Prosthetic joint infection (PJI) of the shoulder is a very uncommon complication after shoulder arthroplasty, with rates in the literature ranging from 0.5-6.7%.1,4, 5, 6, 7,9 In 2018, the International Consensus Meeting on Musculoskeletal infection established guidelines for the diagnosis and management of shoulder PJI.3 These guidelines divided shoulder PJI into one of four categories: definite infection, probable infection, possible infection, and unlikely infection. One criterion that defines a definite infection is the presence of a sinus tract from the skin surface to the prosthesis. Currently, there is little in the literature describing the nature and location of shoulder sinus tracts in connection with PJI. In our experience, sinus tracts associated with PJI are not always located in the peri-incisional area, but instead can be located remotely. This can delay prompt diagnosis of PJI. The purpose of this paper is to present a case series of shoulder PJIs in which the diagnosis was delayed as a result of atypical sinus tracts to the axilla and/or chest wall.
Case 1
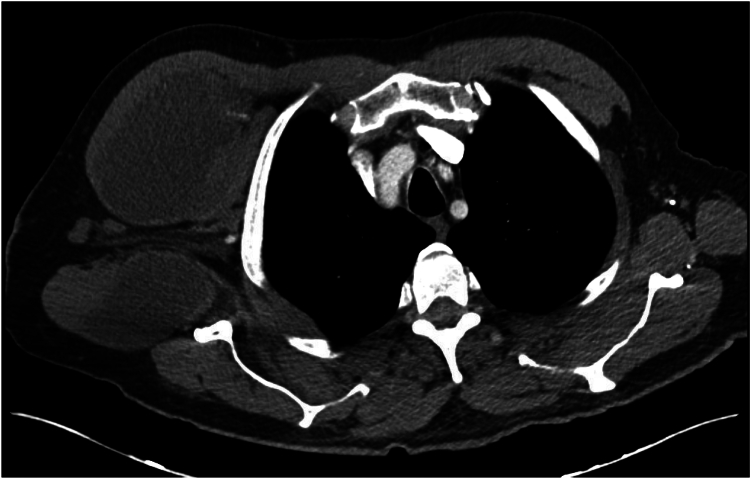
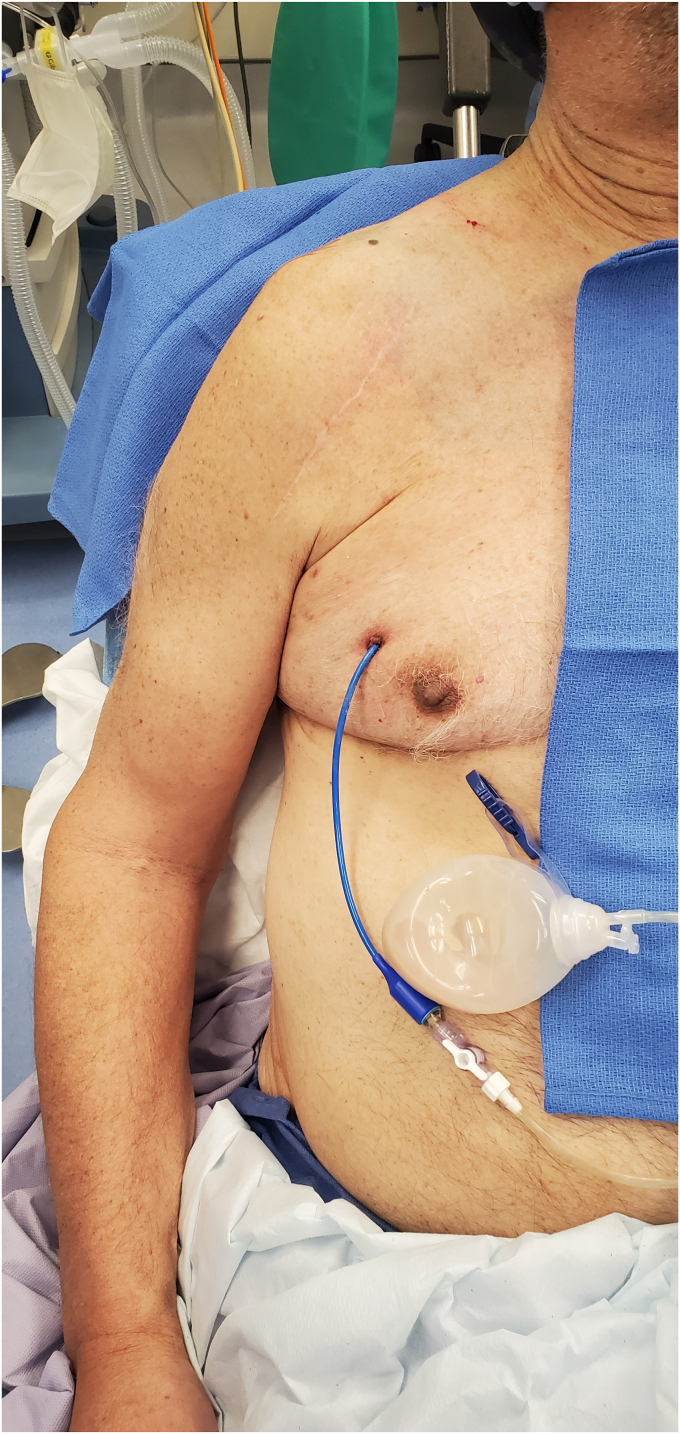
A 74 year-old male with a diagnosis of primary glenohumeral osteoarthritis underwent an uncomplicated right reverse shoulder arthroplasty (RSA) in 2019. He has a history of right shoulder arthroscopic débridement and capsular release 11 months prior to RSA. He presented to the emergency department 3 years after RSA with systemic complains of fevers, chills, and cough. A computed tomography (CT) scan of the chest was