liquid nitrogen for storage.
2.11. Statistical analysis
The associations between clinico‐pathological variables and protein expression were performed using contingency table analysis and Chi square tests. Mantel‐Cox log‐rank test was used to evaluate recurrence‐free survival. Survival curves were generated using the Kaplan–Meier method. Cox proportional hazards regression model was used for multivariate analysis. Two‐sided tests were used for statistical analyses with a limit of significance defined as P value < 0.05. Data analysis was performed using the jmp14.0 (SAS Institute, Inc., Cary, NC, USA) software package.
In all functional studies, the data are presented as means ± standard deviation (SD) derived from triplicates in an independent experiment. This experiment was repeated at least two times, yielding consistent results. Statistical significance was assessed using a two‐tailed Student's t‐test, with a significance threshold set at P < 0.05.
3. Results
3.1. Patient characteristics
Median age of the study population was 38.5 years (range: 6.0–87.6 years), with a male: female ratio of 1 : 3. 32.5% (558/1716) of tumors were bilateral and 50.4% (865/1716) were multifocal. Extrathyroidal extension was noted in 41.4% (710/1716) of DTCs. Regional lymph node metastasis (LNM) was noted in 48.4% (831/1716) of cases and distant metastasis was present in 8.6% (148/1716). Frequency of BRAF and TERT mutations was 54.4% (934/1716) and 12.1% (207/1716), respectively (Table 1).
3.2. PLK1 expression in papillary thyroid cancer and its clinico‐pathological associations
PLK1 protein expression was analyzed immunohistochemically in 1716 PTC samples. However, immunohistochemistry data was interpretable in 1612 samples and hence were included for further analysis. Over‐expression of PLK1 was noted in 54.2% (874/1612) of cases (Fig. 1) and was significantly associated with adverse clinico‐pathological parameters such as extrathyroidal extension (P < 0.0001), lymphovasacular invasion (P = 0.0021), T4 tumors (P < 0.0001), lymph node metastasis (P < 0.0001) and tumor recurrence (P < 0.0001) (Table 2). We also found a significant association between PLK1 expression and molecular markers like BRAF mutation (P < 0.0001) and TERT mutation (P = 0.0021). Interestingly, PLK1 over‐expression was associated with cell cycle regulator, FoxM1 (P < 0.0001) in our cohort (Table 2).
Fig. 1.
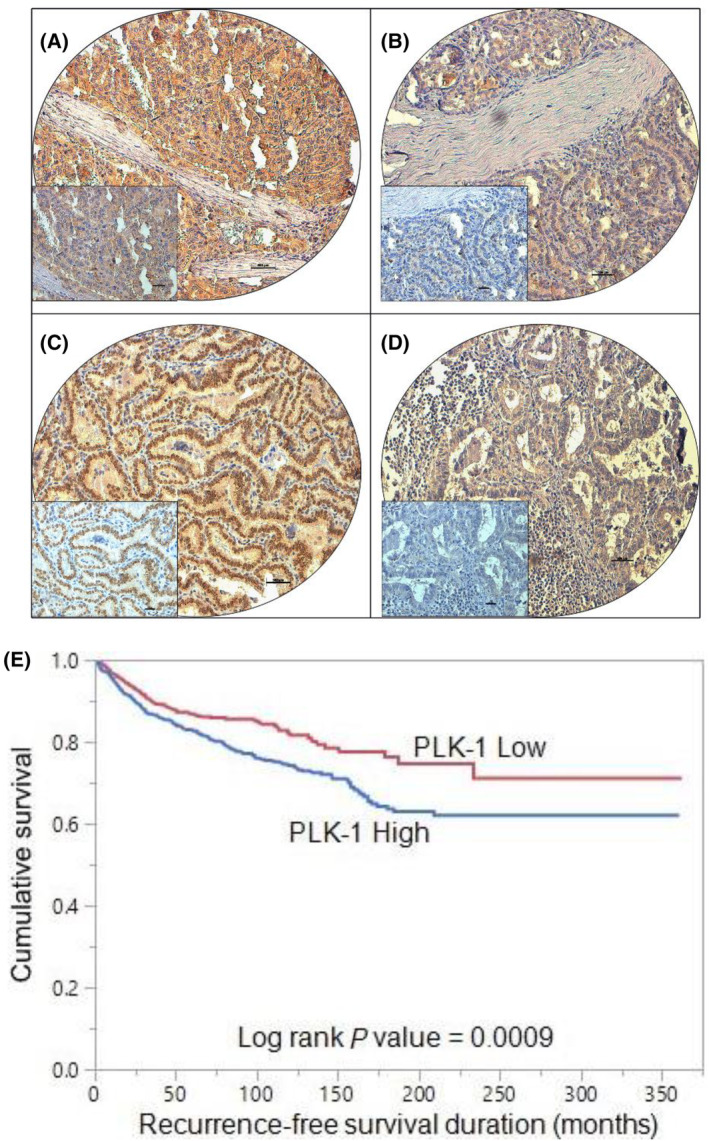
Tissue microarray (TMA) based immunohistochemistry analysis of PLK1 and FoxM1 in papillary thyroid cancer (PTC) patients. PTC TMA spots showing overexpression of PLK1 (A) and FoxM1 (C). In contrast, another set of TMA spots showing reduced expression of PLK1 (B) and FoxM1 (D). 20×/0.70 objective on an Olympus BX 51 microscope (Olympus America Inc, Center Valley, PA, USA) with the inset showing a 40× 0.85 aperture magnified view of the same TMA spot (scale bar = 500 μm). (E) Recurrence‐free survival (RFS). Kaplan–Meier survival plot showing statistically significant poor RFS in PLK1 high expression cases compared to PLK1 low expression (P = 0.0009).
Table 2.
