Abstract
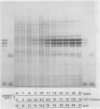
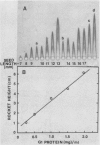
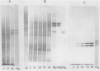
Analysis of total protein, of specific proteins by gel electrophoresis and immunoelectrophoresis, and of protein synthetic activity in vitro confirmed that intense protein synthesis and accumulation occurred as the French bean (Phaseolus vulgaris L). seed grew from 12 to 20 millimeters. These techniques showed that there was no globulin-1 (G1) fraction (requiring high salt for solubility) present in 6-millimeter seeds, and only very small amounts were synthesized in seeds less than 9 millimeters long. The 7- to 9-millimeter stages represent a 2-day transition period over which genetic information for the G1 protein becomes actively expressed, accounting for at least 50% of all protein synthesized in this tissue during the following 14 days. At maturity, the electrophoretic analysis confirmed that G1 globulin was the major storage protein, representing some 50% of the dry seed protein. Cell-free protein synthesis assays, including immunoprecipitation of the in vitro products, clearly showed G1 polypeptides to be among the polysome-directed products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R. C. THE AFTER RESULTS OF CATARACT EXTRACTION. Br J Ophthalmol. 1928 Feb;12(2):85–93. doi: 10.1136/bjo.12.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., McLeester R. C., Bliss F. A. Equal Expression of the Maternal and Paternal Alleles for the Polypeptide Subunits of the Major Storage Protein of the Bean Phaseolus vulgaris L. Plant Physiol. 1977 Jun;59(6):1122–1124. doi: 10.1104/pp.59.6.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Loewenberg J. R. The Development of Bean Seeds (Phaseolus vulgaris L.). Plant Physiol. 1955 May;30(3):244–250. doi: 10.1104/pp.30.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeester R. C., Hall T. C. Simplification of amino acid incorporation and other assays using filter paper techniques. Anal Biochem. 1977 May 1;79(1-2):627–630. doi: 10.1016/0003-2697(77)90447-x. [DOI] [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Romero J., Sun S. M., McLeester R. C., Bliss F. A., Hall T. C. Heritable Variation in a Polypeptide Subunit of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):776–779. doi: 10.1104/pp.56.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Buchbinder B. U., Hall T. C. Cell-free Synthesis of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):780–785. doi: 10.1104/pp.56.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. M., Hall T. C. Solubility characteristics of globulins from Phaseolus seeds in regard to their isolation and characterization. J Agric Food Chem. 1975 Mar-Apr;23(2):184–189. doi: 10.1021/jf60198a004. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]