Abstract
Drinking water quality can be compromised by endocrine-disrupting chemicals (EDCs). Three phenolic compounds [bisphenol A (BPA), nonylphenol (NP), and 4-octylphenol (OP)] and three hormones [17β-estradiol (E2), estrone (E1), and 17α-ethinylestradiol (EE2)] were analyzed as EDCs potentially occurring in source and drinking water from three full-scale drinking water treatment plants (DWTPs) in the Romagna area (Italy) by a combined approach of HPLC-MS/MS target analysis and effect-based tests for estrogenicity and genotoxicity. The EDC removal efficiency was evaluated at different steps along the treatment process in the most advanced DWTP. NP prevailed in all samples, followed by BPA. Sporadic contamination by OP and E1/E2 appeared only in the source waters; EE2 was never detected. No estrogenic or genotoxic activity was found, except for two samples showing estrogenicity well below the effect-based trigger value suggested for drinking water safety (0.9 ng/L EEQ). BPA and NP levels were largely below the threshold value; however, increases were observed after the intermediate steps of the treatment chain. The good quality of the water relied on the last step, i.e. the activated carbon filtration. DWTPs may represent an extra source of EDCs and monitoring chemical occurrence at all steps of the process is advisable to improve efficiency.
Keywords: Endocrine-disrupting chemicals (EDCs), Drinking water, Effect-based methods, HPLC/MS-MS, Water treatment efficiency
Graphical abstract
Highlights
-
•
EDCs in drinking water treatment plants were assessed by HPLC-MS/MS and bioassays.
-
•
The efficiency of the different steps along the treatment chain was evaluated.
-
•
Treatment plants may represent an extra source of BPA and nonylphenol contamination.
-
•
Genotoxicity was absent, low estrogenicity was shown by 2 out of 18 samples.
-
•
Monitoring EDCs occurrence at all steps of the treatment chain is recommended.
1. Introduction
Over the last few decades, an increasing variety of new compounds of anthropogenic origin has been reported in water bodies worldwide. The presence of contaminants of emerging concern (CECs) in drinking water, including endocrine-disrupting chemicals (EDCs), has drawn attention towards possible risks to human health [1,2]. EDCs are found in a wide range of products used in everyday life, including natural compounds (e.g. hormones), chemicals used as industrial additives and plastics (e.g. bisphenol A, BPA), alkylphenols, pesticides, fungicides, and pharmaceutical agents [3]. EDCs typically occur in water at trace concentrations, and their presence is largely attributable to their continuous release into source water (surface and groundwater) from wastewater systems, because of their incomplete elimination, and from other sources like untreated wastewaters, agricultural and industrial discharges. Moreover, materials in contact with water along the treatment plants can represent additional sources of EDC contamination. Several studies have highlighted the leaching of substances such as alkylphenols and BPA from plastic materials used in drinking water treatment plants (DWTPs) and pipelines [[4], [5], [6], [7]].
DWTPs play a key role in supplying high-quality potable water. However, conventional drinking water technologies, including flocculation, precipitation, adsorption, membrane treatment, and other water treatment methods, are not specifically designed to remove CECs [[8], [9], [10]]. Treatment processes have demonstrated different removal rates for EDCs, ranging from less than 30% to greater than 90% [11]. This is because several EDCs are persistent and non-biodegradable, with strong chemical cohesion in the environment [12], and their removal efficiencies depend on the physical and chemical properties of the substances. Recently, there has been an increasing focus on assessing CECs in drinking water [13,14]. However, removal of these compounds by drinking water treatment technologies has proven challenging due to differences in treatment conditions, variability in water characteristics, and variations in waterworks design.
Human health implications arising from EDCs exposure include adverse reproductive outcomes, developmental disabilities, endometriosis, and breast and testicular cancers [[15], [16], [17]]. Contamination by synthetic and natural hormones has been linked to chronic and acute diseases, such as various types of cancer in females and males, disorders of the reproductive system, obesity, and diabetes [[18], [19], [20]]. Phenolic compounds such as BPA and nonylphenol (NP) have been found in human urine, blood, and reproductive tissues worldwide [21,22]. Their ability to interfere with physiological hormonal regulation disrupt at very low doses is also widely documented, as single compounds and in mixture [[23], [24], [25], [26]].
BPA may exert its toxicological effects at the genetic and epigenetic levels, influencing various cell signaling pathways, and altering the biological behaviors of cancer cells, notably proliferation, invasion, growth, survival, migration, and apoptosis [[27], [28], [29], [30], [31], [32], [33]]. Concerns about the potential impact of EDCs on humans and the environment have motivated the European Union to update the list of chemical parameters to be assessed in the Drinking Water Directive [34], adding suspected EDCs for the first time, including Per- and Poly- Fluorinated Alkylated Substances (PFAS) and BPA. In view of their endocrine-disrupting properties, 17 β-estradiol (E2) and NP are included in the first Watch List for drinking water [35].
Chemical analysis is the most used approach for monitoring drinking water quality. Analytical technologies include advanced, precise, and accurate methods for detecting environmental contaminants at sub-trace levels. However, chemical analyses have some limitations, as they cover a wide but finite number of substances. Moreover, the analytical limit of quantification (LOQ) for some compounds is above the proposed threshold guideline values (e.g. hormones like E2 and 17α-ethinylestradiol, EE2) [36], thus their occurrence cannot be determined. Finally, chemical analyses do not account for the biological effects of chemicals or their mixtures. As such, the choice of combining chemical and bioanalytical tools has become of increasing interest and is recommended for water quality monitoring [37,38]. The application of effect-based methods is relevant for drinking water, where chemicals are often present at low concentrations, potentially below the analytical LOQs, nevertheless contributing to biological effects [39,40]. Effect-based methods can also provide valuable information regarding treatment efficiency. To comprehensively assess water quality, a battery of bioassays is recommended to include different health-relevant biological endpoints, such as those related to xenobiotic metabolism, hormone-mediated modes of action (e.g. endocrine activity), reactive modes of action (genotoxicity), and oxidative stress response [41].
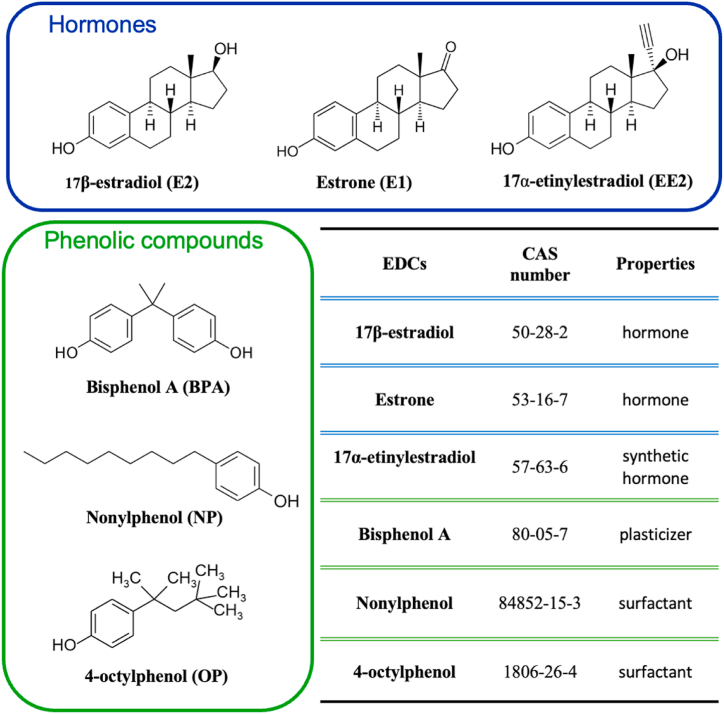
In aquatic environments, estrogenic activity has primarily been ascribed to the natural steroids E2, and estrone (E1), and to the synthetic estrogen EE2 used in contraceptives [42]. However, industrial xenobiotics alone or in mixtures may also contribute to overall estrogenic activity [43,44]. The present study aimed to evaluate the occurrence of representative EDCs [hormones E2, E1, and EE2; phenolic compounds BPA, NP, and 4-octylphenol (OP)] (Fig. 1) in the source and treated water of three DWTPs serving the Romagna area (the southeast part of the Emilia-Romagna region, Italy), and to monitor the potential bioactivity of the drinking water ready to be distributed. Chemical analysis was combined with cell-based assays to assess potential estrogenic activity (E-SCREEN assay) and genotoxicity (micronuclei test) to ascertain the overall drinking water quality. The investigation was conducted in summer over three years (2020–2022). The DWTP applying the most advanced technologies was also selected to perform chemical monitoring throughout intermediate water treatment steps (i.e., pre-chlorination, flocculation, ultrafiltration) in order to analyze their removal efficiencies. To this specific purpose, chemical analyses were performed during summer and also winter periods to examine seasonal variations.
Fig. 1.
Targeted EDCs in the present study.