Clinico‐pathological associations of PLK‐1 expression in papillary thyroid carcinoma.
| Total | PLK‐1 high | PLK‐1 low | P value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| No. of patients | 1612 | 874 | 54.2 | 738 | 45.8 | ||
| Age (years) | |||||||
| ≤ 55 | 1316 | 81.6 | 700 | 80.1 | 616 | 83.5 | 0.0810 |
| > 55 | 296 | 18.4 | 174 | 19.9 | 122 | 16.5 | |
| Sex | |||||||
| Female | 1227 | 76.1 | 666 | 76.2 | 561 | 76.0 | 0.9308 |
| Male | 385 | 23.9 | 208 | 23.8 | 177 | 24.0 | |
| Histology type | |||||||
| Classical variant | 1018 | 63.1 | 598 | 68.4 | 420 | 56.9 | < 0.0001 |
| Follicular variant | 287 | 17.8 | 100 | 11.4 | 187 | 25.3 | |
| Tall‐cell variant | 167 | 10.4 | 103 | 11.8 | 64 | 8.7 | |
| Other variants | 140 | 8.7 | 73 | 8.4 | 67 | 9.1 | |
| Tumor laterality | |||||||
| Unilateral | 1083 | 67.2 | 575 | 65.8 | 508 | 68.8 | 0.1945 |
| Bilateral | 529 | 32.8 | 299 | 34.2 | 230 | 31.2 | |
| Tumor focality | |||||||
| Unifocal | 804 | 49.9 | 425 | 48.6 | 379 | 51.4 | 0.2751 |
| Multifocal | 808 | 50.1 | 449 | 51.4 | 359 | 48.6 | |
| Extrathyroidal extension | |||||||
| Absent | 948 | 58.8 | 472 | 54.0 | 476 | 64.5 | < 0.0001 |
| Present | 664 | 41.2 | 402 | 46.0 | 262 | 35.5 | |
| Lymphovascular invasion | |||||||
| Absent | 1178 | 73.1 | 666 | 76.2 | 512 | 69.4 | 0.0021 |
| Present | 434 | 26.9 | 208 | 23.8 | 226 | 30.6 | |
| pT | |||||||
| T1 | 643 | 40.0 | 371 | 42.5 | 272 | 37.0 | < 0.0001 |
| T2 | 514 | 32.0 | 264 | 30.2 | 250 | 34.0 | |
| T3 | 333 | 20.7 | 157 | 18.0 | 176 | 24.0 | |
| T4 | 118 | 7.3 | 81 | 9.3 | 37 | 5.0 | |
| Lymph node metastasis | |||||||
| Absent | 680 | 46.4 | 331 | 41.4 | 349 | 52.4 | < 0.0001 |
| Present | 786 | 53.6 | 469 | 58.6 | 317 | 47.6 | |
| Distant metastasis | |||||||
| Absent | 1469 | 91.1 | 787 | 90.0 | 682 | 92.4 | 0.0960 |
| Present | 143 | 8.9 | 87 | 10.0 | 56 | 7.6 | |
| Stage | |||||||
| I | 1364 | 85.0 | 723 | 83.2 | 641 | 87.1 | 0.0981 |
| II | 169 | 10.5 | 98 | 11.3 | 71 | 9.6 | |
| III | 22 | 1.4 | 15 | 1.7 | 7 | 1.0 | |
| IV | 50 | 3.1 | 33 | 3.8 | 17 | 2.3 | |
| Tumor recurrence | |||||||
| Yes | 302 | 18.7 | 197 | 22.5 | 105 | 14.2 | < 0.0001 |
| No | 1310 | 81.3 | 677 | 77.5 | 633 | 85.8 | |
| BRAF mutation | |||||||
| Present | 881 | 55.8 | 531 | 61.6 | 350 | 48.9 | < 0.0001 |
| Absent | 697 | 44.2 | 331 | 38.4 | 366 | 51.1 | |
| TERT mutation | |||||||
| Present | 198 | 13.1 | 129 | 15.6 | 69 | 10.2 | 0.0021 |
| Absent | 1309 | 86.9 | 700 | 84.4 | 609 | 89.8 | |
| FOXM1 IHC | |||||||
| High | 469 | 29.8 | 345 | 40.1 | 124 | 17.4 | < 0.0001 |
| Low | 1102 | 70.2 | 515 | 59.9 | 587 | 82.6 | |
3.3. PLK1 expression and clinical outcome
We further analyzed the association of PLK1 over‐expression with clinical outcome in our cohort of PTC. Kaplan–Meier curve analysis showed that patients exhibiting PLK1 over‐expression had a significantly shorter RFS compared to those who had low PLK1 expression (P = 0.0009) (Fig. 1E). This association remained significant on multivariate analysis, after adjusting for other clinico‐pathological variables (Hazard ratio = 1.32, 95% confidence interval = 1.02–1.71, P = 0.0325), suggesting that PLK‐1 was an independent predictor of RFS (Table 3).
Table 3.
Univariate and multivariate analysis of PLK‐1 expression using Cox proportional hazard model for recurrence‐free survival.
| Clinico‐pathological variables | Recurrence‐free survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age | ||||
| Above ≥ 55 years (vs. < 55 years) | 2.90 (2.27–3.68) | < 0.0001 | 2.27 (1.70–3.00) | < 0.0001 |
| Sex | ||||
| Male (vs. female) | 1.77 (1.39–2.23) | < 0.0001 | 1.36 (1.04–1.76) | 0.0244 |
| Histology | ||||
| Aggressive variants (vs. non‐aggressive variants) | 1.43 (1.04–1.93) | 0.0276 | 1.15 (0.79–1.64) | 0.4528 |
| Tumor laterality | ||||
| Bilateral (vs. unilateral) | 1.67 (1.33–2.09) | < 0.0001 | 1.18 (0.92–1.52) | 0.1973 |
| Tumor focality | ||||
| Multifocal (vs. unifocal) | 1.23 (0.98–1.54) | 0.0722 | ||
| Extrathyroidal extension | ||||
| Present (vs. absent) | 2.94 (2.33–3.74) | < 0.0001 | 1.60 (1.21–2.14) | 0.0010 |
| Lymphovascular invasion | ||||
| Present (vs. absent) | 1.31 (1.01–1.69) | 0.0452 | 0.96 (0.70–1.30) | 0.7791 |
| pT | ||||
| T4 (vs. T1–T3) | 1.93 (1.52–2.45) | < 0.0001 | 1.28 (0.98–1.65) | 0.0651 |
| Lymph node metastasis | ||||
| Present (vs. absent) | 2.70 (2.09–3.53) | < 0.0001 | 2.01 (1.51–2.70) | < 0.0001 |
| Distant metastasis | ||||
| Present (vs. absent) | 7.23 (5.28–9.69) | < 0.0001 | 3.55 (2.49–4.96) | < 0.0001 |
| PLK‐1 expression | ||||
| High (vs. Low) | 1.49 (1.18–1.89) | 0.0008 | 1.32 (1.02–1.71) | 0.0325 |
3.4. Inhibition of PLK1 impedes cell proliferation and induces cell cycle arrest and apoptosis
The PLK1 overexpression was observed in our PTC cohort and was significantly correlated with aggressive clinicopathological parameters. Hence, our objective was to explore whether inhibiting PLK1 could serve as a feasible therapeutic approach to impede cell growth and trigger apoptosis in PTC cells. Initially, we assessed PLK1 protein expression in Nthy‐ori 3–1, a normal human primary thyroid follicular epithelial cell line, and other three PTC cell lines (BCPAP, TPC‐1, and K1). Our analysis revealed elevated PLK1 expression in two PTC cell lines (BCPAP and TPC‐1), while K1 and Nthy‐ori 3–1 cell lines displayed low levels of PLK1 expression (Fig. 2A). Subsequently, we treated escalating doses of volasertib to PTC cell lines, BCPAP and TPC‐1, and assessed cell viability using the MTT assay after 24 and 48 h of treatment. As shown in Fig. 2B,C, there was a significant dose‐dependent inhibition of cell viability after 48 h in both cell lines. We selected volasertib doses 250 and 500 nm and 48 h time point for further in vitro experimentation.
