Abstract
Abstract
Congenital diaphragmatic hernia (CDH) is a severe birth defect and a major cause of neonatal respiratory distress. Impacting ~2–3 in 10,000 births, CDH is associated with a high mortality rate, and long-term morbidity in survivors. Despite the significant impact of CDH, its etiology remains incompletely understood. In 2003, Greer et al. proposed the Retinoid Hypothesis, stating that the underlying cause of abnormal diaphragm development in CDH was related to altered retinoid signaling. In this review, we provide a comprehensive update to the Retinoid Hypothesis, discussing work published in support of this hypothesis from the past 20 years. This includes reviewing teratogenic and genetic models of CDH, lessons from the human genetics of CDH and epidemiological studies, as well as current gaps in the literature and important areas for future research. The Retinoid Hypothesis is one of the leading hypotheses to explain the etiology of CDH, as we continue to better understand the role of retinoid signaling in diaphragm development, we hope that this information can be used to improve CDH outcomes.
Impact
This review provides a comprehensive update on the Retinoid Hypothesis, which links abnormal retinoic acid signaling to the etiology of congenital diaphragmatic hernia.
The Retinoid Hypothesis was formulated in 2003. Twenty years later, we extensively review the literature in support of this hypothesis from both animal models and humans.
Introduction
Congenital Diaphragmatic Hernia (CDH) is a life-threatening birth defect with high mortality that affects 2–3 in 10,000 newborns.1–3 CDH is characterized by incomplete formation of the diaphragm and associated lung hypoplasia, contributing to severe neonatal respiratory distress.4,5 In high-resource settings, at least 1 in 5 newborns with CDH will not survive the neonatal period (~20–30% mortality), with outcomes significantly worse in low-resource settings (>90% mortality).6–8 While rare, there is a considerable cost incurred when providing newborns with CDH intensive care, requiring significant healthcare resources.3,9–11 This burden on the healthcare system and patient families is compounded when considering that over half of surviving CDH patients have complex long-term morbidity.12–15 To fully grasp the clinical picture of CDH’s impact, the reader is referred to the recent review by Zani and colleagues.5
The etiology of CDH is complex and remains largely unknown.5,16 Different considerations include the clinical presentation and type of diaphragm defect. For example, CDH can present as an isolated defect (~50–60% of cases) or complex CDH, where additional abnormalities are present.1,17,18 Moreover, CDH can be thought of as an umbrella term incorporating different types of diaphragm defect.19,20 This includes the most commonly occurring Bochdalek CDH that is characterized by a hole in the posterolateral diaphragm (~90–95% of cases), anterior holes through the foramen of Morgagni (Morgagni hernias, ~3% of cases), diaphragm eventrations (~2–3% of cases), and central tendon defects (~1–2% of cases).19,21,22 As discussed below, it is important to consider that etiologic factors may vary between these different presentations of CDH. It is generally considered that the two main factors that contribute to CDH are genetic and/or environmental. Approximately 30–40% of CDH cases have an identifiable genetic cause.3,23 Of this number, chromosomal defects account for about ~10% of CDH cases, and de novo mutations account for 10–22% of cases.24–26 Environmental risk factors for CDH are varied, including associations with maternal age, alcohol use, and smoking.3,16,27–30 As the major focus of this review, one of the leading hypotheses to explain the etiology of CDH is the so-called Retinoid Hypothesis. This hypothesis was crystallized in the landmark paper published by Greer, Babiuk, and Thebaud in 2003, stating that “abnormalities linked with the retinoid signaling pathway early in gestation may contribute to the etiology of CDH”.31 The Retinoid Hypothesis is centered on the importance of the signaling molecule retinoic acid (RA) as a potent regulator of mammalian development.32–34 RA is an active metabolite of dietary vitamin A; the reader is directed to the following reviews that comprehensively describe its complex metabolism and signaling pathway.35–37
The 2003 formulation of the Retinoid Hypothesis was a milestone in CDH research and influenced subsequent work in the field.31 Here, we review discoveries that complement and strengthen the Retinoid Hypothesis, drawing upon work from both animal models and human CDH published in the last 20 years. Note, we have focused on RA signaling in abnormal diaphragm development; while there is an extensive literature linking RA, the lungs, and CDH, it is beyond the scope of this review.