2. Materials and methods
2.1. Chemicals
The natural (E2 and E1) and synthetic (EE2) estrogens, the alkylphenols (NP and OP), and BPA were selected as micropollutants for the present study and were purchased from Merck Life Science (Milan, Italy). E2-d2 and BPA-d6 were used as labeled internal standards, purchased by CDN Isotopes (Quebec, Canada). Stock solutions and their internal standards were prepared in methanol and stored at −20 °C. Mix working standards (1 mg/L), containing all EDCs, were also prepared in methanol by spiking each of the dissolved individual stock solutions. Thereafter, the mix working standard was diluted using water/methanol (50:50 v/v) to the subsequent concentration needed. Solvent reagents purchased from Merck Life Science were of LC-MS analytical grade. Solid phase extraction cartridges (Oasis HLB, 6 cc) with a size of 200 mg/6 mL were purchased from Waters S.p.A. (Milan, Italy).
2.2. Drinking water treatment systems and sampling collection
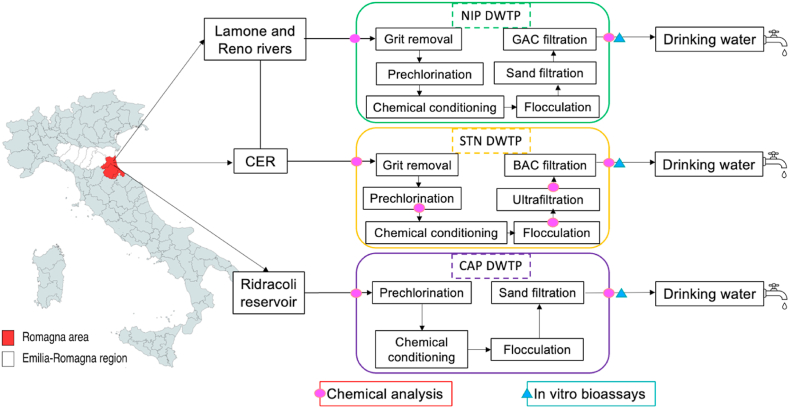
Water samples were collected from the main three DWTPs operating in the Romagna area (Italy) and managed by the company Romagna Acque Società delle Fonti. Schematic representation of the geographical position and treatment processes of DWTPs are shown in Fig. 2.
-
•
DWTP Bassette (NIP), located in an industrial area of Ravenna, has been designed to treat surface water from areas with intense anthropic activities, mixing water from the Lamone and Reno Rivers, and from the CER (“Canale Emiliano-Romagnolo”, an artificial water transfer system that takes water directly from the Po River). Its instant production potential is 1100 L/s. The DWTP includes the following treatment processes: pre-chlorination using chlorine dioxide, followed by chemical conditioning and sedimentation, clariflocculation using aluminum polyhydroxychloride in sludge recirculation basins called “Accelerator”, sand filtration, granular activated carbon filtration, and final chlorination (using chlorine dioxide) before the water enters the distribution network.
-
•
DWTP Standiana (STN), located near Ravenna and active since 2015, applies both conventional and advanced processes to treat water from CER, that crosses a heavily anthropized area. STN DWTP instant production potential is 1100 L/s. STN DWTP consists of micro-sieving followed by pre-chlorination treatment using chlorine dioxide, flocculation, ultrafiltration with membranes, biologically activated carbon (BAC) filtration, and lastly dosing with chlorine dioxide for final disinfection.
-
•
DWTP Capaccio (CAP), located near Forlì, receives surface water from the large artificial lake Ridracoli closed by a dam sited in the National Park of the Casentinesi Forests (an area with low anthropic impact). The CAP plant has a maximum production of approximately 3600 L/s. The DWTP currently employs conventional treatment with pre-chlorination (using chlorine dioxide), clariflocculation using aluminum polyhydroxychloride followed by rapid sand filtration (with filter batteries consisting of 16 units that can be fed individually), and disinfection with sodium hypochlorite.
Fig. 2.
Geographical position and schematic representation of treatment processes at the three drinking water treatment plants (DWTPs) investigated: NIP; Capaccio, CAP; and Standiana, STN. CER: Canale Emiliano-Romagnolo. The sampling points are indicated with dots and triangles. For each sampling point chemical or biological analyses further applied are indicated in the boxes at the bottom.
Raw (IN) and treated (OUT) water samples were collected from the three DWTPs investigated in this study. To evaluate the effectiveness of removal by the intermediate treatments, the most advanced plant STN was investigated more in-depth. Three sampling points were considered along the treatment processes, after pre-chlorination (PRECL), flocculation (FLOCC), and ultrafiltration (ULTR). A total of 6 sampling campaigns were carried out in summer (July and September) of 2020, 2021, and 2022. The summer period was chosen for the above samplings because of the scarce rainfall and high evaporation rates in the area, when EDCs were expected to be more concentrated in water bodies. In addition, samples from the STN DWTP were collected also in winter (January and February/March) over the same three years for a total of 5 additional campaigns. During the wet season, contaminants can be diluted because of the increase in rainfall and river flow rates. The detailed weather and flow rivers’ conditions in the area during the three-year sampling period are provided in Fig. S-1 (Supplementary material Figure S-1). At each sampling point, three replicate water samples (1 L each) were collected in PE bottles pre-washed with methanol. Water samples were then transported to the laboratory, stored in the dark at 4 °C, and processed within 36 h.
2.3. Sample extraction
Before extraction, water samples for chemical analysis were separately spiked with a mixture of labeled internal standards (E2-d2 and BPA-d6) at a concentration of 30 ng/L. Samples were filtered using glass microfiber filters (1.60 μm) and then cellulose acetate filters (0.45 μm) to remove suspended particulate matter [7]. The samples were subsequently extracted using Oasis HLB cartridges as previously reported [7,45]. Briefly, cartridges were activated using 6 mL of methanol and 6 mL of deionized water. Then, the water samples were passed through the preconditioned cartridges at a max flow rate of 14 mL/min. Subsequently, the HLB cartridges were eluted with 6 mL methanol under gravity, after which the eluents were blown down under a gentle stream of nitrogen. Finally, the extracts were re-dissolved with 0.5 mL methanol/water (50:50, v/v) for chemical analysis. For biological analysis extracts were dissolved into the assay medium and transferred into 2 mL vials using 0.22 μm membrane filters for analyses.
2.4. HPLC-MS/MS analysis
Chemical analysis of the selected substances in water samples was performed by high-performance liquid chromatography equipped with a triple quadrupole mass spectrometer (HPLC-MS/MS), according to our previous study [45]. Briefly, the selected EDCs and their corresponding internal standards were analyzed using HPLC-MS/MS (Waters, Milford, MA, USA) using a negative electrospray ionization source (ESI-) in multiple-reaction monitoring (MRM) mode. Separation of compounds was achieved through an XBridge C18 3.5 μm 2.1 × 150 mm column (Waters S.p.A.) and the volume injection was 20 μL. Sample extracts concentrated 2000 times were measured along with calibration standards, which corresponded to a range of 0–300 μg/L for phenols and steroids [45].
2.5. Quality control (QC)/quality assurance (QA)
QC and QA measurements were performed for the entire experimental protocol. The apparatus and glassware were pre-washed by rinsing with methanol. An equal volume (1 L) of deionized water spiked with the same internal standard solution as water samples was simultaneously extracted and analyzed. It was extracted along with every batch of samples as a procedural blank sample to assess contamination during sample extraction and analysis. The limit of detection (LOD) and the LOQ were defined as the concentration of a compound providing a signal-to-noise ratio of three and ten, respectively. Each calibration curve for the target compounds had a high correlation coefficient (r2 > 0.99). Moreover, the standard mixture solution was injected in turn after the injection of every six samples during instrumental analysis as calibration of the data acquisition. HPLC-MS/MS performance and analyte identification were monitored throughout the retention time (within ±2%) of the compound in the samples relative to those in the authentic standards. The performance of the method was evaluated by estimating the linearity, extraction recovery, sensitivity (both LOD and LOQ), precision (repeatability and reproducibility), matrix effects (ionization suppression or enhancement), and relative abundance (quantifier/qualifier transition ratio).
The removal efficiency of the treatment plant was calculated using the inlet and outlet water concentrations as follows [46]:
2.6. Cell-based methods
Drinking water samples were assessed for cytotoxicity using the cell viability MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, for estrogenicity using the cell proliferation assay E-SCREEN, and for genotoxicity using the micronuclei test. Cells were plated into 12 or 24-well plates at an initial concentration of 10,000 cells/well. After 24 h, the seeding medium was replaced by the experimental medium containing water extracts or different concentrations of E2. All in vitro assays were performed as previously described in Valbonesi et al. [45]. The human breast cancer cell line, MCF-7, kindly provided by Prof. M. Marino (University Roma Tre, Rome, Italy), was used for all bioassays. All water samples were tested for cytotoxicity in a dilution series to ensure that experiments were carried out under noncytotoxic conditions. Positive controls were analyzed in parallel with the water samples using an E2 standard curve ranging from 10−15 M to 10−8 M for estrogenicity, and 9 × 10−8 M of mitomycin C for genotoxicity assessment. The negative control (ultrapure water extract in the presence of 0.1% methanol) and the internal positive control with 10−9 M E2 were added to each plate.