performed, showing a large subcutaneous fluid-filled lesion in the right chest wall (Fig. 1). He underwent interventional radiology (IR)-guided drainage with 480 cc of purulent material aspirated and a drain was placed. Cultures from the sample grew Staphylococcus haemolyticus. Infectious disease was consulted and he was placed on intravenous (IV) antibiotics. He followed up periodically with the IR department and underwent sclerotherapy of the drainage catheter multiple times with betadine. Due to a persistent fluid collection after 3 months, he was referred to an orthopedic surgeon. On exam he was noted to have a drain in the anterior chest wall that was draining approximately 30 cc of purulent fluid each day (Fig. 2). Radiographs of the shoulder showed mild bony resorption of the proximal humerus beneath the humeral tray. It was determined that the chest wall fluid collection likely represented a chronic PJI of the right shoulder. He subsequently underwent two stage revision with explant of the RSA and placement of an antibiotic spacer, 6 weeks of IV antibiotics, repeat negative aspiration, and finally reimplantation of an RSA. He was last seen 9 months postoperatively, doing well with 135 degrees of active forward elevation, 35 degrees of external rotation, internal rotation to the sacrum, and no signs of recurrent infection.
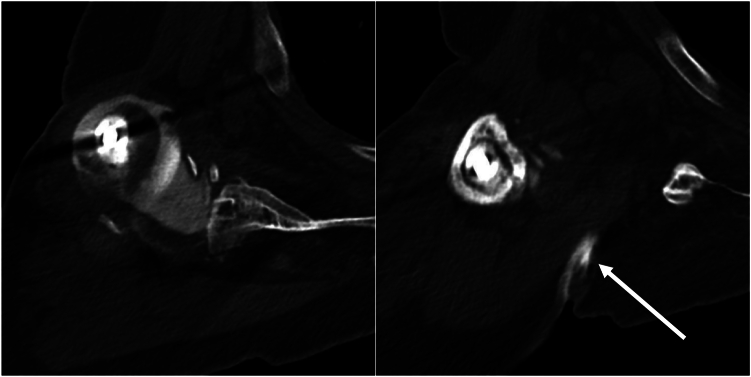
Figure 1.
Case 1. CT scan showing subcutaneous anterior chest wall fluid collection. CT, computed tomography.
Figure 2.
Case 1. Preoperative clinical photo showing anterior chest wall drain with purulent fluid.