History of the Retinoid Hypothesis
The Retinoid Hypothesis was based on research dating back to the 1940s,38 drawing together several threads of evidence to justify the notion that altered retinoid signaling may cause CDH.31 The foundation for the Retinoid Hypothesis included evidence from animal models of CDH, as well as emerging clinical data. In brief, these studies included: (1) the observation that the offspring of vitamin A deficient rats had a high incidence of diaphragmatic defects, and when vitamin A was re-introduced into the diet, the rate of herniation decreased.39–41 (2) Compound Retinoic acid receptor (Rar) knock-out mice have a low incidence of CDH.42,43 (3) Nitrofen, a teratogen widely used to study CDH in rodents, inhibits the RA synthesizing enzyme RALDH2 (also called ALDH1A2) in vitro,44 and suppresses activation of a Rar reporter gene (RARE-LacZ) in vivo.44,45 (4) Nitrofen-induced CDH in rats can be reduced by the co-administration of large doses of vitamin A.46 And (5) in a small cohort of infants with CDH, circulating levels of retinol and its carrier protein in the blood (RBP) were decreased by ~50% in comparison with controls.47 The balance of this evidence led the authors to conclude that altered retinoid signaling may be a contributing factor in the etiology of CDH. As reviewed below, the formulation of the Retinoid Hypothesis had a significant impact on the field and its direction of research.
New insights from animal models of CDH
Animal models of CDH are an important tool in improving our understanding of how CDH develops and its causes. Recent discoveries have helped to define the RA signaling pathway in the developing diaphragm, as well as provide important new insight into the role of vitamin A and its derivatives in the development of CDH gleaned from teratogenic and genetic models.
Defining the retinoic acid signaling pathway in the developing diaphragm
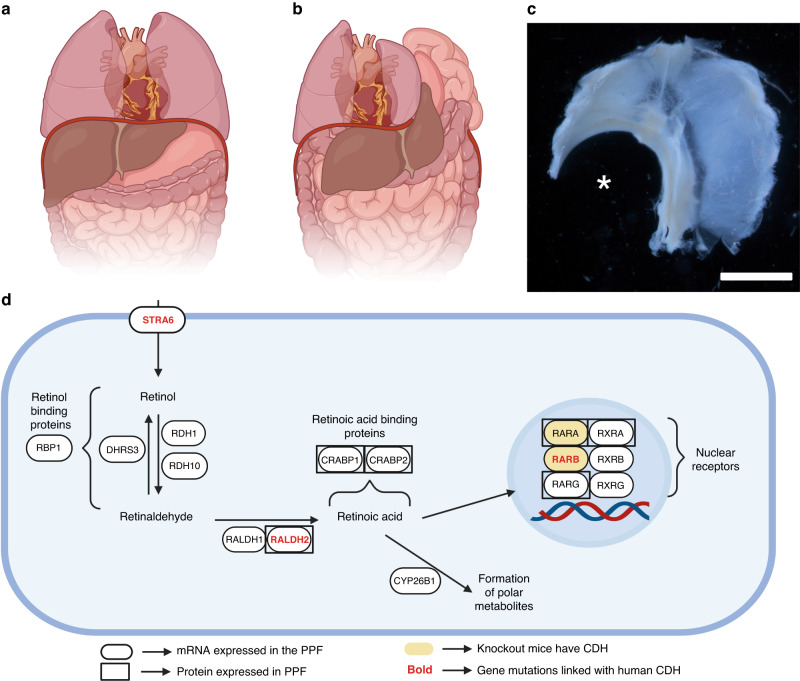
As the Retinoid Hypothesis has developed, it has become increasingly important to define the RA signaling pathway in the developing diaphragm, including identifying which specific components of the pathway are expressed in the developing diaphragm, and in which specific cells. Given our knowledge of CDH pathogenesis, this work has primarily focused on the pleuroperitoneal folds (PPFs); key structures in the developing diaphragm that are known to be disrupted in animal models of CDH.48,49 Immunohistochemical and transcriptomic studies have helped to define the RA signaling pathway in the PPF,23,50 with additional insight gained from other studies.51 Figure 1 describes our current understanding of the RA signaling pathway in the PPF, although we recognize that this pathway does not discriminate between different cellular subtypes within this structure, an important knowledge gap in the field given the suspected importance of specific cell types in the cellular origins of CDH, a topic beyond the scope of the current review.49,52
Fig. 1. Congenital diaphragmatic hernia and retinoic acid signaling.