2.7. Data evaluation
The concentrations of samples were expressed in units of relative enrichment factor (REF), which considers the combination of the extraction factor from the concentration step and dilution factor in the bioassay as reported by Escher et al. [47]. When incubated with the cells, extracts were diluted 40-fold with the cell medium to obtain a final concentration of 0.1% methanol. The resulting REF in the bioassays e.g. 20, means that the samples were 20 times enriched in the cellular medium. Cell-based bioactivities of the water samples and positive controls were normalized to the negative controls on each plate. For the E-SCREEN assay, estrogenic activity was expressed as the Proliferative Effect (PE) compared to the negative control set as 1. A dose-dependent cell proliferation curve was obtained by fitting the data to a four-parameter sigmoidal curve of E2, using a commercial graphical package (SigmaPlot software, ver 13, Systat Software Inc.). The equivalent estradiol concentration (EEQ, ng/L) was calculated by non-linear interpolation from the dose-response curve of E2 in the respective experiment, by inserting the PE of the water samples in the inversion of the four-parameter logistic function fitted with the curve parameters obtained from the E2, following the equation below:
The LOD calculated from the mean effect of the negative control plus three times the standard error was 0.014 ng/L EEQ [47].
The results of the micronuclei test were expressed as the number of micronuclei/1000 binucleated cells scored on each slide and reported as the mean ± standard deviation (SD), obtained from three independent experiments. Data groups were compared using one-way ANOVA and followed by Dunnett post-hoc test; the significance was calculated in comparison to the negative control. A statistical difference was accepted when p < 0.05 (Sigma Stat, SPSS Science, Chicago IL, USA).
3. Results and discussion
The aim of this study has been to evaluate the occurrence of selected EDCs in surface water at the entrance of three DWTPs and in the corresponding treated water. Chemical measures were complemented with biological analysis, to ascertain cumulative estrogenic and/or genotoxic effects of the samples. Particular emphasis was addressed to intermediate water treatments within one of the DWTPs studied i.e. Standiana DWTP. By narrowing our focus to critical stages of water treatment we aimed to provide insight into the fate and behavior of EDCs during the process, offering a deeper understanding of the removal efficiency of the different steps. We also gave attention to seasonality and explored potential variations in the occurrence of EDCs in winter and summer.
3.1. Chemical analysis
3.1.1. Occurrence of selected micropollutants in the source and treated water of the DWTPs
The present work provides a comprehensive picture of the state of EDCs contamination in drinking water in the Romagna area over a three-year period. The EDCs detected in the inlet and outlet water of the studied DWTPs are shown in Table 1, Table 2, Table 3, for NIP, CAP and STN, respectively.
Table 1.
Levels of suspected EDCs (ng/L) measured in water samples from NIP DWTP collected during six sampling campaigns in 2020, 2021 and 2022. IN: pre-treatment water, OUT: post-treatment water; LOQ: limit of quantification (ng/L). Bold numbers: EDCs detected in OUT water samples.
| Sampling | NIP | BPA | NP | OP | E2 | E1 | EE2 |
|---|---|---|---|---|---|---|---|
| July 2020 | IN | 13.4 | 9.0 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | 5.6 | 12.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2020 | IN | 7.4 | 7.5 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | 6.3 | 2.4 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2021 | IN | 3.8 | 8.2 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | 5.2 | 36.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2021 | IN | 3.6 | 99.9 | 1.9 | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 4.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2022 | IN | 3.0 | 6.3 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 22.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2022 | IN | 13.9 | 6.0 | 1.5 | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 14.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| LOQ | 1.0 | 2.0 | 0.7 | 0.8 | 0.9 | 2.7 |
Table 2.
Levels of suspected EDCs (ng/L) measured in water samples from Capaccio DWTP (CAP) collected during six sampling campaigns in 2020, 2021 and 2022. IN: pre-treatment water, OUT: post-treatment water; LOQ: limit of quantification (ng/L). Bold numbers: EDCs detected in OUT water samples.
| Sampling | CAP | BPA | NP | OP | E2 | E1 | EE2 |
|---|---|---|---|---|---|---|---|
| July 2020 | IN | 3.4 | 22.3 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | 1.9 | 3.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2020 | IN | 3.5 | 9.0 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | 3.4 | 2.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2021 | IN | < LOQ | 12.8 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 3.3 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2021 | IN | < LOQ | 6.1 | < LOQ | 5.5 | < LOQ | < LOQ |
| OUT | < LOQ | 3.4 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2022 | IN | 1.7 | 12.0 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 22.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2022 | IN | < LOQ | 42.6 | < LOQ | < LOQ | < LOQ | < LOQ |
| OUT | < LOQ | 11.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| LOQ | 1.0 | 2.0 | 0.7 | 0.8 | 0.9 | 2.7 |
Table 3.
Levels of suspected EDCs (ng/L) measured in water samples from Standiana DWTP (STN) collected during summer campaigns in 2020, 2021 and 2022. IN: pre-treatment water, PRECL: pre-chlorination step, FLOCC: flocculation step, ULTR: ultrafiltration step, OUT: post-treatment water; LOQ: limit of quantification (ng/L). Bold numbers: EDCs detected in OUT water samples.
| Sampling | STN | BPA | NP | OP | E2 | E1 | EE2 |
|---|---|---|---|---|---|---|---|
| July 2020 | IN | 3.0 | 18.3 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 1.6 | 27.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 3.6 | 16.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 5.4 | 15.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 3.5 | 8.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2020 | IN | 4.2 | 12.7 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 2.0 | 16.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 4.4 | 22.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 5.4 | 1.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 2.0 | 6.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2021 | IN | 3.7 | 4.6 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | < LOQ | 2.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | < LOQ | 5.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 2.5 | 132.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | < LOQ | 12.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2021 | IN | 3.8 | 83.4 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | < LOQ | 14.1 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | < LOQ | 7.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 1.5 | 5.3 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | < LOQ | 10.1 | < LOQ | < LOQ | < LOQ | < LOQ | |
| July 2022 | IN | 1.6 | 8.7 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 2.0 | 53.4 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 2.0 | 14.4 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 3.4 | 14.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | < LOQ | 5.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| September 2022 | IN | < LOQ | 30.5 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 1.4 | 143.3 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 1.9 | 15.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | < LOQ | 12.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | < LOQ | 6.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| LOQ | 1.0 | 2.0 | 0.7 | 0.8 | 0.9 | 2.7 |
Among the selected substances analyzed by HPLC MS/MS, NP was the most prominent compound in all samples (100% detection frequency) in both raw and finished drinking water samples from all DWTPs. The highest concentration of NP (36.7 ng/L) was reported in the drinking water of the NIP DWTP in September 2021. It is worth noting that a higher amount of NP was often measured in treated water, implying that NP persisted after water treatment. NP is widely used for the production of industrial and household products such as detergents, emulsifiers, wetting agents, additives, and resins [48]. The widespread use of NP leads to its wide occurrence in water [49]. The NP concentrations detected in this study were similar to those determined in our previous investigation [45] and in line with further reports. In drinking water samples from other Italian DWTPs, the highest concentrations of NP were 84 ng/L [50] and 90 ng/L [51]. The NP level was 126 ng/L in drinking water from Spain [52], 93 ng/L from United States [53], 72 ng/L from Canada [54], 34 ng/L from France [55], and 16 ng/L from Germany [56].
BPA is primarily used as an additive in the production of polycarbonate plastics and epoxy resins to enhance the transparency, durability, flexibility, and longevity of plastics [57]. In this study, BPA occurred in all water samples entering NIP DWTP, it was often detected in STN DWTP, and occasionally found in CAP DWTP. Overall, BPA contamination was moderately to completely removed by the water treatment processes, with concentrations in the treated water often below the analytical LOQ (NIP DWTP: LOQ – 6.3 ng/L; STN DWTP: LOQ – 3.5 ng/L; and CAP DWTP: LOQ – 3.4 ng/L). BPA levels reported in this work were in accordance with those reported previously [45], but lower than those reported by Maggioni et al. [50] (up to 102 ng/L), Riva et al. [58] (BPA median value 23 ng/L), and Russo et al. [51] (BPA median concentration 46 ng/L).
The natural hormones E2 and E1 were detected only in one raw water sample, whereas the synthetic EE2 has never been reported to exceed its analytical LOQ. Sporadic OP contamination was found in the inlet water of NIP. OP and hormones were efficiently removed by water treatment at both DWTPs. BPA levels in drinking water were always below the 2.5 μg/L parametric value set by the regulation. No exceedances of drinking water guidance values proposed by the EU watchlist for NP and E2 (0.3 μg/L and 0.001 μg/L, respectively) were observed. The results obtained for the analyzed treated waters confirmed the suitability of the water treatment process applied to the waterworks investigated.
