Abstract
Background
Blue light therapy (BLT) is a Food and Drug Administration cleared modality used in dermatology as an effective treatment of acne. The primary purpose of this study is to determine if there are dose-dependent antimicrobial effects of BLT against Cutibacterium acnes (C. acnes).
Methods
A known strain of C. acnes was grown on chocolate agar in a controlled laboratory environment under anaerobic conditions for 1 week. After 1 week, 2-3 colonies of C. acnes were isolated and transferred to broth medium to incubate for 2 or 7 days. Broth vials (treatment arm) then underwent 1 of 6 different blue light dosing treatment regimens and a duplicate broth vial served as a control left open to the same environment. The BLT regimens were a single treatment of 25 J/cm2, 50 J/cm2, 75 J/cm2, 100 J/cm2, 2 serial treatments of 50 J/cm2 separated by 24 hours, or 2 serial treatments of 75 J/cm2 separated by 24 hours. The Omnilux Blue device (415 nm wavelength) was used for all BLT treatments and delivered, on average, 1.68 ± 0.004 J/min. Following treatment, the control and treatment broth samples were plated on chocolate agar and allowed to grow for 7 days. After 7 days, plates were counted and colony forming units (CFUs) were calculated. Six trials were completed for each BLT dosing regimen based on an a priori power analysis of 6 individual 2-sided t-tests. Comparisons in the primary outcome were made via mixed-effects analysis of variance with replicate as a random effect.
Results
All BLT treatment regimens resulted in significantly fewer CFUs than their aggregate control plate CFUs (P < .05 for all). Furthermore, in 2-way comparison of CFUs between BLT treatment groups, a single treatment of 75 J/cm2 did lead to significantly less growth than 25 J/cm2 (P = .017) and 50 J/cm2 (P = .017). There were no improved antimicrobial effects with serial treatments when comparing 2 doses of 50 J/cm2 with a single dose of 100J/cm2, nor were 2 doses of 75 J/cm2 more efficacious than 100 J/cm2. Using the Omnilux Blue device, it took 44.8 minutes to deliver a 75 J/cm2 dose.
Conclusion
BLT is an effective antimicrobial agent against this single virulent strain of C. acnes. Treatment dosing of 75 J/cm2 was identified to be the most effective dose per unit time. Serial treatments did not lead to superior antimicrobial effects over a single, high-dose treatment.
Keywords: Cutibacterium acnes, Blue light therapy, Shoulder, Infection, Culture
Infection following shoulder surgery leads to significant patient morbidity and strain on the healthcare system. Cutibacterium acnes (C. acnes) has been identified as one of the most common pathogens leading to deep infections after shoulder surgery.1,18,21,30,33,35,36 The significant clinical challenges in diagnosing and treating shoulder surgical infections caused by C. acnes are well documented, particularly in the arthroplasty setting.6,8,19,27 Given the diagnostic, therapeutic, and cost burden of managing C. acnes shoulder infections, numerous investigations have sought to identify modalities to eradicate or reduce the bioburden of C. acnes prior to shoulder surgery with varying success.7,15,23,26,32, 33, 34
Treatments such as topical benzoyl peroxide gel, topical and oral antibiotics, and presurgical skin preparation with hydrogen peroxide are a few of the more commonly described modalities to diminish bioburden to date, yet infections remain a challenge.15, 16, 17,23,26,33,34,38 A limitation of topical treatments is the depth to which the antimicrobial agent penetrates human skin. To be maximally efficacious, antimicrobial agents need to reach the pilosebaceous glands rich in C. acnes at least 1 mm beneath the epidermal surface. Blue light therapy (BLT) is a Food and Drug Administration cleared modality used in dermatology as an effective treatment against C. acnes, among other bacteria, for patients with mild to moderate inflammatory acne.11, 12, 13,40 The mechanism of the bactericidal effect for BLT is the production of reactive oxygen species when the light (405-470 nm) is absorbed by endogenous porphyrins. Porphyrins are naturally expressed to a greater extent in bacteria compared to human tissues, particularly in C. acnes.3,9,14 An advantage of light-based antimicrobial treatments such as BLT is the ability to penetrate 1.2-1.5 mm beneath the skin surface to eliminate bacteria residing in the sebaceous glands.2 Blue light demonstrates bactericidal effects at doses as low as 5 J/cm2; however, some authors have reported higher doses (> 100 J/cm2) are necessary to injure human tissues.5,14,31 A recent randomized controlled trial in healthy, young male volunteers compared a single BLT treatment followed by chlorhexidine wash to 1 shoulder against 5 serial treatments with 5% benzoyl peroxide gel followed by chlorhexidine wash to the contralateral shoulder.7 The authors sought to determine if a single treatment of BLT would lead to diminished C. acnes bioburden at the deltopectoral interval as determined by swab cultures of the skin. Their results did not show a robust antimicrobial effect of a single treatment of BLT. It is possible that the dose of BLT administered in the study was insufficient to have a significant effect.7 While several prior controlled laboratory studies have reported potent antimicrobial effects of BLT against C. acnes, the lights used, dosing regimens, and methodology have all varied.3,4,9 Furthermore, to our knowledge, there has been limited controlled laboratory investigations examining if serial treatments have more pronounced antimicrobial effects, and if the antimicrobial effects of BLT differ based on if C. acnes is in a rapid growth or stationary phase.3,24,37
The purpose of this study is to (1) determine if there are dose-dependent antimicrobial effects of BLT against C. acnes, (2) if serial exposures to BLT are more efficacious than a single exposure to BLT at eradicating C. acnes at the same radiant exposure, and (3) determine if the antimicrobial properties of BLT are dependent on the growth phase of the bacterium. The authors hypothesized that BLT would have more significant bactericidal effects against C. acnes with increasing radiant exposure. Furthermore, serial exposures to BLT would have significantly greater antimicrobial properties than a single dose at the same radiant exposure, and BLT would have greater bactericidal effects against C. acnes in its exponential growth phase.