Fig. 2.
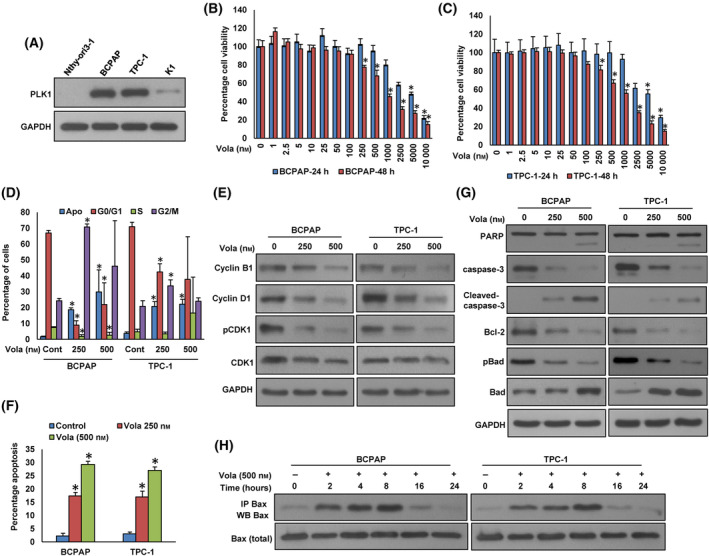
Inhibition of PLK1 impedes cell proliferation and induces cell cycle arrest and apoptosis. (A) Basal expression of PLK1 in Nthy‐ori 3–1 and PTC cell lines. Proteins were extracted from Nthy‐ori 3–1 cell line and three PTC cell lines and subjected to immunoblotting using indicated antibodies (n = 3). (B, C) Volasertib inhibits cell viability. PTC cells (104) were exposed to increasing doses of volasertib for 24 and 48 h and cell viability was assessed using MTT. Data were presented as mean ± SD (n = 8). (D, E) Cell cycle analysis of PTC cells treated with volasertib. BCPAP and TPC‐1 cells were exposed to 250 and 500 nm volasertib for 48 h. Following incubation, either cells were analyzed for cell cycle fractions by flow cytometry (D) or proteins were extracted from cells and were subjected to immuno‐blotting to analyze cell cycle markers (E). Data were presented as mean ± SD (n = 3). (F, G) Volasertib induces apoptosis in PTC cell lines. PTC cells were exposed to indicated doses of volasertib for 48 h. Following incubation, either cells were stained with annexin‐V/PI and analyzed by flow cytometry (F) or proteins were extracted from cells and were subjected immuno‐blotting using antibodies as indicated (G). Data were presented as mean ± SD (n = 3). (H) Effect of volasertib on Bax activation in PTC cells. PTC cells were treated with volasertib for indicated time course. Following treatment, cells were lysed in 1% Chaps lysis buffer and subjected to immuno‐precipitation with anti‐Bax antibody (sc‐493; Santa Cruz) and immunoblotted with anti‐Bax antibody (B8554; SIGMA‐ALDRICH), (n = 3). Statistical analyses were conducted employing two‐tailed Student's t‐tests. *P < 0.05.
To discern whether the reduction in cell viability was attributed to cell cycle arrest or apoptosis, we treated PTC cells with volasertib (250 and 500 nm) for 48 h, followed by analysis for cell cycle and apoptosis. As anticipated, our findings demonstrated that volasertib treatment resulted in a reduction in the G1 phase of the cell cycle, subsequently leading to G2/M phase arrest and apoptosis (Fig. 2D). We also analyzed the expression levels of important cell cycle proteins regulated by PLK1.
The volasertib treatment reduced the levels of cyclin B1, Cyclin D1, and phopho‐CDK1 in these cells (Fig. 2E). To confirm apoptosis in volasertib‐treated PTC cells, we treated BCPAP and TPC‐1 cells with volasertib for 48 h, and stained with annexin V and propidium iodide and analyzed for apoptosis by flow cytometry. As shown in Fig. 2F, there was an increase in apoptotic cells after treatment with volasertib in both the PTC cell lines tested. We also examined the expression of apoptotic protein markers after volasertib treatment on these cells by immunoblotting. The volasertib treatment induced the cleavage of PARP and caspase‐3 in these cells (Fig. 2G). In addition, treatment with volasertib caused an increase in the total Bad and a decrease in phosphorylated Bad as well as Bcl‐2 protein expression in these cells (Fig. 2G).
The treatment of volasertib at different time points also showed Bax protein conformational changes at 8 h in both PTC cell lines (Fig. 2H).
We additionally overexpressed PLK1 in Nthy‐ori 3–1 and evaluated the cell growth by clonogenic assay. As shown in Fig. S1A,B, forced expression of PLK1 significantly increased cell growth. In addition, we examined whether overexpression of PLK1 affects the distribution of cells in the different cell cycle phases (Fig. S1C). We noted a substantial rise in the proportion of cells residing in the S phase, which is the most actively proliferative phase, in Nthy‐ori 3–1 cells with PLK1 overexpression. These results indicate that the upregulation of PLK1 increases the cell proliferation in vitro. On the contrary, the knockdown of PLK1 significantly decreased the cell growth in BCPAP and TPC‐1 cells (Fig. S1D,E).