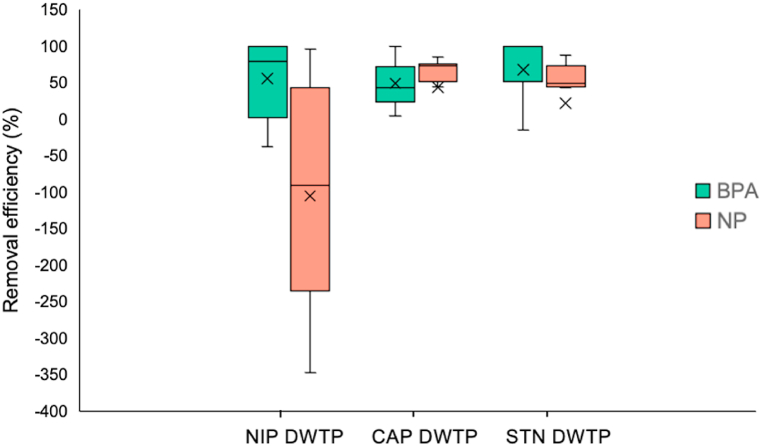
The removal efficiency of each waterwork was calculated as the concentration of finished water compared to the raw water, and the results are shown in Fig. 3. The median removal efficiency of the different DWTPs during the summer season was as follows: NP -90.6% and BPA 78.9% in NIP; NP 73.2% and BPA 42.9% in CAP; NP 49.4% and BPA 100% in STN. The results of this study indicate a wide variability in the effectiveness of each water system among the detected EDCs.
Fig. 3.
BPA and NP removal efficiency of the three DWTPs investigated (NIP; Capaccio, CAP; and Standiana, STN) during the summer campaigns (July and September 2020, 2021, and 2022) (n = 6).
3.1.2. Occurrence of micropollutants and their fate during water treatment processes of STN DWTP
As previously reported [59], no treatment alone can remove all contaminants; some processes significantly reduce the concentrations (e.g. advanced treatments), whereas others have little impact on their removal (e.g. conventional treatments). To determine the fate and removal of micropollutants during water treatment performed by the STN DWTP, samples were collected from raw to finished drinking water, including intermediate conventional treatments, i.e. pre-chlorination, flocculation, and ultrafiltration. An overview of the analyzed EDCs occurrence along the water treatments is shown in Table 3 (summer) and Table 4 (winter). Only two target compounds (BPA and NP) showed detectable levels in the drinking water samples and exhibited variable levels along the treatment process. Increases in BPA and/or NP levels were observed after intermediate treatments. Occasionally the NP level exceeded 100 ng/L. This level was lower than the threshold guideline value of 300 ng/L, but considerably higher than the level measured in the water entering the DWTPs. It is therefore suggested that the DWTP represents an extra source of EDCs contamination in water. This could be related to the leaching of organic chemicals from the plastic materials into the water [60]. Various additives such as lubricants, plasticizers, antioxidants and other stabilizers, softeners, and coloring agents are used in pipes, epoxy coating, valves, and other components of DWTPs to increase the life and stability of the materials [17]. Recent studies have demonstrated that emerging contaminants such as BPA, NP, PFAS and phthalates, widely used as additives in plastic materials, can be released by polymer pipes into drinking water [4,5,57,61]. Thus, the migration of contaminants may adversely influence the quality of the produced drinking water. The leaching of contaminants from pipes and plastic materials into drinking water has poorly been addressed, but it definitively needs to be evaluated for a better understanding of the risk to human health.
Table 4.
Levels of suspected EDCs (ng/L) measured in water samples from Standiana DWTP (STN) collected during winter campaigns in 2020, 2021 and 2022. IN: pre-treatment water, PRECL: pre-chlorination step, FLOCC: flocculation step, ULTR: ultrafiltration step, OUT: post-treatment water; LOQ: limit of quantification (ng/L). Bold numbers: EDCs detected in OUT water samples.
| Sampling | STN | BPA | NP | OP | E2 | E1 | EE2 |
|---|---|---|---|---|---|---|---|
| January 2020 | IN | 6.9 | 7.2 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 11.2 | 10.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 4.4 | 12.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 6.6 | 5.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 4.4 | 7.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| February 2020 | IN | 5.7 | 16.3 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 3.9 | 10.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 3.7 | 12.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 4.2 | 10.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 6.9 | 1.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| January 2021 | IN | 2.6 | 32.0 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 1.9 | 15.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 2.8 | 17.2 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 1.8 | 17.8 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 3.5 | 11.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| March 2021 | IN | 2.7 | 10.2 | < LOQ | < LOQ | < LOQ | < LOQ |
| PRECL | 1.4 | 9.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 3.2 | 16.5 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 2.7 | 13.6 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | 3.1 | 10.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| March 2022 | IN | 3.5 | 10.1 | 2.2 | < LOQ | 3.2 | < LOQ |
| PRECL | 3.4 | 12.0 | < LOQ | < LOQ | < LOQ | < LOQ | |
| FLOCC | 4.9 | 12.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| ULTR | 3.0 | 14.9 | < LOQ | < LOQ | < LOQ | < LOQ | |
| OUT | < LOQ | 5.7 | < LOQ | < LOQ | < LOQ | < LOQ | |
| LOQ | 1.0 | 2.0 | 0.7 | 0.8 | 0.9 | 2.7 |
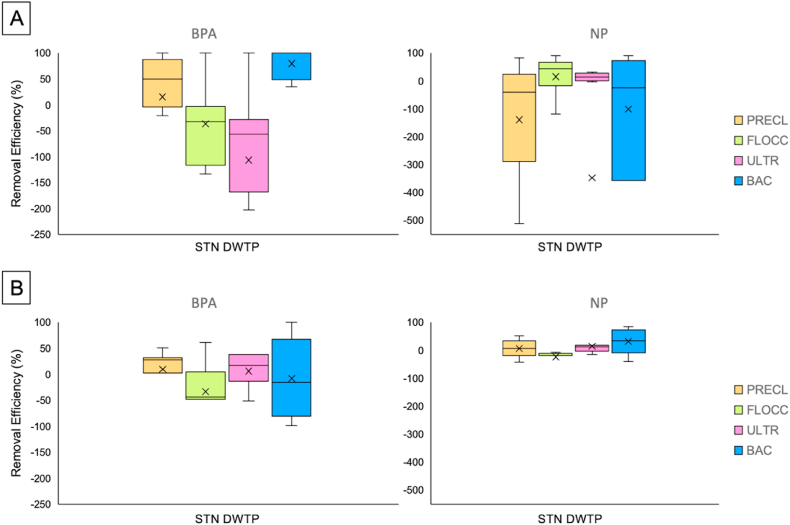
Seasonal variations in the micropollutants measured in the STN DWTP were also investigated. The removal efficiencies of BPA and NP during selected water treatment processes are shown in Fig. 4. EDC removal efficiencies appear to vary depending on the season, despite similar concentrations in the raw water during winter and summer samplings. During the summer (Fig. 4A), pre-chlorination accounted for 50.0% of BPA removal and was ineffective for NP. Flocculation and membrane ultrafiltration showed negative removal efficiency of −32.4% and −56.8% for BPA, respectively. These intermediate treatments also exhibited less than 45% removal efficiency for NP. It has been reported that conventional treatments by DWTPs are largely ineffective in removing EDCs [8,62], and further studies [[63], [64], [65]] showed negative removals of BPA during water treatment processes. Despite the incomplete removal during the conventional treatment processes, present results show that STN DWTP can remove EDCs at the final step. A 100% reduction in the BPA concentration with respect of the water entering that stage was revealed after the last treatment step before the water leaves the waterwork, i.e. BAC filtration. Differently, a median removal efficiency lower than 50% was observed for NP at the same stage.
Fig. 4.
BPA and NP removal efficiency evaluated after PRECL (pre-chlorination), flocculation (FLOCC), ultrafiltration (ULTR), and after biological activated carbon (BAC) filtration treatment steps of Standiana (STN) DWTP. [A] Removal efficiency during the summer campaigns (July and September 2020, 2021, and 2022) (n = 6). [B] Removal efficiency during the winter campaigns (January, February and/or March 2020, 2021, and 2022) (n = 5).
In the winter (Fig. 4B), smaller variations were identified along the treatments, but the overall process exhibited lower removal efficiency for BPA. The possible reason could be their leaching from plastic materials inside the DWTP, as above mentioned. However, the seasonal discrepancy was particularly evident for BAC filtration, which was ineffective in removing BPA during the winter, while showing high efficiency during the summer. Temperature is one of the most important factors that can influence the performance of the microbial population present in BAC, especially low temperatures [66]. Kim and Zoh [10] suggested that low temperatures could decrease the adsorption of activated carbon, thus decreasing the removal efficiency of micropollutants. Additionally, it should be noted that the microporous sizes of ultrafiltration membranes are usually much larger than the size of some EDCs; thus, the sieving mechanism cannot contribute to their elimination [6,62]. Overall, BPA and NP concentrations in finished drinking water were always in the very low ng/L range and well below the suggested limits.
3.2. Bioanalytical tools
The complementation of bioassays and chemical analyses is an excellent tool to target the total biological effects and comprehensively assess water quality [67]. In environmental matrices various substances coexist establishing potential interactions, and mixtures are unintentionally formed, on which chemical analysis alone does not provide information [68]. Cumulative exposure to multiple EDCs may be associated with severe health outcomes demonstrated in animals and humans, even when the concentrations of single chemicals fall below the regulatory dose [23]. Given the relevance of EDC occurrence in water and the importance of DWTPs in supplying high-quality water intended for human consumption, the present study investigated relevant biological endpoints for human health in the finished water. The E-SCREEN assay was applied to determine the total estrogenic potential of drinking water samples collected in summer over three years at the three DWTPs studied. Furthermore, considering that potentially toxic compounds can be formed during disinfection [69], in this work the micronuclei test was employed to determine the genotoxic potentials of finished water samples.