Materials and methods
This was a controlled laboratory in vitro study at a single center and was exempt from institutional review board approval. A known strain of C. acnes (ATCC 6919) was used for this study. All study activities took place at an academic microbiology laboratory with extensive experience working with this bacterium.
Description of materials
Blue light therapy device
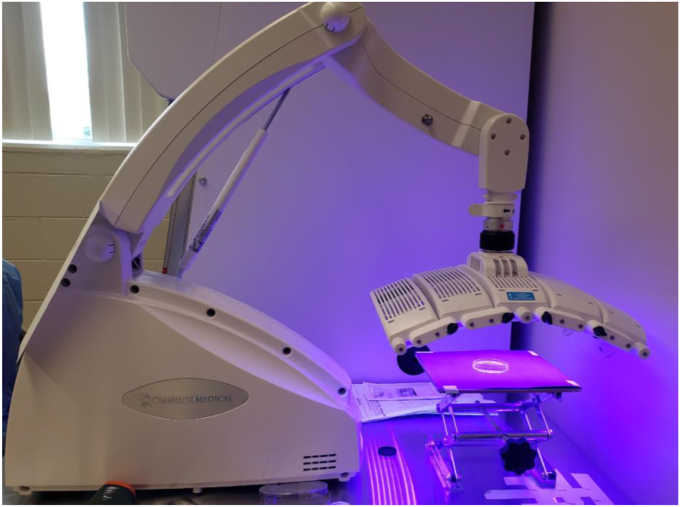
The Omnilux Blue BLT device (GlobalMed Technologies, Napa, CA, USA) was used for all blue light treatments in this study (Fig. 1). This is one of several BLT devices that are Food and Drug Administration–cleared and emits a 415-nanometer (nm) wavelength of light using light-emitting diodes. This device has been used in prior studies.7
Figure 1.
Photograph demonstrating the set up and treatment of a petri dish with the blue light device with the face 5 cm away from the dish surface.
Blue light dosing sensor
This novel sensor device provides a real-time reading of the intensity or irradiance (watts/cm2) of a blue light source emitting a 415 nm wavelength of light (Fig. 2). The device also integrates this signal over time and indicates when a desired “dose” (joules/cm2) of blue light has been received. The photoresistor is covered with a blue gel bandpass filter to reduce its response to any background light. The sensor was calibrated by using a Thorlabs PM100D power meter console (Thorslabs, Inc., Lafayette, CO, USA) with a S120C sensor head and was used to measure the intensity of light from the blue light source at a given distance away. The resistance of the photoresistor was then recorded at the same location. This power meter has had its calibration certified by the National Institute of Standards and Technology. Twenty data points were taken at distances corresponding to an intensity range of 2 to 40 mW/cm2. An intensity versus resistance curve was then fit to these data and is used by the microcontroller to calculate light intensity for a given resistance value of the photoresistor. Once calibrated, the device gives an intensity reading within 1%-3% of the Thorlabs power meter when they are placed at the same distance from the light source.
Figure 2.
Photograph demonstrating the blue light dosing sensor.
Blue light therapy treatment groups
-
•BLT regimens for C. acnes (6 total treatment groups):
-
○Single treatment of 25 J/cm2
-
○Single treatment of 50 J/cm2
-
○Single treatment of 75 J/cm2
-
○Single treatment of 100 J/cm2
-
○Serial treatment of 50 J/cm2 × 2 treatments (total radiant exposure [dose]: 100 J/cm2)
-
▪Separated by 24 hours to simulate what could feasibly be done in clinical practice.
-
▪
-
○Serial treatment of 75 J/cm2 × 2 treatments (total radiant exposure [dose]: 150 J/cm2)
-
▪Separated by 24 hours to simulate what could feasibly be done in clinical practice.
-
▪
-
○
The above doses and time intervals were chosen based on the work of Ashkenazi et al, who demonstrated serial treatments with blue light at 75 J/cm2 led to a 4-log10 decrease in growth (99% decreased growth with a single treatment at 75 J/cm2 and 99.99% for 2 treatments at 75 J/cm2).3 The primary outcome was the estimated number of colony forming units (CFUs) per milliliter (mL) between treatment groups post-treatment. The primary comparison group to the BLT treatment plates of C. acnes was a control plate with aliquots taken from the same initial sample as the BLT-treated plate.
There was a direct linear relationship between dose delivered and time. Time to deliver 25 J, 50 J, 75 J, and 100 J was recorded for 2 trials each on different days (total: 8). The Omnilux Blue BLT device delivered, on average, 1.672 ± 0.004 J/min. To deliver a dose of 25 J/cm2, it took 891.5 seconds (14.85 minutes). For 50 J/cm2, it took 1790 seconds (29.83 minutes), for 75 J/cm2, 2685.5 seconds (44.75 minutes), and for 100 J/cm2, it took 3588 seconds (59.8 minutes).