The diaphragm forms an anatomical barrier between the abdominal and thoracic cavity (a). CDH is characterized by incomplete diaphragm development and herniation of abdominal organs into the thoracic cavity (b). Rodent models are widely used to models to study CDH. Here, a superior view of a whole diaphragm dissected from a teratogen-treated mouse fetus shows a large left-sided posterolateral (Bochdalek) diaphragm defect (asterisk; c). Retinoic acid signaling is thought to be essential for normal diaphragm development. A schematic representing our current knowledge regarding retinoic acid metabolism and signaling in the pleuroperitoneal fold (PPF), a key structure in the developing diaphragm is presented (d). This figure reflects known expression of specific genes and proteins in the PPF, as well as instances where mouse or human gene mutations have been associated with CDH. c scales bar = 500 µm. a, b, and d created with BioRender.com.
Teratogenic models of CDH
Key studies in the original formulation of the Retinoid Hypothesis were based on data generated using the nitrofen model of CDH in rodents.53–55 While much of the early work used rats, mice are increasingly being used. The timing of teratogen administration is similar in both species (i.e., ~gestational day 8–10); however, for unknown reasons mice seem to be more resistant to nitrofen treatment, necessitating the use of teratogenic combinations to generate a high incidence of CDH.50,56,57 Recent work has strengthened the Retinoid Hypothesis by exploring how nitrofen produces CDH through the disruption of RA signaling, how exogenous retinoids can rescue teratogen-induced CDH, and how direct targeting of RAR signaling can causes CDH.
Mechanisms of teratogen-induced CDH
The Retinoid Hypothesis was founded on reports that nitrofen can inhibit RALDH2 and suppress activation of RAR signaling.44,45 Noble and colleagues conducted a series of mechanistic studies to better understand how nitrofen disrupted retinoid signaling to induce CDH.58 This work provided further evidence that nitrofen inhibits RALDH2 using a RARE-LacZ reporter system in P19 cells, which could be overcome by adding excess enzyme substrate in the form of retinal.58 It was subsequently shown in rats that nitrofen treatment on gestational day 9 significantly decreased fetal RA levels between gestational day 11 and 13, which coincides with a critical time in diaphragm development and provided unequivocal evidence that nitrofen treatment could inhibit RA synthesis in vivo.58 This decrease in RA synthesis complemented the previously observed decrease in activation of the RARE-LacZ reported gene in nitrofen-treated mice.45 Indeed, nitrofen and three other CDH-inducing teratogens that inhibit RALDH2 all decrease embryonic retinoid acid signaling throughout the critical period of diaphragm development, as subsequently shown in RARE-LacZ mice.50 While alternative mechanisms have been suggested,59,60 the evidence suggests that nitrofen primarily induces CDH by inhibiting RALDH2, thereby decreasing RA synthesis and inhibiting downstream signaling through the RARs.