3.2.1. Cytotoxicity
The MTT assay was performed to determine the highest non-cytotoxic concentrations of the drinking water samples in the MCF-7 cell line. Cytotoxicity was defined as cell viability below 80% compared to the negative control. Initially, the SPE extracts were tested at different dilution levels ranging from 10 to 80 REFs (Supplementary material Fig. S-2). Due to the decreased cell viability observed in some samples above REF40, drinking water samples were then tested for their estrogenic/genotoxic potential at the concentration corresponding to REF20, in order to assess all samples under conditions in which cell viability was not compromised.
3.2.2. Estrogenic activity
The E-SCREEN assay using MCF-7 cell line is a sensitive and stable tool for quantitatively analyzing the estrogenic activity of environmental water samples [42]. The overall estrogenicity of the drinking water extracts is shown in Fig. 5A. Notably, samples from CAP DWTP outlet waters often induced a slight reduction in cell viability, which was significant in September 2020. This tendency may have resulted from variations in the contaminant mixtures after the water treatment. Disinfectants used during water treatment may react with micropollutants to form transformation products (e.g. disinfection by-products), which may be more or less toxic than their parent compounds. However, it is unclear which chemicals induce the slight cytotoxic response presently observed. Nevertheless, the cytotoxicity did not interfere with the measurement of estrogenic activity because cell viability did not drop below 80%.
Fig. 5.
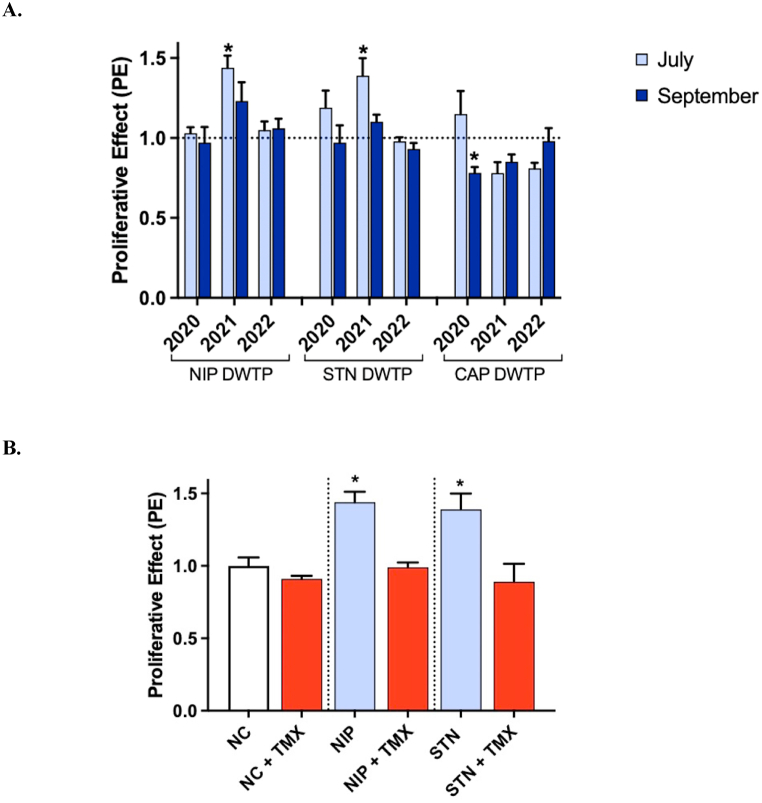
[A] Estrogenic activity observed at REF 20 after exposure of MCF-7 cells to drinking water samples from three DWTPs (NIP; Standiana, STN; Capaccio, CAP) collected during six campaigns in 2020, 2021 and 2022. Data are expressed as the mean of proliferative effect (PE) ± SE of different experiments, each conducted in quadruplicate (n = 4). Activity is normalized to negative control, which was set at 1.0 (dotted line). [B] Estrogenic activity in drinking water samples from NIP and STN DWTPs in July 2021 (violet bars) compared in the presence of 10−7 M Tamoxifen (TMX; red bars). NC: negative control (1.0 ± 0.06). Data are expressed as the mean of PE ± SE of different experiments, each conducted in quadruplicate (n = 3). *P < 0.05 vs negative control.
A significant increase in cell proliferation was observed in two drinking water samples (NIP and STN DWTPs) collected in July 2021, compared to the negative control. For these samples, the EEQ was 0.004 and 0.003 ng/L, respectively. The estrogenic activity was suppressed in the presence of the estrogen receptor antagonist tamoxifen, confirming that the two water samples possessed agonistic activity through estrogen receptors (Fig. 5B). The measured levels were similar to other samplings, but the biological effects were observed only in NIP and STN DWTPs in July 2021. It becomes challenging to directly attribute observed effects to BPA and NP. We could speculate the temporary occurrence of a potential mixture containing BPA, NP and further not assessed chemicals that could induce additive/synergistic effects. Such observation supports the importance of coupling effect-based tests with chemical analysis to enable risk assessment of real exposure to EDC mixtures. Generally, the observed estrogenic activities of the samples were low, and within the similar range previously measured in our laboratory (0.009 ng/L EEQ) [45]. This observation is also in accordance with results from previous studies (estrogenic activity in the range of 0.002–0.014 ng EEQ/L) [50,70], but lower than that reported by other authors (0.012–5.30 ng/L EEQ) [69,[71], [72], [73], [74], [75]].
Overall, the detection of an effect does not necessarily indicate poor water quality [76,77]. The effect-based trigger (EBT) value for drinking water derived by Escher and coworkers [78], recommended a safety levels of 0.9 ng/L EEQ to establish that the effects are at an acceptable level. In this study, drinking water samples showing estrogenic effect had activities considerably lower than the proposed EBT value, suggesting that they do not pose a risk by interfering with the estrogenic pathway. Compared with the acceptable daily intake (ADI) of 50 ng/kg/day (E2 equivalent) for humans, assuming complete absorption, a person would have to consume approximately 1,000,000 L of treated water to reach the ADI.
3.2.3. Genotoxicity
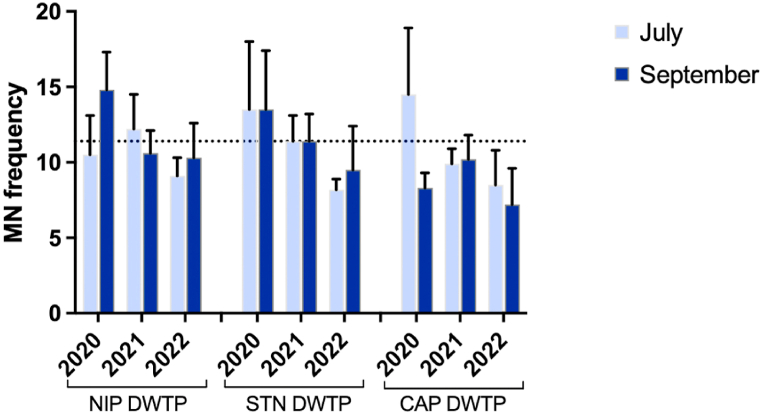
The quality of the drinking water is generally different from that of the DWTPs inlet water, since micropollutants and microorganisms present in source water can be removed by water treatment processes. However, various disinfection by-products can be generated after chlorination, as a result of the combination of disinfectants and naturally occurring inorganic and organic materials [[79], [80], [81]]. Such disinfection by-products may exert toxic and genotoxic/mutagenic effects on living organisms, including humans [82,83]. Moreover, corrosion products, pipeline material leaching, and residual contaminants can pose a threat to the safety of water quality and to human health [84]. In water quality assessment, genotoxicity tests provide a strong basis for assessing the reactive toxicity of such complex mixtures [47,85]. In this study, the micronuclei test was applied to analyze damage as chromosome aberrations and disturbances in the mitotic cycle in water extracts after water treatment. None of the water samples induced a statistically significant increase in micronuclei frequency compared to the negative control (Fig. 6), suggesting the absence of critical issues relating the drinking water quality.
Fig. 6.
Genotoxic response of drinking water samples from three DWTPs (NIP; Standiana, STN; and Capaccio, CAP) collected during summer campaigns in 2020, 2021, and 2022. Data are expressed as the mean of micronuclei (n°/1000 binucleated cells) ± SD (n = 3). Genotoxicity is compared to negative control (0.1 % methanol), which was set at 11.4 ± 2.8 (dotted line). Positive control using Mitomycin C showed a MN frequency of 29.4 ± 3.3.
4. Conclusions
This study investigated the efficiency of treatment processes by assessing EDC removal, and comprehensively evaluated the quality of the finished drinking water ready to be distributed across the Romagna area (Italy) by integrated chemical and biological screening approaches.