In vitro culture protocol
Single blue light therapy treatment
Before treatment, C. acnes (ATCC 6919) was inoculated on chocolate agar and allowed to incubate anaerobically for 1 week to maximize porphyrin expression and replicate, as closely as possible, conditions of the bacterium as it colonizes human skin.3 This was done a total of 36 times for 6 “replicates” for each of the 6 BLT treatment groups outlined above. After 1 week, 5 mL of thioglycolate broth (Difco Inc., Plymouth, MI, USA) was added to a 14 mL Falcon tube and inoculated with 2-3 C. acnes colonies. The inoculated broth (termed Day 0 broth) was then incubated at 37 °C under anaerobic conditions for either 48 hours (2 days) or 168 hours (7 days) before BLT treatment of the bacterium to investigate if BLT effects differ based on where C. acnes is in its growth cycle. It has been established that bacteria in exponential growth phases are more susceptible to antibiotics but little work has been done to see if similar effects result with light-based antimicrobial treatments.24,37 We conducted a growth phase analysis in broth and identified Day 2 as the exponential phase of growth for this strain of C. acnes and by Day 5 the bacteria had reached stationary phase (Supplementary Appendix S1).
Following either 2 days (exponential) or 7 days (stationary) as defined by the prelude cell density experiments (Supplementary Appendix S1), 0.5 mL of the inoculated base broth was transferred to 2 separate 1.5 mL microcentrifuge tubes (1 control and 1 treatment tube) and centrifuged at 13,000xg for 10 minutes. The supernatant was removed and the pellet resuspended in 5 mL of 1x phosphate buffered saline. This solution was then transferred to a 5 mL petri dish such that there was a control plate and a “treatment” plate for both the Day 2 and Day 7 time points for each replicate and each of the 6 treatment regimens. The “treatment” petri dish was then placed under the blue light on the bench top and its corresponding control plate was left out in the same environment but not exposed to BLT. The treatment plate then underwent the prescribed dosing until the blue light dosing sensor read the desired joules for the given trial (25 J/cm2, 50 J/cm2, 75 J/cm2, and 100 J/cm2). Once the BLT was completed, the contents of the petri dishes were transferred to sterile 14 mL falcon tubes in broth. The broth was then transferred to the anaerobic chamber.
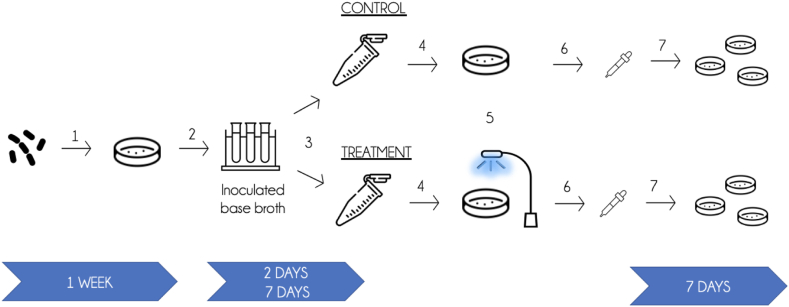
In the anaerobic chamber, a series of serial dilutions were performed by adding 0.1 mL of broth to 0.9 mL of 1x phosphate buffered saline. Dilutions were performed until there were 2 diluted broths that produce approximately 30-300 colonies, as is standard to ensure accuracy.28 After completing the serial dilutions, 0.1 mL of each of these solutions was transferred in duplicate to 2 chocolate agar plates and spread with L-shaped spreader to allow for growth of isolated colonies. This procedure was completed for both treatment and cont°rol samples. Then, the plates were parafilmed and incubated at 37 °C under anaerobic conditions for 7 days. Following 7 days of incubation, the number of C. acnes colonies was counted. A graphic depiction of the treatment protocol and timeline can be found in Fig. 3.
Figure 3.
Treatment protocol (1) C. acnes (ATCC 6919) was inoculated on chocolate agar and allowed to incubate anaerobically for 1 week. (2) 5 mL of Thioglycolate broth was added to a 14 mL falcon tube and inoculated with 2-3 C. acnes colonies. The inoculated base broth (termed Day 0 broth) was then incubated at 37 °C under anaerobic conditions for either 48 hours (2 days) or 168 hours (7 days) before further manipulation or BLT treatment (3) 0.5 mL of inoculated base broth was transferred to 2 separate 1.5 mL microcentrifuge tubes (one control and one treatment tube) and centrifuged at 13,000xg for 10 minutes. (4) The supernatant was removed and the pellet resuspended in 5 mL of 1x phosphate buffered saline (PBS). This solution was then transferred to a 5 mL petri dish. (5) The “treatment” petri dish was then placed under the blue light on the bench top and its corresponding control plate was left out in the same environment but not exposed to BLT. The treatment plate underwent the prescribed dosing until the blue light dosing sensor read the desired joules. (6) Once the BLT was completed, the contents of the petri dishes were transferred to sterile 14 mL falcon tubes in broth, which were transferred to the anaerobic chamber. (7) Broth was and allowed to incubate anaerobically for 7 days, and then counted. BLT, blue light therapy.