Case 2
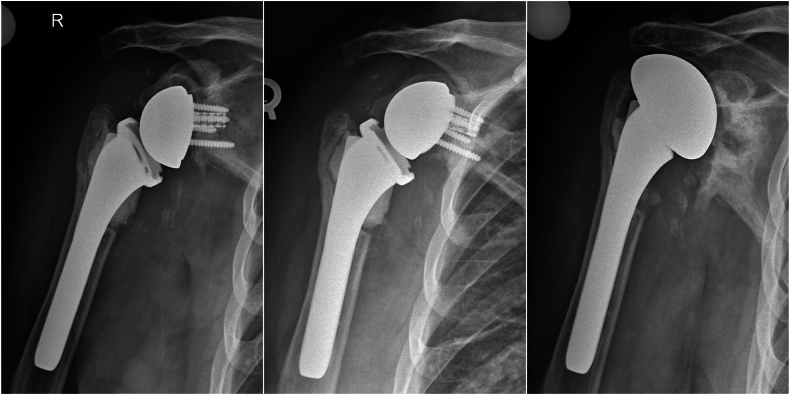
An 80 year-old male with a diagnosis of right primary glenohumeral osteoarthritis underwent an uncomplicated RSA in 2011. There was no history of previous surgery on this shoulder. Of note, he had a history of contralateral shoulder PJI and underwent two stage revision and was fully recovered prior to the current RSA. Five years postoperatively, he developed a large posterior “axillary cyst” that was removed by dermatology as an epidermal cyst. The patient noted that he had hematoma formation and drainage following cyst removal. After repeated treatments and débridements by the dermatologist, the patient decided to return to the orthopedic surgeon. At that time there was concern for possible deeper underlying infection and PJI. The patient underwent IR-guided aspiration of the shoulder with less than 1 cc of fluid aspirated. Extended cultures yielded no growth. At that time, two stage revision surgery was recommended; however, the patient canceled and was lost to follow-up. Two years later, the patient represented to the office with recurrence of the cyst. A magnetic resonance imaging (MRI) scan was obtained, showing a 5.2 cm posterior fluid collection with concern for connection to the glenohumeral joint (Fig. 3). The patient subsequently underwent two stage revision with explantation and antibiotic spacer. Intraoperative cultures were positive for Cutibacterium acnes. After 6 weeks of IV antibiotics followed by a negative repeat aspiration, revision to RSA was performed. Unfortunately, 7 months after revision RSA, the patient presented with 2 months of increasing atraumatic shoulder pain and radiographs revealed superior displacement of the baseplate. The patient subsequently underwent conversion to a hemiarthroplasty (Fig. 4). Cultures from that revision were taken and grew C. acnes and he was treated with 6 weeks of IV antibiotics per infectious disease recommendations. He was last seen 18 months after revision to hemiarthroplasty, noted to be doing well with 150 degrees of active forward elevation, 30 degrees of external rotation, and internal rotation to the sacrum, with no signs of recurrent infection.
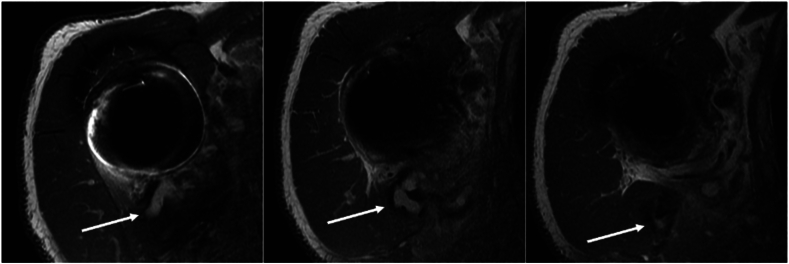
Figure 3.
Case 2. MRI showing communication of a posterior fluid collection to the shoulder joint. MRI, magnetic resonance imaging.
Figure 4.
Case 2. Revision RSA (Left). Displacement of baseplate (Center). Revision to hemiarthroplasty (Right). RSA, reverse shoulder arthroplasty.
Case 3
A 76 year-old male with an initial diagnosis of rotator cuff arthropathy underwent RSA in 2013 following a failed arthroscopic rotator cuff repair. The primary RSA was complicated by infection and was treated with early débridement and implant retention. He subsequently developed a drainage tract at the inferior end of the posterior axilla. His primary care physician referred him to a general surgeon who performed multiple local débridements without improvement. He was then referred to our institution for further evaluation. Findings were consistent with chronic infection of the RSA. He underwent explant, placement of antibiotic spacer, and débridement of the posterior abscess and sinus tract. Cultures were positive for Enterococcus raffinosus and he was treated with 6 weeks of IV antibiotics. Two months after the explantation he was noted to have scant drainage from the posterior wound. A CT sinogram was obtained, showing a 3 cm sinus tract from the posterior skin surface towards the axillary recess but without definite tracking to the glenohumeral joint possibly due to sinus collapse and/or scar tissue (Fig. 5). Following the sinogram the patient did not return for follow-up. He presented 9 months later with increased drainage from the persistent sinus tract. Operative management was recommended and he underwent débridement, excision of the posterior sinus tract and exchange of the antibiotic spacer, followed by a second 6 week course of IV antibiotics. Due to other medical concerns, the patient declined second stage reimplantation. He was last seen 10 months after the second débridement. Physical exam showed 40 degrees of active forward elevation, 20 degrees of external rotation, and internal rotation to the hip. He had no signs of recurrent infection and the posterior axillary sinus tract was noted to be resolved.