3.5. PLK1 interacts directly with FoxM1 in vitro
Our clinical data revealed a significant correlation between PLK1 and FoxM1 in PTC cases (Table 2). To investigate this interaction in vitro, we initially evaluated the expression of PLK1 and FoxM1 in Nthy‐ori 3–1, BCPAP, TPC‐1, and K1 cell lines by western blotting. We found associated expression of PLK1 and FoxM1 in two PTC cell lines (BCPAP and TPC‐1), whereas cells with low or negligible expression of PLK1 showed low expression of FoxM1 (Fig. 3A). The physical interaction between PLK1 and FoxM1 was examined by immunoprecipitation (IP) analysis in vitro. The result shows that PLK1 binds to FoxM1 (Fig. 3B) and vice versa (Fig. 3C) in PTC cell lines.
Fig. 3.
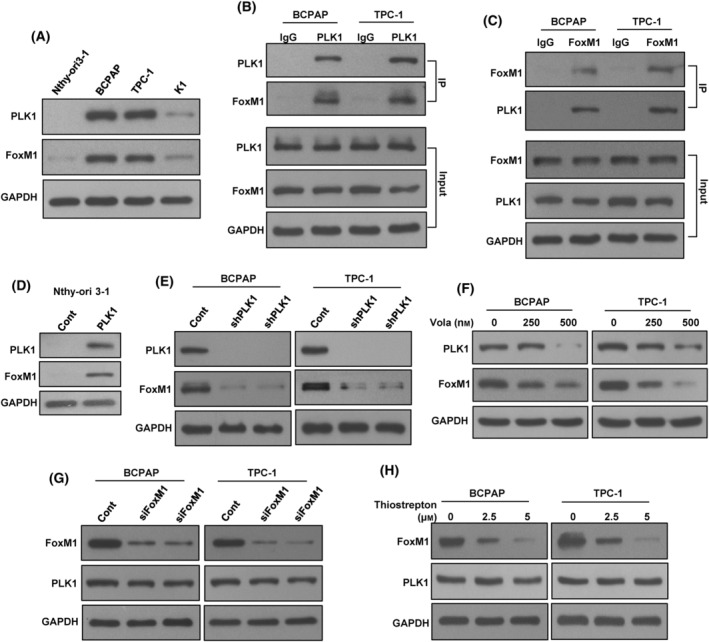
PLK1 interacts with FoxM1 in vitro. (A) Basal expression of PLK1 and FoxM1 in PTC cell lines. Proteins were isolated from Nthy‐ori 3–1 cell line and three PTC cell lines and immunoblotted with antibodies against PLK1, FoxM1, and GAPDH (n = 3). (B) PLK1 interact with FoxM1. Cell lysates extracted from PTC cells were immunoprecipitated with PLK1 or IgG antibody. Interaction of endogenous PLK1 and FoxM1 was detected by immunoblotting (n = 3). (C) FoxM1 interact with PLK1. Cell lysates extracted from PTC cells were immunoprecipitated with FoxM1 or IgG antibody. Interaction of endogenous FoxM1 and PLK1 was detected by immunoblotting (n = 3). (D) Forced expression of PLK1 triggers the activation of FoxM1 expression. Nthy‐ori 3–1 cells were transfected with either an empty vector or PLK1 cDNA for 48 h. Proteins were extracted from overexpression clones and were subjected immunoblotting using antibodies as indicated (n = 3). (E) Knockdown of PLK1 inhibits FoxM1. PTC cells underwent transfection with two distinct PLK1 shRNA sequences, and proteins from the selected clones were later analyzed using immunoblotting (n = 3). (F) Volasertib treatment reduce the expression of PLK1 and FoxM1 in PTC cells. PTC cells were treated with specified doses of volasertib for 48 h. Proteins were extracted after cell lysis and were subjected to immunoblotting, as indicated (n = 3). (G, H) Inhibition of FoxM1 has no effect on PLK1 expression. PTC cells were either transfected with FoxM1 siRNA (20 nm) or treated with thiostrepton (2.5 and 5 μm). Following 48 h, the cells were lysed, and the extracted proteins were subsequently subjected to immunoblotting using antibodies as indicated (n = 3).
To confirm the PLK1 and FoxM1 association in vitro, we stably overexpressed PLK1 in Nthy‐ori 3–1 cell line and examined the FoxM1 expression. As shown in Fig. 3D, forced expression of PLK1 dramatically increased the FoxM1 expression in this cell line. Next, we stably knockdown PLK1 using two separate sequences of shRNA's in both BCPAP and TPC‐1 cells and analyzed PLK1 and FoxM1 expression. Knockdown of PLK1 markedly down‐regulated the expression of FoxM1 in these cells (Fig. 3E). Similar results were observed when PLK1 was inhibited using specific PLK1 inhibitor, volasertib (Fig. 3F). We also inhibited FoxM1 expression using specific siRNA or inhibitor to see the effect on PLK1 expression. As shown in Fig. 3G,H, inhibition of FoxM1 using either siRNA or specific inhibitor, thiostrepton successfully down‐regulated the expression of FoxM1, whereas PLK1 expression remained unchanged. All these results indicate the association of PLK1 with FoxM1 in vitro.
3.6. Combined inhibition of PLK1 and FoxM1 markedly attenuates PTC cell growth in vitro
We showed a strong association between PLK1 and FoxM1 protein expression in PTC cell lines and patient tissues. Therefore, we assume that combined inhibition of PLK1 and FoxM1 expression could induce an efficient cytotoxic effect in PTC cells. The combination of varying doses of volasertib with sub‐optimal dose of thiostrepton exhibited a synergistic reduction in cell viability in PTC (Fig. S2). Using calcusyn software [34], we determined that in the BCPAP cell line, a concentration of 250 nm volasertib combined with 2.5 μm thiostrepton resulted in a combination index (CI) of 0.673, indicating synergy (Fig. S3). In the TPC‐1 cell line, the same combination had a CI of 0.624, also indicating synergy in inhibiting cell viability (Fig. S4). We selected these specific doses for further experimental investigations. We showed that combination of volasertib and thiostrepton significantly (P < 0.05) decreased the colony number of PTC cells as compared to treatment alone (Fig. 4A,B). Furthermore, combination of volasertib and thiostrepton synergistically potentiated apoptosis in PTC cells (Fig. 4C). As shown in Fig. 4D, the combination of volasertib and thiostrepton also induced the cleavage of PARP and caspase‐3 as well as reduced the expression of anti‐apoptotic proteins, Bcl‐2 and Bcl‐xL in these cells.