Exogenous retinoids can rescue teratogen-induced CDH
Powerful evidence to support nitrofen’s mechanism of action and the importance of retinoid signaling in diaphragm development came from the observation that administration of vitamin A could lower the incidence of nitrofen-induced CDH in rats.46 Using a dose of 15,000 IU of vitamin A, Thebaud et al. decreased the incidence of nitrofen-induced CDH by over half.46 Using the same dose of vitamin A, other groups confirmed that co-administration of vitamin A could lower the incidence of nitrofen induced CDH in rats, while also showing beneficial effects of exogenous vitamin A on the developing lung.61,62 Based on the model that nitrofen inhibits RA synthesis, subsequent studies tested the hypothesis that administration of RA would be more efficacious than vitamin A (retinol) administration in preventing teratogen-induced CDH.63 These studies showed that while vitamin A administration (25,000 IU vitamin A) reduced the incidence of CDH by ~40%, RA had a more potent rescue effect, lowering the incidence by ~80%.63 The potent effect of direct supplementation with RA to prevent CDH was further emphasized in teratogen-treated mice.50 Here, a high incidence of CDH (~60% CDH) was almost completely abrogated by co-administration with RA (~1% CDH), with the authors further showing that while teratogen treatment decreased embryonic RARE-lacZ expression, RAR signaling was restored by RA treatment in accord with the decrease in CDH incidence.50
A limitation of the rescue studies discussed above was the high dose of vitamin A (e.g., 15,000–25,000 IU vitamin A) required to reverse the effects of nitrofen. Interestingly, based on the observation that low maternal dietary vitamin A intake is a risk factor for developing CDH in humans,64,65 it has been shown that altered dietary vitamin A intake can modulate the incidence of teratogen-induced CDH, such that mice consuming a vitamin A deficient diet have a higher incidence of CDH.57 This study is important because it shows that high, pharmacological doses of vitamin A are not required to prevent teratogen-induced CDH, rather, it can be achieved through increased dietary vitamin A intake. As discussed below, this is important in the context of low maternal dietary vitamin A intake in humans as a potential risk factor for CDH.
Taken together, several studies have shown the efficacy of high doses of vitamin A to prevent teratogen-induced CDH, with more recent work suggesting that adequate dietary vitamin A intake can achieve a similar result. These studies highlight the importance of RA signaling in the development of teratogen-induced CDH and the feasibility of preventing CDH, at least in animal models.
Directly targeting retinoic acid receptor signaling induces CDH
As discussed above, there is strong evidence to suggest that nitrofen induces CDH by inhibiting RA synthesis via RALDH2. Other potential mechanisms of nitrofen’s action have been suggested,58–60 representing a potential limitation in the interpretation of these studies. On the other hand, studies using the pan-RAR antagonist BMS493 provide direct evidence in support of the Retinoid Hypothesis, showing that specific inhibition of RA signaling is a potent inducer of Bochdalek CDH in rats.50 Indeed, a 5 mg/kg dose of BMS493 induced almost 100% herniation when given to pregnant dams on gestational day 11. BMS493 administration was further used to define the critical period of diaphragm development in rats, which spanned gestational days 8 to 13. Remarkably, separate critical periods were identified for the left and right hemi-diaphragms, with the left side of the diaphragm being preferentially affected with BMS493 administration between gestational days 8 and 9, the right side preferentially affected between gestational days 11 and 13, and bilateral hernias produced with administration on gestational day 10. Thus, direct targeting of RAR signaling on different gestational days induced CDH in a temporally and spatially restricted manner.50 While BMS493 was first used to induce CDH in rats, subsequent research has shown that this compound can also induce CDH in mice, albeit at a lower incidence (~6%).66 Similarly, <1% of mice treated with the pan-RAR antagonist BMS-189453 also developed CDH.67
Genetic models of CDH
The observation that Rar knock-out mice developed CDH was integral to the original formulation of the Retinoid Hypothesis.31 Surprisingly, there has been a lack of studies genetically dissecting the RA signaling pathway in the developing diaphragm; however, several other mouse models with an indirect relationship to RA signaling have been described that are supportive of the Retinoid Hypothesis.
Genetic models directly related to retinoic acid signaling
The only direct genetic evidence supporting the Retinoid Hypothesis obtained from mice with targeted mutations in RA signaling come from studies of Rar compound mutant mice (Fig. 1). There are three Rars encoded in the mammalian genome, Rara, Rarb, and Rarg.32,68 Single knock outs for each Rar do not produce diaphragm defects; however, a low incidence of CDH has been reported in Rara−/−:Rarb−/− compound mutant mice, which was not observed in Rara−/−:Rarg−/− or Rarb−/−:Rarg−/−compound mutants.42,43 Interestingly, diaphragm defects were also reported in Rxra transgenic mice that lack their activation function domain (AF-2), highlighting the importance of this heterodimeric partner of the RARs.69 To our knowledge, there are no other published studies describing diaphragm defects in mice carrying mutations in genes directly associated with RA signaling. Indeed, many knock-out mice in this pathway are grossly normal and viable (e.g., Lrat, Crbp1, and Rbp4).70–72 The lack of a diaphragm phenotype in these mice may reflect robustness/compensation in the RA signaling pathway, or evidence that they are not essential in diaphragm development. Conversely, some knock-out mice in this pathway are embryonic lethal (e.g., Raldh2, Cyp26a1, and Dhrs3)73–75 precluding an assessment of diaphragm formation. In this latter case, experimental approaches using conditional deletion of specific genes could allow testing of their hypothetical importance in the developing diaphragm.