Serial blue light therapy treatment culture protocol
For the 2 serial BLT treatment regimens spanning 24 hours (50 J/cm2 and 75 J/cm2 × 2), the same culture protocol was followed precisely as the single treatment protocol with few differences. The C. acnes (ATCC 6919) strain was inoculated on chocolate agar and incubated under anaerobic conditions for 1 week. At 1 week, 2-3 colonies were transferred to 5 mL of thioglycolate broth to create a Day 0 broth. This broth was incubated for 24 hours before the first BLT treatment (Day 1) was completed as outlined by the single treatment protocol with the following differences: after exposure, the control and treatment broths were added to 14 mL falcon tubes, centrifuged at 4000xg at 4 °C for 10 minutes, and the supernatant removed. The tubes were moved back to the anaerobic chamber and the pellet resuspended in 5 mL of thioglycolate broth before incubating for 24 hours. After 24 hours, the second exposure was completed (Day 2) as outlined in the single treatment protocol and likewise plated. All of the above steps were completed a second time at Days 6 and 7 using the original inoculated base broth.
Statistical analysis
An a priori power analysis was conducted using the parameters of 6 individual 2-sided t-tests with adjusted alpha level of 0.0083. To achieve 80% power to detect mean differences of 2 standard deviation between treatment and control, this study required 6 unique C. acnes bacterial samples per treatment regimen and 6 corresponding control samples. Secondary comparisons of the primary outcome were made between treatment groups and between treatment and control plates at varying time points described below. Prior to analysis, skewedness of the outcome was assessed and transformations of the data to achieve normality were explored. Comparisons in the primary outcome between treatments and between time points were made via mixed-effects analysis of variance with replicate as a random effect. Log-based estimates of coefficients and confidence intervals were exponentiated and reported for ease of interpretation. For comparison between treatment and control groups based on growth phase of bacterium, a mixed-effects analysis of variance with growth phase, treatment status, and their interaction term were used as fixed effects and replicate as a random effect. A model was fit separately for each dosing mechanism. Log ratios were calculated for both growth phases to estimate the effect of treatment on outcomes of BLT using control as a baseline. Since all treated samples showed a reduction in BLT compared to control the log ratio denotes a percentage of the control BLT. For example, a log ratio of 0.24 reports the quantity of growth of the BLT-treated group was 24% of the corresponding control group. The mixed model with interaction term estimated a P value for the comparison of the log ratios between the 2 growth phases. This was used to assess for differences in treatment affect based on growth phase. Holm adjustment for multiple hypothesis testing was used to mitigate risk of Type I error inflation. A P < .05 was considered statistically significant. All statistical analyses were performed using R for statistical computing version 4.1.
Results
Overall treatment versus control effects
All BLT treatment regimens except for the 25 J/cm2 dose resulted in significantly fewer CFUs/mL than their aggregate control broth CFUs when day 2 and day 7 data were aggregated for each treatment dose [P < .05 for all (Table 1)].
Table I.
Blue light treatment effects compared to internal controls for each of the 6 treatment doses as an estimated geometric mean colony forming unit aggregating 6 replicates per treatment.
| Dose (J/cm2) | Treatment | Mean CFUs/mL (95% CI)∗ | P value |
|---|---|---|---|
| 25 | Control | 9.42 × 106 (1399653, 63790370) | .067 |
| Treatment | 8.49 × 105 (131983, 5459029) | ||
| 50 | Control | 2.30 × 107 (8958714, 58839153) | .002 |
| Treatment | 8.39 × 105 (327363, 2150056) | ||
| 75 | Control | 2.91 × 107 (682492, 1244434394) | .015 |
| Treatment | 1.23 × 104 (287, 524057) | ||
| 100 | Control | 2.82 × 107 (6228530, 127714135) | <.001 |
| Treatment | 7.94 × 102 (175, 3594) | ||
| 50-50 | Control | 4.09 × 106 (687346, 24308980) | .001 |
| Treatment | 1.86 × 103 (312, 11045) | ||
| 75-75 | Control | 1.10 × 106 (389485, 3098225) | <.001 |
| Treatment | 2.17 × 103 (768, 6108) |
J, Joules; cm, centimeters; CFU, colony forming units; CI, confidence interval; ANOVA, analysis of variance.
Estimated geometric mean from mixed effects ANOVA, on logarithmic transformed data.
Between treatment group comparisons and serial treatment effects
In a 2-way comparison of CFUs/mL between BLT treatment groups again aggregating all data (day 2 and day 7 broth) for each treatment group, there were no increased antimicrobial effects delivering a single 50 J/cm2 treatment compared to 25 J/cm2. A single treatment of 75 J/cm2 did lead to significantly less growth than 25 J/cm2 and 50 J/cm2 (P = .017 for each comparison, respectively). There were no improved antimicrobial effects with serial treatments when comparing 2 doses of 50 J/cm2 with a single dose of 100 J/cm2 nor were 2 doses of 75 J/cm2 more efficacious than 100 J/cm2 (Table 2).
Table II.
Two-way comparisons of geometric means for each of the 6 blue light treatment groups.
| Dose (J/cm2) | Mean CFUs/mL (95% CI)∗ | Between dose comparisons | P value |
|---|---|---|---|
| 25 | 8.49 × 105 (75474, 9546376) | 25-50 | 1 |
| 50 | 8.39 × 105 (74596, 9435423) | 25-75 | .017 |
| 75 | 1.23 × 104 (1091, 138027) | 25-100 | < .001 |
| 100 | 7.94 × 102 (71, 8927) | 25 to 50-50 | .001 |
| 50-50 | 1.86 × 103 (165, 20888) | 25 to 75-75 | .001 |
| 75-75 | 2.17 × 103 (174, 27009) | 50-75 | .017 |
| 50-100 | < .001 | ||
| 50 to 50-50 | .001 | ||
| 50 to 75-75 | .001 | ||
| 75 to 100 | .2 | ||
| 75 to 50-50 | .699 | ||
| 75 to 75-75 | .775 | ||
| 100 to 50-50 | 1 | ||
| 100 to 75-75 | 1 | ||
| 50-50 to 75-75 | 1 | ||
CFU, colony forming units; CI, confidence interval; ANOVA, analysis of variance.