Fig. 4.
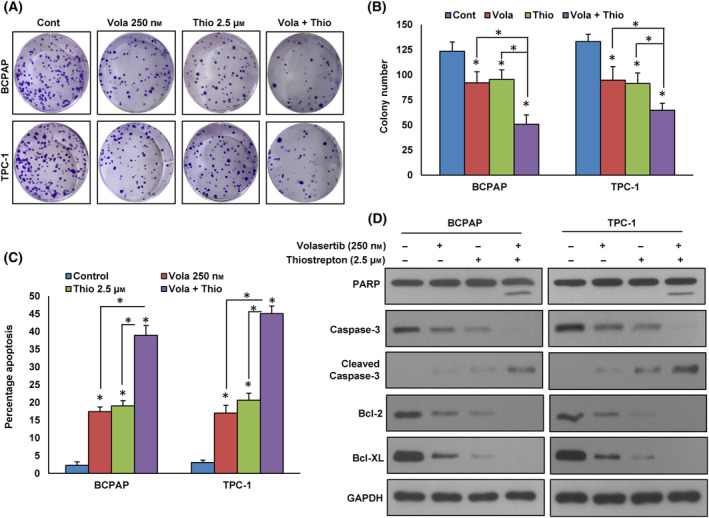
Combined inhibition of PLK1 and FoxM1 markedly attenuates PTC cell growth in vitro. (A–B) Volasertib and thiostrepton synergistically inhibits clonogenicity. PTC cells (5 × 102) after volasertib and thiostrepton treatments were seeded into each of three dishes (60 mm diameter), and grown for an additional 10 days, then stained with crystal violet and colonies were counted. Data were presented as mean ± SD (n = 3). Statistical analyses were performed using two‐tailed Student's t‐tests. *P < 0.05. (C) Volasertib and thiostrepton synergistically induce apoptosis. PTC cells were exposed to specified doses either of volasertib and thiostrepton, single or in combination, for 48 h. Subsequently, these cells were stained with annexin‐V/PI and analyzed by flow cytometry. Data were presented as mean ± SD (n = 3). Statistical analyses were performed using two‐tailed Student's t‐tests. *P < 0.05. (D) Volasertib and thiostrepton synergistically induce the cleavage of caspase‐3 and PARP. PTC cells after 48 h of indicated treatment, lysed and proteins were subjected to immunoblot analysis using antibodies as indicated (n = 3).
3.7. Inhibition of PLK1 decreases the self‐renewal potential of spheroids formed from PTC cells
A recent study demonstrates that PLK1 plays a critical role in maintaining cancer stemness [35]. To explore the role of PLK1 in spheroid growth in PTC, we established stable PLK1 knockdown PTC cell lines and cultured them in spheroid medium. Intriguingly, the knockdown of PLK1 led to a significant reduction in spheroid growth (Fig. 5A,B) and a downregulation in the expression of stem cell markers such as CD133, CD44, and NANOG (Fig. 5C). Moreover, the forced expression of PLK1 in the Nthy‐ori 3–1 cell line resulted in enhanced spheroid growth (Fig. 5D) and an increased expression of PLK1, FoxM1, CD44, CD133, and NANOG, compared to cells transfected with an empty vector (Fig. 5E). Furthermore, our findings demonstrate that the combined inhibition of PLK1 and FoxM1, using volasertib and thiostrepton, respectively, led to a significant reduction in spheroid growth (Fig. 5F) and a decrease in the stemness properties (Fig. 5G) of both PTC cell lines.
Fig. 5.
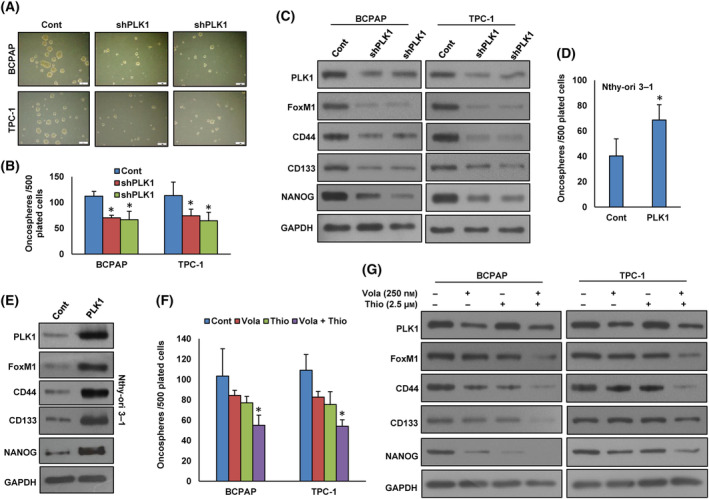
Inhibition of PLK1 decreases the self‐renewal ability of spheroids generated from PTC cells. (A, B) Knockdown of PLK1 impairs the self‐renewal capacity of spheroids. PTC cells were transfected with PLK1 shRNA and subsequently subjected to a sphere‐forming assay (scale bar = 1 mm). The total number of spheroids in the entire well was quantified. Data were presented as mean ± SD (n = 3). (C) Knockdown of PLK1 reduces the stem cell characteristics of spheroids. PTC cells were transfected with PLK1 shRNA and cultured in a sphere‐forming medium. Subsequently, proteins were extracted from the spheroids and analyzed via immunoblotting using antibodies as indicated (n = 3). (D) Forced PLK1 expression enhances the growth of spheroids. Nthy‐ori 3–1 cells were transfected with either an empty vector or PLK1 cDNA, and subsequently, these cells were subjected to a sphere‐forming assay. The total number of spheroids in the entire dish was quantified. Data were presented as mean ± SD (n = 3). (E) Forced expression of PLK1 enhances the stem cell properties of spheroids, as validated through immunoblotting employing stem cell markers. Nthy‐ori 3–1 cells were transfected with either an empty vector or PLK1 cDNA and subsequently cultured in a sphere‐forming medium. Proteins were isolated from spheroids and immunoblotted with antibodies against PLK1, FoxM1, CD44, CD133, NANOG, and GAPDH (n = 3). (F) Volasertib and thiostrepton synergistically reduces spheroid growth. PTC cells were exposed to specified doses of volasertib and thiostrepton, either single or in combination, for 48 h. Following this treatment, the cells were subjected to a sphere‐forming assay. Spheroids in the entire dish were counted. Data were presented as mean ± SD (n = 3). (G) PTC cells were exposed to specified doses either of volasertib and thiostrepton, single or in combination, for 48 h. Subsequently, these treated cells were cultured in a sphere‐forming medium and proteins isolated from spheroids were subjected to immunoblotting using antibodies as indicated (n = 3). Statistical analyses were performed using two‐tailed Student's t‐tests. *P < 0.05.