Genetic models indirectly related to retinoic acid signaling
In addition to Rar compound mutant mice, diaphragm defects have been described in other genetic models, including several genes indirectly linked to RA signaling.76 This link is most clear with Wt1, which encodes a transcription factor with a broad expression pattern, including the PPF.77–79 The original description of Wt1−/− mice emphasized its role in urogenital development but also mentioned diaphragm defects that were later found to be like Bochdalek CDH.80,81 Because of its broad importance in embryonic development,82 Wt1−/− mice typically do not survive into late gestation, limiting the ability to study diaphragm development.81 This limitation was overcome by conditional deletion of Wt1. Carmona et al. used an enhancer of Gata4 to conditionally delete Wt1 in the lateral plate mesoderm, leading to a ~80% incidence of Bochdalek CDH.51 Similarly, Cleal et al. conditionally deleted Wt1 in the PPF to generate offspring with an 80–90% penetrance of Bochdalek CDH.77 Regarding RA signaling, WT1 regulates the expression of the RA synthesizing enzymes RALDH2,83 and Wt1 itself is thought to be a RA target gene.84 Interestingly, dietary RA supplementation has been shown to relieve the incidence of CDH in mice with conditional Wt1 deletion and decrease the size of diaphragmatic defects in embryos that still displayed CDH.51
Like WT1, COUP transcription factor 2 (COUP-TF2, encoded by Nr2f2) is a developmentally important transcription factor with links to RA signaling.85,86 It has been shown that COUP-TF2 is upregulated by RA, and it can regulate gene expression by modulating the dimerization of RAR/RXRs.84,87–90 Global Coup-tf2−/− mice are embryonically lethal; however, conditional deletion of Coup-tf2 in the PPF induces Bochdalek CDH in ~50% of offspring.91 Two other genes that encode transcription factors that functionally interact with each other and have been linked to RA signaling and CDH are Gata4 and Fog2 (also known as Zfpm2). GATA4 is involved in many different developmental processes, the most important one being proper cardiac development.92–94 Gata4−/− mice are embryonic lethal, but mice heterozygous for a Gata4 mutation (exon 2 deletion) develop midline diaphragm defects, impacting the central tendon in ~30% of offspring and causing overt herniation in ~15% of offspring.95 Furthermore, conditional deletion of Gata4 in the PPF caused diaphragm defects in 100% of mutant mice, 2/3 of which were Bochdalek hernias, and the remaining 1/3 Morgagni hernias.96 FOG2 is thought of as a co-factor of the GATA transcription factors and has been shown to interact with GATA4 in heart development.97 Mice with a mutation in Fog2 present diaphragm abnormalities characterized by incomplete muscularization of the posterolateral diaphragm in 100% of mutant mice.98 Regarding the Retinoid Hypothesis, both Gata4 and Fog2 are thought to be RA target genes,84,87,99,100 and it has been shown that retinoids can regulate downstream gene expression by interacting with GATA4 and FOG2.101 Interestingly, FOG2 has also been shown to interact with COUP-TF2, suggesting complex interactions with multiple transcription factors that are important in diaphragm development.102
The presence of diaphragm defects in Wt1, Coup-tf2, Gata4, and Fog2 mutant mice indicates their individual importance in normal diaphragm development. Links between these genes and RA signaling support the Retinoid Hypothesis, as recently discussed by others.103 Future studies integrating our understanding of how these genes interact should provide insight into the cellular pathogenesis and etiology of CDH.
New Insights from human cases of CDH
When Greer and colleagues formulated the Retinoid Hypothesis, there was sparse evidence linking it with CDH in infants.31 This represented a limitation of the hypothesis; however, in the intervening 20 years new insights driven by advances in clinical genetics, as well as epidemiological studies have strengthened the relevance of the hypothesis to CDH in humans.