Day 2 and day 7 growth phase cycle data were all aggregated for a comprehensive picture of treatment effects.
P values are Holm adjusted for 15 tests.
Estimated geometric mean from mixed effects ANOVA, on logarithmic transformed data.
Growth phase antimicrobial effects
When comparing the antimicrobial effects of BLT based on growth phase (exponential vs. steady state), significantly less growth was seen for all treatment dosing regimens no matter if the bacteria were in the exponential or steady state growth phase. Comparison of log ratio differences between treatment and control based on whether the bacteria in the exponential growth phase (day 2) and those in the steady state phase (day 7) demonstrated significantly greater BLT antimicrobial effects in the bacteria treated in the steady state phase for doses: 25 J/cm2 (P = .002), 75 J/cm2 (P < .001), 100 J/cm2 (P < .001), and 50-50 J/cm2 (P < .001) (Table 3).
Table III.
Comparison of blue light therapy treatment effects between dosing regimens based on whether the bacterium was in the exponential growth phase (day 2) or stationary growth phase (day 7).
| Dose (J/cm2) | Growth phase | Treatment | Mean CFUs/mL (95% CI)∗ | Log ratio (95% CI)† | P value |
|---|---|---|---|---|---|
| 25 | Exponential | Control | 1.32 × 107 (2207217, 80093594) | .002 | |
| Exponential | Treated | 5.85 × 106 (970322, 35210191) | 0.439613 (0.085640, 2.256640) | ||
| Stationary | Control | 1.47 × 107 (2155393, 100454744) | |||
| Stationary | Treated | 1.23 × 105 (20370, 745905) | 0.008377 (0.001444, 0.048608) | ||
| 50 | Exponential | Control | 2.38 × 107 (8777822, 64792559) | ||
| Exponential | Treated | 1.54 × 106 (566562, 4182020) | 0.064545 (0.022929, 0.181696) | .126 | |
| Stationary | Control | 2.21 × 107 (8115076, 60203216) | |||
| Stationary | Treated | 4.57 × 105 (167880, 1245447) | 0.020687 (0.007349, 0.058236) | ||
| 75 | Exponential | Control | 1.37 × 107 (2504687, 75057278) | ||
| Exponential | Treated | 2.64 × 105 (48260, 1446194) | 0.019268 (0.003765, 0.098601) | <.001 | |
| Stationary | Control | 6.19 × 107 (11267183, 340547400) | |||
| Stationary | Treated | 5.70 × 102 (104, 3134) | 0.000009 (0.000002, 0.000047) | ||
| 100 | Exponential | Control | 5.83 × 107 (15096228, 225285073) | <.001 | |
| Exponential | Treated | 7.51 × 103 (1945, 29025) | 0.000129 (4e-05, 0.000412) | ||
| Stationary | Control | 1.36 × 107 (3518944, 52873232) | |||
| Stationary | Treated | 8.4 × 101 (22, 325) | 0.000006 (2e-06, 0.000020) | ||
| 50-50 | Exponential | Control | 1.39 × 106 (359583, 5369182) | <.001 | |
| Exponential | Treated | 3.77 × 103 (976, 14572) | 0.002714 (0.000935, 0.007877) | ||
| Stationary | Control | 1.20 × 107 (3101373, 46625430) | |||
| Stationary | Treated | 9.15 × 102 (236, 3547) | 0.000076 (0.000026, 0.000221) | ||
| 75-75 | Exponential | Control | 4.61 × 105 (145017, 1468407) | .985 | |
| Exponential | Treated | 9.01 × 102 (283, 2867) | 0.001952 (0.000462, 0.008243) | ||
| Stationary | Control | 2.61 × 106 (818202, 8357598) | |||
| Stationary | Treated | 5.21 × 103 (1629, 16641) | 0.001991 (0.000472, 0.008407) |
CFU, colony forming units; CI, confidence interval; ANOVA, analysis of variance.
Comparisons between control and treatment groups were performed for both the exponential growth and the stationary growth phases and the log ratios of those differences in growth compared.
P value is comparison of log ratios from exponential versus steady growth states.
Estimated geometric mean from mixed-effects ANOVA, on logarithmic transformed data.
Estimated log ratio of treated/control from mixed-effects ANOVA, on logarithmic transformed data.
Discussion
This study demonstrates that a single treatment of BLT at a dose of at least 50 J/cm2 results in significantly decreased C. acnes CFUs in vitro, with 75 J/cm2 having the most robust antimicrobial effects in the shortest amount of treatment time. Serial treatments did not lead to greater antimicrobial effects. The bactericidal effects of BLT were found to be more pronounced if C. acnes was in stationary phase of the growth cycle for nearly all treatment doses.