3.8. Combined inhibition of PLK1 and FoxM1 delays PTC tumor growth in vivo
We showed that synergistic inhibition of PLK1 and FoxM1 significantly suppressed PTC cell growth in vitro. We also investigated whether the combined administration of volasertib and thiostrepton could delay the growth of PTC tumors in NU/J mice. In the xenograft study, we inoculated TPC‐1 cells (4 × 106 cells per mouse) into the flanks of 6‐week‐old female NU/J mice. After the tumors had developed (about 100 mm3), the mice were injected (i.p) with volasertib (20 mg·kg−1) and thiostrepton (20 mg·kg−1) either alone or in combination, twice a week for 30 days. DMSO (0.1%, i.p) was served as vehicle control. We found that co‐treatment of volasertib with thiostrepton delayed tumor growth as shown by reduced tumor size and volume (Fig. 6A,B) as well as tumor weight (Fig. 6C) but had no effect on the body weight of mice (data not shown). Further, co‐treatment of volasertib with thiostrepton markedly suppressed the expression of PLK1, FoxM1 and induced cleavage of PARP and caspase‐3 as well as downregulation of Bcl‐2 and Bcl‐xL expression in tumor tissues (Fig. 6D,E). These data clearly demonstrate that combined inhibition of PLK1 and FoxM1 augment antitumor effects in TPC‐1 cell xenografts in NU/J mice.
Fig. 6.
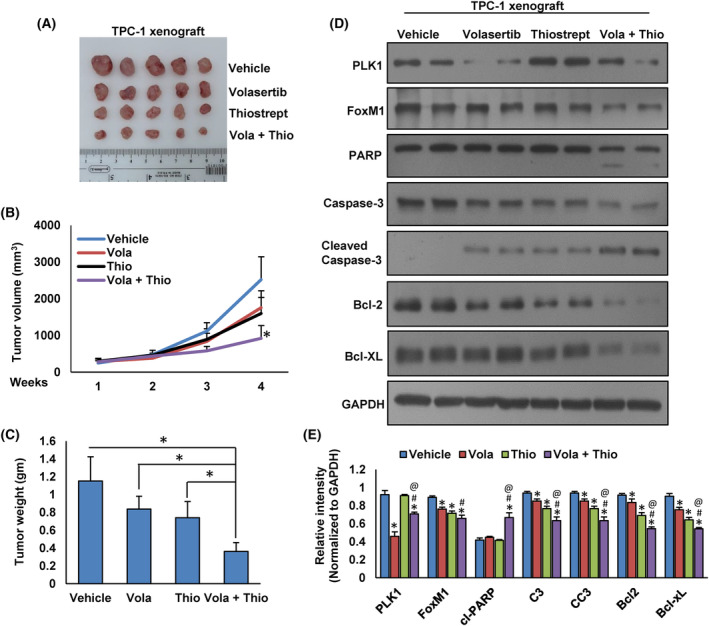
Volasertib and thiostrepton synergistically inhibit PTC tumor growth in vivo. TPC‐1 cells were subcutaneously injected into the flanks of 6‐week‐old NU/J mice (4 × 106 cells per mouse). Once the tumors reached approximately 100 mm3 in size, the mice were subjected to intraperitoneal treatment with the specified doses of volasertib (20 mg·kg−1) and thiostrepton (20 mg·kg−1), either administered individually or in combination. This treatment regimen was repeated twice a week and continued for a duration of 30 days. (A) Representative images of tumors from each group of mice. (B) The tumor volume measurements were taken every week. The average (n = 5) tumor volume (±SD) in each group of mice was calculated, *P < 0.05. (C) Following 4 weeks of treatment, the mice were euthanized, and the mean tumor weight (±SD) was determined for each group, *P < 0.05. (D) Tissue lysates from tumors were immuno‐blotted with antibodies against PLK1, FoxM1, PARP, Caspase‐3, Cleaved‐caspase‐3, Bcl‐2, Bcl‐xL, and GAPDH. (E) Western blots were quantified and data are the mean ± SD (n = 3). Statistical analyses were performed using two‐tailed Student's t‐tests. *Statistically significant difference compared to vehicle control with P < 0.05. #Statistically significant difference compared to volasertib treatment with P < 0.05. @Statistically significant difference compared to thiostrepton treatment with P < 0.05.
4. Discussion
PLK1 dysregulation has been reported in different tumor types and its overexpression has been associated poor patients' outcome, resistance to chemotherapy and radiotherapy [11, 36, 37, 38]. However, the role of PLK1 in PTC pathogenesis is unclear. In this study, we reveal that PLK1 is highly expressed in 54.2% of PTC tumor sample (874/1612). Elevated PLK1 expression has been notably correlated with several aggressive clinicopathological factors, including extrathyroidal extension, advanced stage, lymph node metastasis, and tumor recurrence.
Interestingly, PLK1 overexpression was associated with important cell regulator FoxM1 which has been shown previously by us [18] and others [39] to play an important role in Middle Eastern PTC aggressiveness. However, after adjusting for clinical factor, a multivariant COX proportional hazard model indicated that only PLK1 is able independently to serve as independent marker for recurrence free survival.
Inhibition of PLK1 in PTC cell lines with volasertib caused G2/M phase arrest and mitotic block. Our study provides several lines of evidence supporting the oncogenic role of PLK1 in PTC. First, inhibition of PLK1 reduces PTC cell growth in vitro and in vivo. Second, our in vitro data show a potential role of PLK1 in regulation of cancer‐stem cell‐like cells in PTC. Inhibition of PLK1 significantly suppressed the spheroid growth and downregulated the expression of stem cell markers such as CD44, CD133, NANOG in PTC. This shows that PLK1 might have a potential role in maintaining stemness properties in PTC.