Figure 5.
Case 3. CT sinogram showing posterior sinus tract and contrast dye in the glenohumeral joint. CT, computed tomography.
Case 4
An 84 year-old male underwent right RSA in 2014 at an outside institution. He presented to our office 6 years postoperatively for evaluation of a draining wound from the posterior axilla which had been present for several months. During this time he was being treated by a general surgeon with multiple débridements. An MRI was obtained prior to our evaluation which reported fluid extending from the glenohumeral joint to a posterior axillary fluid collection (Fig. 6). Two stage revision surgery was recommended. The patient underwent explant, antibiotic spacer, and débridement of the posterior sinus tract. Cultures were positive for Staphylococcus epidermidis and he was treated with 6 weeks of IV antibiotics. After a period off of antibiotics he underwent reimplantation of RSA. The patient was noted to be doing well 3 months postoperatively with no recurrent signs of infection. He presented again two years later noting a gradual increase in pain. An IR-guided aspiration was obtained with cultures positive for C. acnes. On exam, it was noted that the posterior axillary sinus was no longer present. Two stage revision surgery was recommended. He underwent débridement, explant and antibiotic spacer, 6 weeks of IV antibiotics, and finally revision to RSA. At 5 months postoperatively he was noted to be doing well without signs of recurrent infection. He is being maintained on long term suppressive oral antibiotics suppression as recommended by the infectious disease consultants.
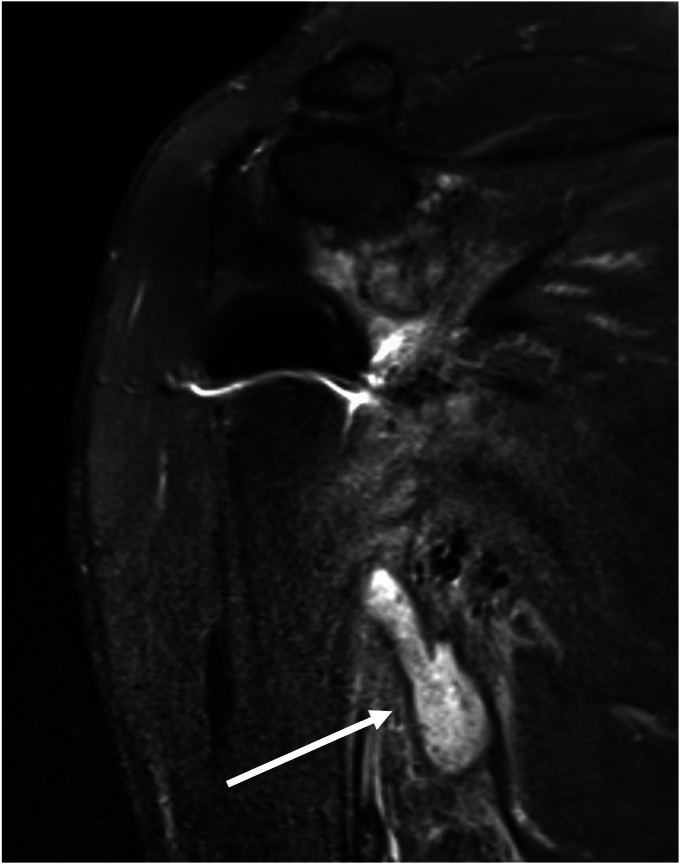
Figure 6.
Case 4. MRI showing posterior sinus tracking from the glenohumeral joint. MRI, magnetic resonance imaging.