Investigators continue to study treatments to diminish C. acnes bioburden prior to shoulder surgery, particularly open shoulder surgery, with hopes reduction in C. acnes will translate into lower postoperative infection rates with varying success.7,15,23,26,32, 33, 34 It is possible that C. acnes persists after treatment with topical antimicrobial agents because they do not penetrate beyond the epidermal surface. It may be time to rethink our strategies turning focus from topical applications or systemic medications to technology that can have both targeted effects at a specific area and penetrate beyond the epidermal surface. As such, a recent study has looked to translate dermatologic applications for use in orthopedics surgery.8 Within dermatology, studies have proven that BLT can decrease macroscopic acne burden.11,12 However, few have specifically evaluated BLT in a controlled laboratory setting to examine its antimicrobial properties against C. acnes.3 The present study adds granular data detailing specific doses of BLT necessary to diminish CFUs by 1000x.
While some authors have investigated BLT’s antimicrobial properties against many different microbes, Ashkenazi et al in 2003 conducted a study evaluating antimicrobial effects of blue light (407-420 nm) against C. acnes.3 In their investigation, a single strain of C. acnes (ATCC 6919) was grown in clostridial broth under anaerobic conditions. The authors had 2 groups, one C. acnes group grown in broth reinforced by a photosensitizing agent 5-Aminolevulinic acid (ALA) and one without ALA. C. acnes was allowed to grow for 96 hours with CFU per ml-1 counted every 24 hours. After each 24-hour interval of anaerobic growth in liquid medium, the vials were illuminated by 75 J/cm2 of BLT (ie, serial treatments). The authors noted this dose decreased bacterial counts by 1-2 orders of magnitude at 24 hours and by 4 orders of magnitude after a second BLT treatment at 48 hours. After 3 consecutive treatments, there was a decrease in viability by 5 orders of magnitude. Furthermore, cultures grown for 48 hours with ALA and then treated with a single 75 J/cm2 exhibited a decrease in bacterial CFU by a factor of 7. Their results demonstrate strong antimicrobial properties of BLT at a dose of 75 J/cm2 that were enhanced further by augmenting their medium with ALA, a precursor for porphyrin synthesis.3 The results of the present study are concordant with those of Ashkenazi et al in demonstrating the strong antimicrobial properties of BLT in vitro at a dose of 75 J/cm2.3 However, the present study did not find serial treatments to have additive antimicrobial effects as Ashkenazi et al reported.3
Exogenous application of photosensitizers can lead to increased apoptosis of both bacteria and human cells when illuminated by visible light.3 These agents are taken up by cells and lead to a buildup of an endogenous photosensitizer, protoporphyrin IX (PpIX). Increased PpIX expression causes greater production of reactive oxygen species in these cells.29,39 This results in cell apoptosis and necrosis.39 While rapidly dividing cells more strongly take up ALA, healthy human tissues are also affected. The side effects of ALA use include erythema, pain, edema, stinging, burning, scaling/crusting, dyspigmentation, pruritis, erosion, urticaria, ulceration, bleeding, scaling, dysesthesias, and pustules.25 In contrast, BLT alone, at doses of less than 100 J/cm2 leads to no detrimental effects of human tissues both on gross inspection and at a histologic level allowing this treatment to be employed in a perioperative setting.22 Given that C. acnes naturally expresses porphyrins, particularly when grown in culture for at least 3 days, we did not find it necessary to use photosensitizers in this work.3
Although the growth phase of commensal organisms on human skin has not been well defined, it can be postulated that bacteria on skin may be in various stages of their growth cycle. Our results lend support that BLT is an efficacious antimicrobial treatment no matter the growth phase of C. acnes. However, the antimicrobial effects of blue light on C. acnes are orders of magnitude greater in the stationary phase of growth. These findings are in contrast to a large body of literature reporting faster-growing bacteria are more susceptible to antibacterial effects of antibiotics such as β-lactams.24,37 It is possible that this finding is due to BLT having a completely different mechanism of bactericidal effect. Furthermore, it may also be that a greater proportion of bacteria in the stationary phase of growth in the present study were naturally closer to microbe death than bacteria in the exponential phase. More work is needed to better delineate if the growth phase of C. acnes is germane to the clinical application of BLT.
The findings of this study are a positive step forward, but significant work remains to translate this technology to the clinical setting. To us, the clinical application of BLT may be best served in the preoperative holding area or in clinic a day or two prior to surgery. As the results of the present study indicate, a single treatment of BLT at 75 J/cm2 can significantly reduce C. acnes bioburden. As others have described, numerous subtypes of C. acnes exist. It is not known at this time if different subtypes express porphyrins to varying degrees and by extension would be more or less sensitive to BLT.10,20 Additional investigation is needed to understand how the complex microenvironment including various chromophores of human skin may scatter BLT, which may alter duration of treatment needed for the pilosebaceous glands to receive a target dose.2 Further work should consider more detailed evaluation of the growth and examine if time-dependent BLT antimicrobial effects exist.
Limitations
There are several limitations of this study. This was an in vitro laboratory study in a controlled environment, and it is unknown if C. acnes would respond differently to BLT treatment in the complex microenvironment of human skin. Furthermore, the present investigation used a single subtype of C. acnes; it is unknown if other subtypes would respond differently to the same BLT treatments. A bacterial bioburden threshold below which the likelihood of clinically manifesting a prosthetic joint infection is diminished remains unknown. While the intent of this investigation and others was to demonstrate decreased bacterial load as a surrogate for decreasing infection risk, more work needs to be done to draw firm correlations between bioburden and clinically symptomatic infection with C. acnes.