The significant association between PLK1 and FoxM1 overexpression in the study cohort is not surprising. PLK1 and FoxM1 overexpression has been observed in a variety of several types of cancer [16, 40, 41]. However, their relationship within PTC had not fully illustrated. Here, we have demonstrated that both PLK1 and FoxM1 are overexpressed in PTC samples and cell lines. Our research extends to targeting two interrelated and mutually reinforcing pathways (PLK1 and FoxM1) within PTC cells, aiming to achieve a more effective anti‐proliferative response. The concurrent inhibition of these pivotal pathways has not been previously explored in PTC. In our study, we combined volasertib and thiostrepton in PTC cell lines, resulting in a synergistic anti‐proliferative effect and inhibition of cell growth observed in both in vitro and in vivo experiments.
Another interesting finding of this study is the identification of regulatory relationship between PLK1 and FoxM1 in PTC. Our data show that PLK1 co‐immunoprecipitated with FoxM1 in PTC. In addition, ectopic expression of PLK1 increase FoxM1 expression whereas PLK1 inhibition decrease FoxM1 expression. Interestingly, FoxM1 inhibition has no effect on PLK1 expression, suggesting that PLK1 functions via FoxM1, which is in line with previous studies, where PLK1 positively regulates FoxM1 in different tumor types [16, 39]. The above finding suggests the importance of using therapeutic interventions that focuses on targeting PLK1‐FoxM1 mediated signaling network.
The strategy of combining therapeutic targets may offer enhanced efficacy, particularly in specific aggressive subtypes of thyroid cancer. Our study places emphasis on targeting the intertwined pathways (PLK1 and FoxM1) in PTC, with the goal of achieving a superior anti‐proliferative response. In PTC cell lines, we observed a synergistic anti‐proliferative effect when inhibiting both PLK1 and FoxM1, leading to reduced cell viability and the induction of apoptosis. These results were further supported by in vivo studies where the combination of both volasertib and thiostrepton therapy significantly delayed xenograft tumor growth when compare with either single agent or control treatment.
5. Conclusion
Taken together, our study showed that PLK1 plays an important prognostic role in Middle Eastern PTC. We also found promising therapeutic effect of volasertib and thiostrepton combination in the treatment of PTC tumors, in particular, the subset of patient that overexpress FoxM1. These findings can potentially expand the clinical applications of volasertib in the treatment of PTC. The studies involving combination with FoxM1 inhibitor hold significant translational potential, paving the way for novel therapeutic approaches in the treatment of thyroid cancer.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
PKP and AKS designed, performed experiments, and wrote the manuscript. SKP prepared the TMA and conducted all the immunohistochemistry experiments and scoring of IHC spots. DP, ST, KA, RD, RB, and WH performed experiments. FA‐D and SSA‐S collected and analyzed all the clinical samples and data. KSA‐K made substantial contributions to conception, design, and acquisition of data along with analysis and interpretation of data; prepared and wrote the manuscript. KSA‐K gave the final approval for the submission of the manuscript. This is to confirm that all authors read and approved the final manuscript.
Peer review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/1878‐0261.13610.
Supporting information
Fig. S1. PLK1 promotes cell growth in vitro.
Fig. S2. Synergistic effect of volasertib and thiostrepton on PTC cell viability.
Fig. S3. Synergistic inhibition of cell viability by volasertib and thiostrepton in BCPAP cells.
Fig. S4. Synergistic inhibition of cell viability by volasertib and thiostrepton in TPC‐1 cells.
Acknowledgements
This study was not supported by any funding agency; therefore, the authors declare that there is no funding information to be disclosed for this manuscript.
Pratheesh Kumar Poyil and Abdul K. Siraj contributed equally to this article
Data accessibility
All data generated or analyzed during this study are included in this published article.
References
- 1. O'Sullivan B, Burton T, Ziad F, Meyer Rochow G. Papillary thyroid cancer metastases to the parathyroid gland. Clin Pathol. 2022;15:2632010X221134569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Carvalho AY, Kohler HF, Gomes CC, Vartanian JG, Kowalski LP. Predictive factors for recurrence of papillary thyroid carcinoma: analysis of 4,085 patients. Acta Otorhinolaryngol Ital. 2021;41:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr FA, Silljé HH, Nigg EA. Polo‐like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–441. [DOI] [PubMed] [Google Scholar]
- 4. Cholewa BD, Liu X, Ahmad N. The role of polo‐like kinase 1 in carcinogenesis: cause or consequence? Plk1 in carcinogenesis. Cancer Res. 2013;73:6848–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. González‐Martínez J, Cwetsch AW, Gilabert‐Juan J, Gómez J, Garaulet G, Schneider P, et al. Genetic interaction between PLK1 and downstream MCPH proteins in the control of centrosome asymmetry and cell fate during neural progenitor division. Cell Death Differ. 2022;29:1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Martino D, Yilmaz E, Orlacchio A, Ranieri M, Zhao K, Di Cristofano A. PI3K blockage synergizes with PLK1 inhibition preventing endoreduplication and enhancing apoptosis in anaplastic thyroid cancer. Cancer Lett. 2018;439:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dang S‐C, Fan Y‐Y, Cui L, Chen J‐X, Qu J‐G, Gu M. PLK1 as a potential prognostic marker of gastric cancer through MEK‐ERK pathway on PDTX models. Onco Targets Ther. 2018;11:6239–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maire V, Némati F, Richardson M, Vincent‐Salomon A, Tesson B, Rigaill G, et al. Polo‐like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple‐negative breast cancer. Cancer Res. 2013;73:813–823. [DOI] [PubMed] [Google Scholar]
- 9. Ramani P, Nash R, Sowa‐Avugrah E, Rogers C. High levels of polo‐like kinase 1 and phosphorylated translationally controlled tumor protein indicate poor prognosis in neuroblastomas. J Neurooncol. 2015;125:103–111. [DOI] [PubMed] [Google Scholar]