The EDC removal rates by conventional and advanced treatment processes was highly variable, also between seasons, and BPA and NP were not always efficiently eliminated. However, the final concentrations of target EDCs in drinking water were considerably lower than the guideline parameters proposed by the Drinking Water Directive [34], thus indicating the good quality of the water. Data showed that the quality of drinking water is not entirely dependent on the contamination level of the source water, because of potential leaching of chemicals along the plants. These results highlight that DWTPs may represent an extra source of EDCs contamination and emphasize the need for assessing chemical occurrence at all steps of the treatment chain aiming to improve the implants materials. Drinking water samples showed no genotoxic potential after treatment, whereas estrogenic activity was detected in two of the collected water samples, that was however well below the established EBT value. Although the analyzed samples did not show any critical issues, the use of integrated chemical and effect-based approaches for the assessment of drinking water quality is recommended, to counteract the risk associated with unknown chemicals and chemical mixtures, which are poorly examined and non-regulated.
Data availability statement
There is no data need to deposit into a publicly available repository.
CRediT authorship contribution statement
M. Profita: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. E. Fabbri: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Conceptualization. I. Vasumini: Supervision, Resources, Methodology. P. Valbonesi: Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Romagna Acque, Società delle Fonti (Italy) for allowing water sampling and providing technical information. This work was funded by MIUR Italy (RFO 2020 and 2021 to EF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26785.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lazofsky A., Buckley B. Recent trends in multiclass analysis of emerging endocrine disrupting contaminants (EDCs) in drinking water. Molecules. 2022;27:8835. doi: 10.3390/molecules27248835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonsioroski A., Mourikes V.E., Flaws J.A. Endocrine disruptors in water and their effects on the reproductive system. Int. J. Mol. Sci. 2020;21:1929. doi: 10.3390/ijms21061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jager C., Aneck-Hahn N., Swart P., Truebody B., Van Zijl M. Water Research Commission; 2013. Estrogenic Activity and Endocrine Disrupting Chemical (EDC) Status in Water Obtained from Selected Distribution Points in Pretoria and Cape Town: Report to the Water Research Commission (Report No. KV 317/13) Gezina (South Africa) [Google Scholar]
- 4.Cantoni B., Cappello Riguzzi A., Turolla A., Antonelli M. Bisphenol A leaching from epoxy resins in the drinking water distribution networks as human health risk determinant. Sci. Total Environ. 2021;783 doi: 10.1016/j.scitotenv.2021.146908. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y.-C., Chen H.-W., Chen W.-L., Chen C.-Y., Wang G.-S. Occurrence of nonylphenol and bisphenol A in household water pipes made of different materials. Environ. Monit. Assess. 2016;188:562. doi: 10.1007/s10661-016-5556-0. [DOI] [PubMed] [Google Scholar]
- 6.Muhamad M.S., Salim M.R., Lau W.J., Yusop Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. 2016;23:11549–11567. doi: 10.1007/s11356-016-6357-2. [DOI] [PubMed] [Google Scholar]
- 7.Achene L., Bogialli S., Lucentini L. Italian Institute of Health; Rome: 2011. Interferenti endocrini nelle acque da destinare al consumo umano in Italia: strumenti metodologici per un’indagine conoscitiva estesa a diversi sistemi idrici.https://www.iss.it/rapporti-istisan ISTISAN Reports 11/18) [Google Scholar]
- 8.Priya A.K., Gnanasekaran L., Rajendran S., Qin J., Vasseghian Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment- A review. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112298. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Narvaez O.M., Peralta-Hernandez J.M., Goonetilleke A., Bandala E.R. Treatment technologies for emerging contaminants in water: a review. Chem. Eng. J. 2017;323:361–380. doi: 10.1016/j.cej.2017.04.106. [DOI] [Google Scholar]
- 10.Kim M.-K., Zoh K.-D. Occurrence and removals of micropollutants in water environment. Environ Eng Res. 2016;21:319–332. doi: 10.4491/eer.2016.115. [DOI] [Google Scholar]
- 11.Sorlini S., Collivignarelli M.C., Miino M.C. Technologies for the control of emerging contaminants in drinking water treatment plants. Environ Eng Manag J. 2019;18:2203–2216. http://www.eemj.icpm.tuiasi.ro/pdfs/vol18/no10/11_85_Sorlini_19.pdf [Google Scholar]
- 12.Canle M., Fernández Pérez M.I., Santaballa J.A. Photocatalyzed degradation/abatement of endocrine disruptors. Curr. Opin. Green Sustainable Chem. 2017;6:101–138. doi: 10.1016/j.cogsc.2017.06.008. [DOI] [Google Scholar]
- 13.Borrull J., Colom A., Fabregas J., Borrull F., Pocurull E. Presence, behaviour and removal of selected organic micropollutants through drinking water treatment. Chemosphere. 2021;276 doi: 10.1016/j.chemosphere.2021.130023. [DOI] [PubMed] [Google Scholar]
- 14.Tröger R., Ren H., Yin D., Postigo C., Nguyen P.D., Baduel C., Golovko O., Been F., Joerss H., Boleda M.R., Polesello S., Roncoroni M., Taniyasu S., Menger F., Ahrens L., Yin Lai F., Wiberg K. What's in the water? – Target and suspect screening of contaminants of emerging concern in raw water and drinking water from Europe and Asia. Water Res. 2021;198 doi: 10.1016/j.watres.2021.117099. [DOI] [PubMed] [Google Scholar]
- 15.Kahn L.G., Philippat C., Nakayama S.F., Slama R., Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8:703–718. doi: 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giulivo M., Lopez de Alda M., Capri E., Barceló D. Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Gomes R., Lester Jason. In: Endocrine Disrupters in Wastewater and Sludge Treatment Processes. Birkett J., Lester John, editors. CRC Press; 2002. Endocrine disrupters in drinking water and water reuse. [DOI] [Google Scholar]
- 18.Predieri B., Iughetti L., Bernasconi S., Street M.E. Endocrine disrupting chemicals' effects in children: what we know and what we need to learn? Iran. J. Med. Sci. 2022;23 doi: 10.3390/ijms231911899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goralczyk K.A. Review of the impact of selected anthropogenic chemicals from the group of endocrine disruptors on human health. Toxics. 2021;9:146. doi: 10.3390/toxics9070146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S., Homaei A., Raju A.B., Meher B.R. Estrogen: the necessary evil for human health, and ways to tame it. Biomed. Pharmacother. 2018;102:403–411. doi: 10.1016/j.biopha.2018.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz B., Terekeci H., Sandal S., Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020;21:127–147. doi: 10.1007/s11154-019-09521-z. [DOI] [PubMed] [Google Scholar]
- 22.Jalal N., Surendranath A.R., Pathak J.L., Yu S., Chung C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol Rep. 2017;5:76–84. doi: 10.1016/j.toxrep.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caporale N., Leemans M., Birgersson L., Germain P.-L., Cheroni C., Borbély G., Engdahl E., Lindh C., Bressan R.B., Cavallo F., Chorev N.E., D'Agostino G.A., Pollard S.M., Rigoli M.T., Tenderini E., Tobon A.L., Trattaro S., Troglio F., Zanella M., Bergman Å., Damdimopoulou P., Jönsson M., Kiess W., Kitraki E., Kiviranta H., Nånberg E., Öberg M., Rantakokko P., Rudén C., Söder O., Bornehag C.-G., Demeneix B., Fini J.-B., Gennings C., Rüegg J., Sturve J., Testa G. From cohorts to molecules: adverse impacts of endocrine disrupting mixtures. Science. 2022;375:eabe8244. doi: 10.1126/science.abe8244. [DOI] [PubMed] [Google Scholar]
- 24.Catenza C.J., Farooq A., Shubear N.S., Donkor K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.129273. [DOI] [PubMed] [Google Scholar]
- 25.Noorimotlagh Z., Mirzaee S.A., Martinez S.S., Rachoń D., Hoseinzadeh M., Jaafarzadeh N. Environmental exposure to nonylphenol and cancer progression Risk–A systematic review. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109263. [DOI] [PubMed] [Google Scholar]
- 26.Cimmino I., Fiory F., Perruolo G., Miele C., Beguinot F., Formisano P., Oriente F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci. 2020;21:5761. doi: 10.3390/ijms21165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan N.G., Correia J., Adiga D., Rai P.S., Dsouza H.S., Chakrabarty S., Kabekkodu S.P. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ. Sci. Pollut. Res. 2021;28:19643–19663. doi: 10.1007/s11356-021-13071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Profita M., Fabbri E., Spisni E., Valbonesi P. Comparing effects and action mechanisms of BPA and BPS on HTR-8/SVneo placental cells. Biol. Reprod. 2021;105:1355–1364. doi: 10.1093/biolre/ioab139. [DOI] [PubMed] [Google Scholar]