Conclusion
BLT is an effective antimicrobial agent against a single virulent strain of C. acnes. Treatment dosing of 75 J/cm2 was identified to be the most effective dose per unit time. Serial treatments did not lead to superior antimicrobial effects over a single, high-dose treatment.
Disclaimers:
Funding: Orthopaedic Research and Education Foundation Resident Research Grant. American Shoulder Elbow Surgeons Prosthetic Joint Infection Grant. University of Wisconsin Skin Disease Research Center Pilot Grant.
Conflicts of interest: Brian F. Grogan, MD: American Shoulder and Elbow Surgeons: Board or Committee Member; Stryker: paid consultant, presenter or speaker. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this basic science study.
The project was supported by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health National Center for Advancing Translational Sciences (NCATS), grant ULI TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jseint.2023.11.020.
Supplementary data
References
- 1.Achermann Y., Sahin F., Schwyzer H.K., Kolling C., Wust J., Vogt M. Characteristics and outcome of 16 periprosthetic shoulder joint infections. Infection. 2013;41:613–620. doi: 10.1007/s15010-012-0360-4. [DOI] [PubMed] [Google Scholar]
- 2.Ash C., Dubec M., Donne K., Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci. 2017;32:1909–1918. doi: 10.1007/s10103-017-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi H., Malik Z., Harth Y., Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava S., Listopadzki T., Diletti S., Crane J.K., Duquin T.R., Boyle K.K. Effect of blue light and photosensitizers on Cutibacterium acnes on shoulder periprosthetic joint infection Isolates. J Bone Jt Infect. 2020;5:187–197. doi: 10.7150/jbji.46199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bumah V.V., Masson-Meyers D.S., Tong W., Castel C., Enwemeka C.S. Optimizing the bactericidal effect of pulsed blue light on Propionibacterium acnes - a correlative fluorescence spectroscopy study. J Photochem Photobiol B. 2020;202 doi: 10.1016/j.jphotobiol.2019.111701. [DOI] [PubMed] [Google Scholar]
- 6.Cooper M.E., Trivedi N.N., Sivasundaram L., Karns M.R., Voos J.E., Gillespie R.J. Diagnosis and management of periprosthetic joint infection after shoulder arthroplasty. JBJS Rev. 2019;7:e3. doi: 10.2106/JBJS.RVW.18.00152. [DOI] [PubMed] [Google Scholar]
- 7.Cotter E.J., Cotter L.M., Franczek E.B., Godfrey J.J., Hetzel S.J., Safdar N., et al. Efficacy of combinational therapy using blue light and benzoyl peroxide in reducing Cutibacterium acnes bioburden at the deltopectoral interval: a randomized controlled trial. J Shoulder Elbow Surg. 2021 doi: 10.1016/j.jse.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Cotter E.J., Winzenried A.E., Polania-Gonzalez E., Song D., Waterman B.R., Grogan B.F. Role of pre-revision tissue biopsy in evaluation of painful shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2021;30:1445–1457. doi: 10.1016/j.jse.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Dai T., Gupta A., Murray C.K., Vrahas M.S., Tegos G.P., Hamblin M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15:223–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitz-Gibbon S., Tomida S., Chiu B.-H., Nguyen L., Du C., Liu M., et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold M.H., Andriessen A., Biron J., Andriessen H. Clinical efficacy of self-applied blue light therapy for mild-to-moderate Facial acne. J Clin Aesthet Dermatol. 2009;2:44–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Gold M.H., Biron J.A., Sensing W. Clinical and usability study to determine the safety and efficacy of the Silk’n Blue Device for the treatment of mild to moderate inflammatory acne vulgaris. J Cosmet Laser Ther. 2014;16:108–113. doi: 10.3109/14764172.2013.854638. [DOI] [PubMed] [Google Scholar]
- 13.Gwynne P.J., Gallagher M.P. Light as a broad-spectrum antimicrobial. Front Microbiol. 2018;9:119. doi: 10.3389/fmicb.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halstead F.D., Thwaite J.E., Burt R., Laws T.R., Raguse M., Moeller R., et al. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol. 2016;82:4006–4016. doi: 10.1128/AEM.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock D.S., Rupasinghe S.L., Elkinson I., Bloomfield M.G., Larsen P.D. Benzoyl peroxide + chlorhexidine versus chlorhexidine alone skin preparation to reduce Propionibacterium acnes: a randomized controlled trial. ANZ J Surg. 2018;88:1182–1186. doi: 10.1111/ans.14848. [DOI] [PubMed] [Google Scholar]
- 16.Heckmann N., Heidari K.S., Jalali O., Weber A.E., She R., Omid R., et al. Cutibacterium acnes persists despite topical clindamycin and benzoyl peroxide. J Shoulder Elbow Surg. 2019;28:2279–2283. doi: 10.1016/j.jse.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Heckmann N., Sivasundaram L., Heidari K.S., Weber A.E., Mayer E.N., Omid R., et al. Propionibacterium acnes persists despite various skin preparation techniques. Arthroscopy. 2018;34:1786–1789. doi: 10.1016/j.arthro.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Horneff J.G., Hsu J.E., Voleti P.B., O’Donnell J., Huffman G.R. Propionibacterium acnes infection in shoulder arthroscopy patients with postoperative pain. J Shoulder Elbow Surg. 2015;24:838–843. doi: 10.1016/j.jse.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Hsu J.E., Bumgarner R.E., Matsen F.A., 3rd Propionibacterium in shoulder arthroplasty: what We Think We Know Today. J Bone Joint Surg Am. 2016;98:597–606. doi: 10.2106/JBJS.15.00568. [DOI] [PubMed] [Google Scholar]