Case 5
A 60 year-old female with a diagnosis of rotator cuff arthropathy underwent primary RSA in 2016. She had a history of rotator cuff deficiency with multiple ipsilateral shoulder surgeries including latissimus dorsi transfer and arthroscopic débridement. She had an uneventful initial recovery following RSA. Three years after RSA she presented to an outside urgent care facility after noting a “lump” in the axilla. She was diagnosed with a superficial abscess and underwent local débridement and packing of the wound. Seven months later, she presented to a plastic surgeon due to a persistent axillary wound. She underwent another débridement by the plastic surgeon, at which time they noted a fistula that tracked to the humerus. She was then referred to our facility. Our evaluation showed findings consistent with a chronic PJI. She underwent débridement, explant and antibiotic spacer, along with débridement of the axillary sinus tract. Cultures were positive for Staphylococcus lugdunensis and she was treated with 6 weeks of IV antibiotics. The sinus tract healed. She then underwent reimplantation of the RSA. She did well until 2 years postop when she presented with concern for increasing pain and decreased function. Of note, at that time there was no recurrence of the axillary sinus. Radiographs obtained at that time showed concern for a loose humeral component (Fig. 7) and clinical findings consistent with recurrent infection. She then underwent a repeat two stage revision. Initial cultures were once again positive for S. lugdunensis. At latest follow-up 4 months following the second reimplantation she was noted to be doing well with 160 degrees of active forward elevation, 40 degrees of external rotation, and internal rotation to the lumbar spine, with no signs of recurrent infection. The current plan is to maintain her on long term oral antibiotic suppression.
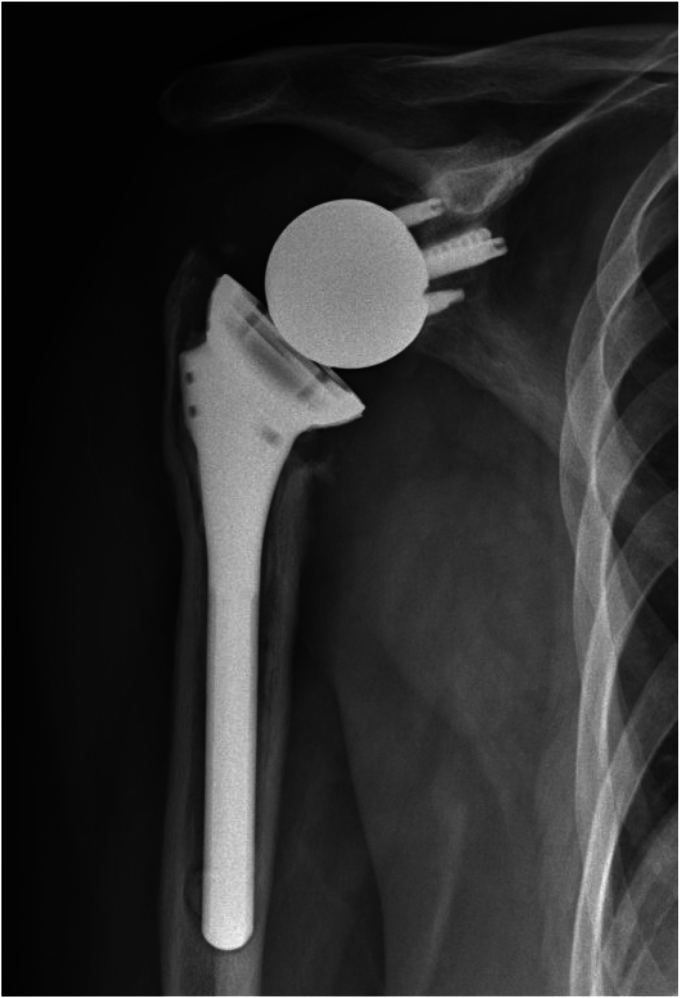
Figure 7.
Case 5. Radiolucencies around the humeral stem, concerning for loosening and possible infection.
Case 6
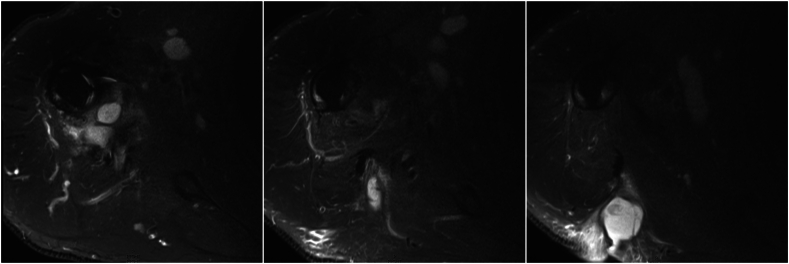
A 72 year-old male with an original diagnosis of primary glenohumeral osteoarthritis underwent anatomic total shoulder replacement in 2020. Unfortunately he sustained subscapularis failure and required revision to RSA 15 months postoperatively. He had an uneventful early recovery. He presented 14 months postop with concern for development of an axillary mass. He was evaluated by both a general and oncologic surgeon. An MRI was obtained, showing a 9 × 2 cm axillary fluid collection with communication to the glenohumeral joint (Fig. 8). IR-guided aspiration was obtained with cultures positive for C. acnes. We recommended two stage revision with 6 weeks of IV antibiotics which was performed. His latest follow-up was 3 months postoperatively and he was noted to be recovering well without signs of recurrent infection.