- 29.Dumitrascu M.C., Mares C., Petca R.-C., Sandru F., Popescu R.-I., Mehedintu C., Petca A. Carcinogenic effects of bisphenol A in breast and ovarian cancers. Oncol. Lett. 2020;20:282. doi: 10.3892/ol.2020.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata M., Kang J.-H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018;36:311–327. doi: 10.1016/j.biotechadv.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Sowlat M.H., Lotfi S., Yunesian M., Ahmadkhaniha R., Rastkari N. The association between bisphenol A exposure and type-2 diabetes: a world systematic review. Environ. Sci. Pollut. Res. 2016;23:21125–21140. doi: 10.1007/s11356-016-7525-0. [DOI] [PubMed] [Google Scholar]
- 33.Ptak A., Hoffmann M., Gruca I., Barć J. Bisphenol A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signalling pathways. Toxicol. Lett. (Shannon) 2014;229:357–365. doi: 10.1016/j.toxlet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 34.EU 2020/2184. https://eur-lex.europa.eu/eli/dir/2020/2184/oj Directive 2020/2184/EU of the European Parliament and of the Council of 16 December 20202 on the quality of water intended for human consumption (recast)
- 35.EU Commission . 2022. COMMISSION IMPLEMENTING DECISION of 19.1.2022 Establishing a Watch List of Substances and Compounds of Concern for Water Intended for Human Consumption as provided for in Directive (EU) 2020/2184 of the European Parliament and of the Council.https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022D0679 [Google Scholar]
- 36.Kase R., Javurkova B., Simon E., Swart K., Buchinger S., Konemann S., Escher B.I., Carere M., Dulio V., Ait-Aissa S., Hollert H., Valsecchi S., Polesello S., Behnisch P., Di Paolo C., Olbrich D., Sychrova E., Gundlach M., Schlichting R., Leborgne L., Clara M., Scheffknecht C., Marneffe Y., Chalon C., Tusil P., Soldan P., Von Danwitz B., Schwaiger J., Palao A.M., Bersani F., Perceval O., Kienle C., Vermeissen E., Hilscherova K., Reifferscheid G., Werner I. Screening and risk management solution for steroidal estrogens in surface and wastewater. TRAC. 2018;102:343–358. doi: 10.1016/j.trac.2018.02.013. [DOI] [Google Scholar]
- 37.Brack W., Aissa S.A., Backhaus T., Dulio V., Escher B.I., Faust M., Hilscherova K., Hollender J., Hollert H., Müller C., Munthe J., Posthuma L., Seiler T.-B., Slobodnik J., Teodorovic I., Tindall A.J., de Aragão Umbuzeiro G., Zhang X., Altenburger R. Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality. Environ. Sci. Eur. 2019;31:10. doi: 10.1186/s12302-019-0192-2. [DOI] [Google Scholar]
- 38.Wernersson A.-S., Carere M., Maggi C., Tusil P., Soldan P., James A., Sanchez W., Dulio V., Broeg K., Reifferscheid G., Buchinger S., Maas H., Van Der Grinten E., O'Toole S., Ausili A., Manfra L., Marziali L., Polesello S., Lacchetti I., Mancini L., Lilja K., Linderoth M., Lundeberg T., Fjällborg B., Porsbring T., Larsson D.J., Bengtsson-Palme J., Förlin L., Kienle C., Kunz P., Vermeirssen E., Werner I., Robinson C.D., Lyons B., Katsiadaki I., Whalley C., den Haan K., Messiaen M., Clayton H., Lettieri T., Carvalho R.N., Gawlik B.M., Hollert H., Di Paolo C., Brack W., Kammann U., Kase R. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ. Sci. Eur. 2015;27:7. doi: 10.1186/s12302-015-0039-4. [DOI] [Google Scholar]
- 39.Neale P.A., Escher B.I., de Baat M.L., Enault J., Leusch F.D.L. Effect-based trigger values are essential for the uptake of effect-based methods in water safety planning. Environ. Toxicol. Chem. 2023;42:714–726. doi: 10.1002/etc.5544. [DOI] [PubMed] [Google Scholar]
- 40.Neale P.A., Escher B.I. In vitro bioassays to assess drinking water quality. Curr Opin Environ Sci Health. 2019;7:1–7. doi: 10.1016/j.coesh.2018.06.006. [DOI] [Google Scholar]
- 41.Escher B.I., Allinson M., Altenburger R., Bain P.A., Balaguer P., Busch W., Crago J., Denslow N.D., Dopp E., Hilscherova K., Humpage A.R., Kumar A., Grimaldi M., Jayasinghe B.S., Jarosova B., Jia A., Makarov S., Maruya K.A., Medvedev A., Mehinto A.C., Mendez J.E., Poulsen A., Prochazka E., Richard J., Schifferli A., Schlenk D., Scholz S., Shiraishi F., Snyder S., Su G., Tang J.Y.M., Burg B. van der, Linden S.C. van der, Werner I., Westerheide S.D., Wong C.K.C., Yang M., Yeung B.H.Y., Zhang X., Leusch F.D.L. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol. 2014;48:1940–1956. doi: 10.1021/es403899t. [DOI] [PubMed] [Google Scholar]
- 42.Kinnberg K. Danish Environmental Protection Agency; Copenhagen, Denmark: 2003. Evaluation of in Vitro Assays for Determination of Estrogenic Activity in the Environment. [Google Scholar]
- 43.Gómez L., Niegowska M., Navarro A., Amendola L., Arukwe A., Ait-Aissa S., Balzamo S., Barreca S., Belkin S., Bittner M., Blaha L., Buchinger S., Busetto M., Carere M., Colzani L., Dellavedova P., Denslow N., Escher B.I., Hogstrand C., Khan E.A., König M., Kroll K.J., Lacchetti I., Maillot-Marechal E., Moscovici L., Potalivo M., Sanseverino I., Santos R., Schifferli A., Schlichting R., Sforzini S., Simon E., Shpigel E., Sturzenbaum S., Vermeirssen E., Viarengo A., Werner I., Lettieri T. Estrogenicity of chemical mixtures revealed by a panel of bioassays. Sci. Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunz P.Y., Simon E., Creusot N., Jayasinghe B.S., Kienle C., Maletz S., Schifferli A., Schönlau C., Aït-Aïssa S., Denslow N.D., Hollert H., Werner I., Vermeirssen E.L.M. Effect-based tools for monitoring estrogenic mixtures: evaluation of five in vitro bioassays. Water Res. 2017;110:378–388. doi: 10.1016/j.watres.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 45.Valbonesi P., Profita M., Vasumini I., Fabbri E. Contaminants of emerging concern in drinking water: quality assessment by combining chemical and biological analysis. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143624. [DOI] [PubMed] [Google Scholar]
- 46.Desye B., Belete B., Asfaw Gebrezgi Z., Terefe Reda T. Efficiency of treatment plant and drinking water quality assessment from source to household, Gondar city, Northwest Ethiopia. J Environ Public Health. 2021;2021:1–8. doi: 10.1155/2021/9974064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escher B., Neale P., Leusch F. IWA Publishing; 2021. Bioanalytical Tools in Water Quality Assessment. [DOI] [Google Scholar]
- 48.Priac A., Morin-Crini N., Druart C., Gavoille S., Bradu C., Lagarrigue C., Torri G., Winterton P., Crini G. Alkylphenol and alkylphenol polyethoxylates in water and wastewater: a review of options for their elimination. Arab. J. Chem. 2014;10:3749–3773. doi: 10.1016/j.arabjc.2014.05.011. [DOI] [Google Scholar]
- 49.Bhandari G., Bagheri A.R., Bhatt P., Bilal M. Occurrence, potential ecological risks, and degradation of endocrine disrupter, nonylphenol, from the aqueous environment. Chemosphere. 2021;275 doi: 10.1016/j.chemosphere.2021.130013. [DOI] [PubMed] [Google Scholar]
- 50.Maggioni S., Balaguer P., Chiozzotto C., Benfenati E. Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ. Sci. Pollut. Res. 2013;20:1649–1660. doi: 10.1007/s11356-012-1075-x. [DOI] [PubMed] [Google Scholar]
- 51.Russo G., Laneri S., Di Lorenzo R., Neri I., Dini I., Ciampaglia R., Grumetto L. Monitoring of pollutants content in bottled and tap drinking water in Italy. Molecules. 2022;27:3990. doi: 10.3390/molecules27133990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valcárcel Y., Valdehíta A., Becerra E., López de Alda M., Gil A., Gorga M., Petrovic M., Barceló D., Navas J.M. Determining the presence of chemicals with suspected endocrine activity in drinking water from the Madrid region (Spain) and assessment of their estrogenic, androgenic and thyroidal activities. Chemosphere. 2018;201:388–398. doi: 10.1016/j.chemosphere.2018.02.099. [DOI] [PubMed] [Google Scholar]
- 53.Benotti M.J., Trenholm R.A., Vanderford B.J., Holady J.C., Stanford B.D., Snyder S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. Drinking water. Environ. Sci. Technol. 2009;43:597–603. doi: 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- 54.Chen M., Ohman K., Metcalfe C., Ikonomou M.G., Amatya P.L., Wilson J. Pharmaceuticals and endocrine disruptors in wastewater treatment effluents and in the water supply system of Calgary, Alberta, Canada. Water Qual Res J. 2006;41:351–364. doi: 10.2166/wqrj.2006.039. [DOI] [Google Scholar]
- 55.Leusch F.D.L., Neale P.A., Arnal C., Aneck-Hahn N.H., Balaguer P., Bruchet A., Escher B.I., Esperanza M., Grimaldi M., Leroy G., Scheurer M., Schlichting R., Schriks M., Hebert A. Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Res. 2018;139:10–18. doi: 10.1016/j.watres.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 56.Kuch H.M., Ballschmiter K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC−(NCI)−MS in the picogram per liter range. Environ. Sci. Technol. 2001;35:3201–3206. doi: 10.1021/es010034m. [DOI] [PubMed] [Google Scholar]