- 20.Huh J., Krueger C.A., Medvecky M.J., Hsu J.R. Medial elbow exposure for coronoid fractures: FCU-split versus over-the-top. J Orthop Trauma. 2013;27:730–734. doi: 10.1097/BOT.0b013e31828ba91c. [DOI] [PubMed] [Google Scholar]
- 21.Kelly J.D., 2nd, Hobgood E.R. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467:2343–2348. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinpenning M.M., Smits T., Frunt M.H.A., van Erp P.E.J., van de Kerkhof P.C.M., Gerritsen R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 23.Kolakowski L., Lai J.K., Duvall G.T., Jauregui J.J., Dubina A.G., Jones D.L., et al. Neer award 2018: benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2018;27:1539–1544. doi: 10.1016/j.jse.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Lee A.J., Wang S., Meredith H.R., Zhuang B., Dai Z., You L. Robust, linear correlations between growth rates and β-lactam-mediated lysis rates. Proc Natl Acad Sci U S A. 2018;115:4069–4074. doi: 10.1073/pnas.1719504115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P.K., Kloser A. Current methods for photodynamic therapy in the US: comparison of MAL/PDT and ALA/PDT. J Drugs Dermatol. 2013;12:925–930. [PubMed] [Google Scholar]
- 26.Leyden J.J. Effect of topical benzoyl peroxide/clindamycin versus topical clindamycin and vehicle in the reduction of Propionibacterium acnes. Cutis. 2002;69:475–480. [PubMed] [Google Scholar]
- 27.Mook W.R., Garrigues G.E. Diagnosis and management of periprosthetic shoulder infections. J Bone Joint Surg Am. 2014;96:956–965. doi: 10.2106/JBJS.M.00402. [DOI] [PubMed] [Google Scholar]
- 28.O’Toole G.A. Classic Spotlight: plate counting you can count on. J Bacteriol. 2016;198:3127. doi: 10.1128/JB.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ormrod D., Jarvis B. Topical aminolevulinic acid HCl photodynamic therapy. Am J Clin Dermatol. 2000;1:131–133. doi: 10.2165/00128071-200001020-00009. [DOI] [PubMed] [Google Scholar]
- 30.Pottinger P., Butler-Wu S., Neradilek M.B., Merritt A., Bertelsen A., Jette J.L., et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94:2075–2083. doi: 10.2106/JBJS.K.00861. [DOI] [PubMed] [Google Scholar]
- 31.Ramakrishnan P., Maclean M., MacGregor S.J., Anderson J.G., Grant M.H. Cytotoxic responses to 405nm light exposure in mammalian and bacterial cells: involvement of reactive oxygen species. Toxicol Vitro. 2016;33:54–62. doi: 10.1016/j.tiv.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Rao A.J., Chalmers P.N., Cvetanovich G.L., O’Brien M.C., Newgren J.M., Cole B.J., et al. Preoperative doxycycline does not reduce Propionibacterium acnes in shoulder arthroplasty. J Bone Joint Surg Am. 2018;100:958–964. doi: 10.2106/JBJS.17.00584. [DOI] [PubMed] [Google Scholar]
- 33.Sabetta J.R., Rana V.P., Vadasdi K.B., Greene R.T., Cunningham J.G., Miller S.R., et al. Efficacy of topical benzoyl peroxide on the reduction of Propionibacterium acnes during shoulder surgery. J Shoulder Elbow Surg. 2015;24:995–1004. doi: 10.1016/j.jse.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Scheer V.M., Bergman Jungestrom M., Lerm M., Serrander L., Kalen A. Topical benzoyl peroxide application on the shoulder reduces Propionibacterium acnes: a randomized study. J Shoulder Elbow Surg. 2018;27:957–961. doi: 10.1016/j.jse.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 35.Singh J.A., Sperling J.W., Schleck C., Harmsen W., Cofield R.H. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2012;21:1304–1309. doi: 10.1016/j.jse.2011.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh J.A., Sperling J.W., Schleck C., Harmsen W.S., Cofield R.H. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21:1534–1541. doi: 10.1016/j.jse.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes J.M., Lopatkin A.J., Lobritz M.A., Collins J.J. Bacterial Metabolism and antibiotic efficacy. Cell Metab. 2019;30:251–259. doi: 10.1016/j.cmet.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stull J.D., Nicholson T.A., Davis D.E., Namdari S. Addition of 3% hydrogen peroxide to standard skin preparation reduces Cutibacterium acnes–positive culture rate in shoulder surgery: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2020;29:212–216. doi: 10.1016/j.jse.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 39.Taylor E.L., Brown S.B. The advantages of aminolevulinic acid photodynamic therapy in dermatology. J Dermatolog Treat. 2002;13:S3–S11. doi: 10.1080/095466302317414645. [DOI] [PubMed] [Google Scholar]
- 40.Wheeland R.G., Koreck A. Safety and effectiveness of a new blue light device for the self-treatment of mild-to-moderate acne. J Clin Aesthet Dermatol. 2012;5:25–31. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.