- 10. Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Köbel M, et al. Polo‐like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Z, Sun Q, Wang X. PLK1, a potential target for cancer therapy. Transl Oncol. 2017;10:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu Y‐L, Wu M‐H, Lee Y‐Y, Chou T‐C, Wong RJ, Lin S‐F. Efficacy and biomarker analysis of adavosertib in differentiated thyroid cancer. Cancers (Basel). 2021;13:3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Yuan C, Wu J, Elsayed Z, Fu Z. Polo‐like kinase 1‐mediated phosphorylation of Forkhead box protein M1b antagonizes its SUMOylation and facilitates its mitotic function. J Biol Chem. 2015;290:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y‐J, Dominguez‐Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, et al. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin‐CDK1. J Biol Chem. 2009;284:30695–30707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu Z, Malureanu L, Huang J, Wang W, Li H, Van Deursen JM, et al. Plk1‐dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed M, Hussain AR, Siraj AK, Uddin S, Al‐Sanea N, Al‐Dayel F, et al. Co‐targeting of cyclooxygenase‐2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol Cancer. 2015;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmed M, Uddin S, Hussain AR, Alyan A, Jehan Z, Al‐Dayel F, et al. FoxM1 and its association with matrix metalloproteinases (MMP) signaling pathway in papillary thyroid carcinoma. J Clin Endocrinol Metabol. 2012;97:E1–E13. [DOI] [PubMed] [Google Scholar]
- 19. Siraj AK, Pratheeshkumar P, Parvathareddy SK, Qadri Z, Thangavel S, Ahmed S, et al. FoxM1 is an independent poor prognostic marker and therapeutic target for advanced Middle Eastern breast cancer. Oncotarget. 2018;9:17466–17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahn H, Sim J, Abdul R, Chung MS, Paik SS, Oh Y‐H, et al. Increased expression of forkhead box M1 is associated with aggressive phenotype and poor prognosis in estrogen receptor‐positive breast cancer. J Korean Med Sci. 2015;30:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bai C, Liu X, Qiu C, Zheng J. FoxM1 is regulated by both HIF‐1α and HIF‐2α and contributes to gastrointestinal stromal tumor progression. Gastric Cancer. 2019;22:91–103. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Barger CJ, Karpf AR. FOXM1: a multifunctional oncoprotein and emerging therapeutic target in ovarian cancer. Cancers (Basel). 2021;13:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tassi RA, Todeschini P, Siegel ER, Calza S, Cappella P, Ardighieri L, et al. FOXM1 expression is significantly associated with chemotherapy resistance and adverse prognosis in non‐serous epithelial ovarian cancer patients. J Exp Clin Cancer Res. 2017;36:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wen N, Wang Y, Wen L, Zhao S‐H, Ai Z‐H, Wang Y, et al. Overexpression of FOXM1 predicts poor prognosis and promotes cancer cell proliferation, migration and invasion in epithelial ovarian cancer. J Transl Med. 2014;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 26. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al‐Rasheed M, et al. Telomerase reverse transcriptase mutations are independent predictor of disease‐free survival in Middle Eastern papillary thyroid cancer. Int J Cancer. 2018;142:2028–2039. [DOI] [PubMed] [Google Scholar]
- 27. Parvathareddy SK, Siraj AK, Iqbal K, Qadri Z, Ahmed SO, Al‐Rasheed M, et al. TERT promoter mutations are an independent predictor of distant metastasis in Middle Eastern papillary thyroid microcarcinoma. Front Endocrinol. 2022;13:808298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al‐Sobhi SS, Al‐Dayel F, et al. PD‐L1 is an independent prognostic marker in Middle Eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancer. 2021;13:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siraj A, Bavi P, Abubaker J, Jehan Z, Sultana M, Al‐Dayel F, et al. Genome‐wide expression analysis of Middle Eastern papillary thyroid cancer reveals c‐MET as a novel target for cancer therapy. J Pathol. 2007;213:190–199. [DOI] [PubMed] [Google Scholar]
- 30. Bavi P, Jehan Z, Atizado V, Al‐Dossari H, Al‐Dayel F, Tulbah A, et al. Prevalence of fragile histidine triad expression in tumors from Saudi Arabia: a tissue microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1708–1718. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Wang H, Sun Z, Guo Q, Shi H, Jia Y. The clinical and prognostic value of polo‐like kinase 1 in lung squamous cell carcinoma patients: immunohistochemical analysis. Biosci Rep. 2017;37:BSR20170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camp RL, Dolled‐Filhart M, Rimm DL. X‐tile: a new bio‐informatics tool for biomarker assessment and outcome‐based cut‐point optimization. Clin Cancer Res. 2004;10:7252–7259. [DOI] [PubMed] [Google Scholar]
- 33. Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chou T‐C, Talalay P. Quantitative analysis of dose‐effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Chen G, Liu B, Tao Z, Wu Y, Zhang K, et al. PLK1 inhibition promotes apoptosis and DNA damage in glioma stem cells by regulating the nuclear translocation of YBX1. Cell Death Discov. 2023;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagege A, Ambrosetti D, Boyer J, Bozec A, Doyen J, Chamorey E, et al. The Polo‐like kinase 1 inhibitor onvansertib represents a relevant treatment for head and neck squamous cell carcinoma resistant to cisplatin and radiotherapy. Theranostics. 2021;11:9571–9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rödel F, Keppner S, Capalbo G, Bashary R, Kaufmann M, Rödel C, et al. Polo‐like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010;177:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu L, Lang Y, Hsu C‐C, Chen W‐M, Chiang J‐C, Hsieh J‐T, et al. Mitotic phosphorylation of tumor suppressor DAB2IP maintains spindle assembly checkpoint and chromosomal stability through activating PLK1‐Mps1 signal pathway and stabilizing mitotic checkpoint complex. Oncogene. 2022;41:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Z, Zhang G, Kong C. FOXM1 participates in PLK1‐regulated cell cycle progression in renal cell cancer cells. Oncol Lett. 2016;11:2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dibb M, Han N, Choudhury J, Hayes S, Valentine H, West C, et al. The FOXM1‐PLK1 axis is commonly upregulated in oesophageal adenocarcinoma. Br J Cancer. 2012;107:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fan W, Ma H, Jin B. Expression of FOXM1 and PLK1 predicts prognosis of patients with hepatocellular carcinoma. Oncol Lett. 2022;23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. PLK1 promotes cell growth in vitro.
Fig. S2. Synergistic effect of volasertib and thiostrepton on PTC cell viability.
Fig. S3. Synergistic inhibition of cell viability by volasertib and thiostrepton in BCPAP cells.
Fig. S4. Synergistic inhibition of cell viability by volasertib and thiostrepton in TPC‐1 cells.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