- 57.Mohammadi A., Dobaradaran S., Schmidt T.C., Malakootian M., Spitz J. Emerging contaminants migration from pipes used in drinking water distribution systems: a review of the scientific literature. Environ. Sci. Pollut. Res. 2022;29:75134–75160. doi: 10.1007/s11356-022-23085-7. [DOI] [PubMed] [Google Scholar]
- 58.Riva F., Castiglioni S., Fattore E., Manenti A., Davoli E., Zuccato E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg Environ. Health. 2018;221:451–457. doi: 10.1016/j.ijheh.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Snyder S., Wert E., Lei H., Westerhoff P., Yoon Y. American Water Works Research Foundation; 2007. Removal of EDCs and Pharmaceuticals in Drinking and Reuse Treatment Processes. [Google Scholar]
- 60.Hammodat A.R., Nassar S., Mortula M.M., Shamsuzzaman M. Factors affecting the leaching of micro and nanoplastics in the water distribution system. J. Environ. Manag. 2023;345 doi: 10.1016/j.jenvman.2023.118779. [DOI] [PubMed] [Google Scholar]
- 61.Rajasärkkä J., Pernica M., Kuta J., Lašňák J., Šimek Z., Bláha L. Drinking water contaminants from epoxy resin-coated pipes: a field study. Water Res. 2016;103:133–140. doi: 10.1016/j.watres.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 62.Katibi K.K., Yunos K.F., Che Man H., Aris A.Z., Mohd Nor M.Z., Azis R.S., Umar A.M. Contemporary techniques for remediating endocrine-disrupting compounds in various water sources: advances in treatment methods and their limitations. Polymers. 2021;13:3229. doi: 10.3390/polym13193229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Z., Liu Z., Wang H., Wan Y., Dang Z., Guo P., Song Y., Chen S. Twelve natural estrogens and ten bisphenol analogues in eight drinking water treatment plants: analytical method, their occurrence and risk evaluation. Water Res. 2023 doi: 10.1016/j.watres.2023.120310. [DOI] [PubMed] [Google Scholar]
- 64.Kumar M., Ngasepam J., Dhangar K., Mahlknecht J., Manna S. Critical review on negative emerging contaminant removal efficiency of wastewater treatment systems: concept, consistency and consequences. Bioresour. Technol. 2022;352 doi: 10.1016/j.biortech.2022.127054. [DOI] [PubMed] [Google Scholar]
- 65.Lv X., Xiao S., Zhang G., Jiang P., Tang F. Occurrence and removal of phenolic endocrine disrupting chemicals in the water treatment processes. Sci. Rep. 2016;6 doi: 10.1038/srep22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim T.G., Yun J., Hong S.-H., Cho K.-S. Effects of water temperature and backwashing on bacterial population and community in a biological activated carbon process at a water treatment plant. Appl. Microbiol. Biotechnol. 2014;98:1417–1427. doi: 10.1007/s00253-013-5057-9. [DOI] [PubMed] [Google Scholar]
- 67.Dingemans M.M., Baken K.A., van der Oost R., Schriks M., van Wezel A.P. Risk‐based approach in the revised European Union drinking water legislation: opportunities for bioanalytical tools. Integrated Environ. Assess. Manag. 2019;15:126–134. doi: 10.1002/ieam.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kienzler A., Bopp S.K., van der Linden S., Berggren E., Worth A. Regulatory assessment of chemical mixtures: requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016;80:321–334. doi: 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Neale P.A., Feliers C., Glauch L., König M., Lecarpentier C., Schlichting R., Thibert S., Escher B.I. Application of in vitro bioassays for water quality monitoring in three drinking water treatment plants using different treatment processes including biological treatment, nanofiltration and ozonation coupled with disinfection. Environ. Sci.: Water Res Technol. 2020;6:2444–2453. doi: 10.1039/C9EW00987F. [DOI] [Google Scholar]
- 70.Wagner M., Oehlmann J. Endocrine disruptors in bottled mineral water: estrogenic activity in the E-Screen. J. Steroid Biochem. Mol. Biol. 2011;127:128–135. doi: 10.1016/j.jsbmb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Oskarsson A., Rosenmai A.K., Mandava G., Johannisson A., Holmes A., Tröger R., Lundqvist J. Assessment of source and treated water quality in seven drinking water treatment plants by in vitro bioassays - oxidative stress and antiandrogenic effects after artificial infiltration. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.144001. [DOI] [PubMed] [Google Scholar]
- 72.Rosenmai A.K., Lundqvist J., le Godec T., Ohlsson Å., Tröger R., Hellman B., Oskarsson A. In vitro bioanalysis of drinking water from source to tap. Water Res. 2018;139:272–280. doi: 10.1016/j.watres.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Shi P., Zhou S., Xiao H., Qiu J., Li A., Zhou Q., Pan Y., Hollert H. Toxicological and chemical insights into representative source and drinking water in eastern China. Environ. Pollut. 2018;233:35–44. doi: 10.1016/j.envpol.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 74.Conley J.M., Evans N., Mash H., Rosenblum L., Schenck K., Glassmeyer S., Furlong E.T., Kolpin D.W., Wilson V.S. Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 U.S. drinking water treatment plants. Sci. Total Environ. 2017;579:1610–1617. doi: 10.1016/j.scitotenv.2016.02.093. [DOI] [PubMed] [Google Scholar]
- 75.Gou Y.-Y., Lin S., Que D.E., Tayo L.L., Lin D.-Y., Chen K.-C., Chen F.-A., Chiang P.-C., Wang G.-S., Hsu Y.-C., Chuang K.P., Chuang C.-Y., Tsou T.-C., Chao H.-R. Estrogenic effects in the influents and effluents of the drinking water treatment plants. Environ. Sci. Pollut. Res. 2016;23:8518–8528. doi: 10.1007/s11356-015-5946-9. [DOI] [PubMed] [Google Scholar]
- 76.Been F., Pronk T., Louisse J., Houtman C., van der Velden-Slootweg T., van der Oost R., Dingemans M.M.L. Development of a framework to derive effect-based trigger values to interpret CALUX data for drinking water quality. Water Res. 2021;193 doi: 10.1016/j.watres.2021.116859. [DOI] [PubMed] [Google Scholar]
- 77.Escher B.I., Neale P.A. Effect‐based trigger values for mixtures of chemicals in surface water detected with in vitro bioassays. Environ. Toxicol. Chem. 2021;40:487–499. doi: 10.1002/etc.4944. [DOI] [PubMed] [Google Scholar]
- 78.Escher B.I., Neale P.A., Leusch F.D.L. Effect-based trigger values for in vitro bioassays: reading across from existing water quality guideline values. Water Res. 2015;81:137–148. doi: 10.1016/j.watres.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 79.Leusch F.D.L., Neale P.A., Busetti F., Card M., Humpage A., Orbell J.D., Ridgway H.F., Stewart M.B., van de Merwe J.P., Escher B.I. Transformation of endocrine disrupting chemicals, pharmaceutical and personal care products during drinking water disinfection. Sci. Total Environ. 2019;657:1480–1490. doi: 10.1016/j.scitotenv.2018.12.106. [DOI] [PubMed] [Google Scholar]
- 80.Lundqvist J., Andersson A., Johannisson A., Lavonen E., Mandava G., Kylin H., Bastviken D., Oskarsson A. Innovative drinking water treatment techniques reduce the disinfection-induced oxidative stress and genotoxic activity. Water Res. 2019;155:182–192. doi: 10.1016/j.watres.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 81.Maffei F., Carbone F., Forti G.C., Buschini A., Poli P., Rossi C., Marabini L., Radice S., Chiesara E., Hrelia P. Drinking water quality: an in vitro approach for the assessment of cytotoxic and genotoxic load in water sampled along distribution system. Environ. Int. 2009;35:1053–1061. doi: 10.1016/j.envint.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Alias C., Feretti D., Benassi L., Zerbini I., Zani C., Sorlini S. Tools for monitoring toxicological and genotoxicological changes in a drinking water treatment plant in Northeast Italy. Water Environ. J. 2022;37:81–94. doi: 10.1111/wej.12819. [DOI] [Google Scholar]
- 83.Sui S., Liu H., Yang X. Research progress of the endocrine-disrupting effects of disinfection byproducts. JoX. 2022;12:145–157. doi: 10.3390/jox12030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan X., Lin T., Wang X., Zhang S., Zhou K. Effects of pipe materials on the characteristic recognition, disinfection byproduct formation, and toxicity risk of pipe wall biofilms during chlorination in water supply pipelines. Water Res. 2022;210 doi: 10.1016/j.watres.2021.117980. [DOI] [PubMed] [Google Scholar]
- 85.Ceretti E., Moretti M., Zerbini I., Villarini M., Zani C., Monarca S., Feretti D. Occurrence and control of genotoxins in drinking water: a monitoring proposal. Publ. Health Res. 2016;5:769. doi: 10.4081/jphr.2016.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There is no data need to deposit into a publicly available repository.