Figure 8.
Case 6. MRI showing axillary fluid collection. MRI, magnetic resonance imaging.
Discussion
Although PJI following shoulder arthroplasty is a well-known complication, the possibility of atypical presentation with sinus tracts remote to the shoulder area and axillary masses that can confuse and delay the diagnosis has not been emphasized. Given the location in the axilla and/or chest wall, patients in this series frequently presented to physicians other than orthopedic surgeons, not realizing that the axillary mass may be related to the previous shoulder replacement. This lack of recognition was often compounded but the treating physician who also did not consider a relationship between the soft tissue problem and the previous shoulder arthroplasty. This led to multiple treatments of the isolated soft tissue problem which ultimately delayed definitive diagnosis and treatment. Successful treatment of PJI can be difficult to achieve and delays in diagnosis make treatment even more challenging.
This series of 6 patients emphasizes the importance of considering PJI for any patient who presents with an axillary mass and/or sinus tract and a history of shoulder arthroplasty, even if the sinus tract is remote to the shoulder as in our patient with a chest wall location. Workup may include advanced imaging such as CT scan or MRI with metal reduction sequences.2 If there is a fistula present to the skin surface, a CT sinogram can be performed. Ultrasound may also be used and has the benefit of being able to track the location of a sinus tract in real time.8 Of course, definitive diagnosis of the PJI is essential to initiate treatment. As this case series, shows successful treatment with two stage revision is not assured and recurrence was common.
Conclusions
Ultimately, the goal of our series is to provide awareness that an axillary and/or chest wall fluid collection, with or without a sinus tract to the skin surface, in a patient with a history of shoulder arthroplasty may be a sign of underlying PJI. These patients should be under the care of an orthopedic surgeon experienced in caring for patients with shoulder PJI and undergo appropriate diagnostic evaluation and management.
Disclaimers
Funding: No outside funding or grant monies were used in the execution of this study or in preparation of this manuscript.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.Patient consent: Consent was obtained for publication.
Footnotes
This study was approved by the NYU Langone IRB. Study #i21-01089.Investigation performed at NYU Langone Department of Orthopedic Surgery, New York, NY, USA.
References
- 1.Contreras E.S., Frantz T.L., Bishop J.Y., Cvetanovich G.L. Periprosthetic infection after reverse shoulder arthroplasty: a review. Curr Rev Musculoskelet Med. 2020;13:757–768. doi: 10.1007/s12178-020-09670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrigues G.E., Zmistowski B., Cooper A.M., Green A., Abboud J., Beazley J., et al. Proceedings from the 2018 international consensus meeting on orthopedic infections: evaluation of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28:S32–S66. doi: 10.1016/j.jse.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Garrigues G.E., Zmistowski B., Cooper A.M., Green A., Hsu J., Ricchetti E., et al. Proceedings from the 2018 international consensus meeting on orthopedic infections: the definition of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28:S8–S12. doi: 10.1016/j.jse.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Kunutsor S.K., Barrett M.C., Whitehouse M.R., Craig R.S., Lenguerrand E., Beswick A.D., et al. Incidence, temporal trends and potential risk factors for prosthetic joint infection after primary total shoulder and elbow replacement: systematic review and meta-analysis. J Infect. 2020;80:426–436. doi: 10.1016/j.jinf.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Lovy A.J., Keswani A., Beck C., Dowdell J.E., Parsons B.O. Risk factors for and timing of adverse events after total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1003–1010. doi: 10.1016/j.jse.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Parada S.A., Flurin P.-H., Wright T.W., Zuckerman J.D., Elwell J.A., Roche C.P., et al. Comparison of complication types and rates associated with anatomic and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30:811–818. doi: 10.1016/j.jse.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Shah S.S., Gaal B.T., Roche A.M., Namdari S., Grawe B.M., Lawler M., et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: part I. JSES Int. 2020;4:929–943. doi: 10.1016/j.jseint.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman S.B., Davis J.J., Muh S.J., Vohra S.T., Patel A., van Holsbeeck M.T. Ultrasound evaluations and guided procedures of the painful joint arthroplasty. Skeletal Radiol. 2022;51:2105–2120. doi: 10.1007/s00256-022-04080-y. [DOI] [PubMed] [Google Scholar]
- 9.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]