Retinoid status of infants with CDH
The only clinical data in support of the Retinoid Hypothesis when it was formulated came from a small study of vitamin A status in 11 cases of CDH.31,47 This study found that newborns with CDH had a ~50% decrease in circulating retinol and retinol binding protein (RBP) concentrations compared to healthy controls.22 A follow-up study including 22 newborns with CDH confirmed this observation, reporting significantly lower retinol and RBP concentrations in newborns with CDH compared to matched controls; however, the magnitude of this decrease was relatively smaller (~20–25% decrease).104 Neither study found a decrease in maternal markers of vitamin A status, suggesting that maternal vitamin A deficiency was not a contributing factor to CDH in these patient populations. Thus, these two studies suggest that the vitamin A status of newborns with CDH is somehow impaired, although the reason for this remains elusive and requires further study. To our knowledge, there has been no other comprehensive examination of vitamin A status in CDH. One autopsy study including nine cases of CDH attempted to indirectly measure retinoid stores in the liver and lung via CRBP1 immunoreactivity.105 These results were interpreted in the context of “retinoic acid status”, which we have questioned as incorrect,106 nevertheless, this study hints at the possibility of impaired vitamin A status of fetuses with CDH. A physiological decline in circulating maternal retinol levels has been reported in human pregnancy, with a rebound to non-pregnant levels after birth.107–109 A similar pattern is seen in pregnant mice and rats.110,111 This has led to the suggestion that these declines correspond with a critical period in diaphragm development and may render this structure more susceptible to aberrant RA signaling.31,103 Variations in circulating maternal retinol levels, fetal retinoid status, and their importance in CDH require further study.
The genetics of CDH and the Retinoid Hypothesis
The genetic etiology of CDH is complex and continues to be an active area of research and discovery.16,76,112 When the Retinoid Hypothesis was first proposed, our understanding of the genetics of CDH was limited; however, since then, there has been progress in understanding the genetic etiology of CDH, as well as how this new knowledge relates to the Retinoid Hypothesis.103,113 Like the mouse genetics, two themes emerge from the human genetics of CDH in relation to the Retinoid Hypothesis: a subset of genes associated with CDH are directly involved in RA signaling, while others can be indirectly linked to RA signaling either as target genes or factors that potentially interact with RAR signaling.
Human genetic disorders directly linking retinoid acid signaling and CDH
Goumy et al. systematically discussed the RA signaling pathway and drew links of varying strength with several CDH-associated genes.113 The strongest evidence linking RA signaling and CDH in humans exists for STRA6, RALDH2, and RARB (Fig. 1). STRA6 (OMIM: 610745) encodes the membrane receptor for RBP, which acts as a transporter for the cellular uptake of retinol.114 STRA6 mutations cause Matthew Wood syndrome, which includes microphthalmia, pulmonary hypoplasia, cardiac malformations, as well as diaphragm defects variously reported as CDH/diaphragm eventration in ~25% of cases.115–117 Diaphragm defects are frequently associated with STRA6 mutations, and this gene is known to be expressed in the developing diaphragm, strongly linking STRA6 with CDH.23,118 ALDH1A2 (RALDH2; OMIM: 603687) encodes a protein which synthesizes RA.119 Mutations in this gene cause a variety of different phenotypes, including tetralogy of Fallot, absent thymus, kidney defects, as well as diaphragm eventration/Bochdalek CDH.120,121 Four known patients with mutations in this gene have diaphragm defects, it is expressed in the developing diaphragm, and its inhibition causes CDH in rodents, strongly linking RALDH2 with CDH.23,44,58,120–122 RARB (OMIM: 180220) encodes for the β isoform of the RAR, a nuclear receptor activated by RA that regulates gene expression.123 Mutations in this gene are associated with diaphragmatic hernias as well as eventrations.124–126 RARB mutations have been reported in 9 patients with CDH, its mutation is linked with CDH in mice, and it is expressed in the developing diaphragm, strongly linking it with CDH.124,125 As discussed by Goumy et al. and others, RBP1, RBP2, RBP5, and LRAT have all been linked with CDH, although the evidence is relatively weak.113,127 These links are primarily driven by the proximity of these genes to chromosomal regions recurrently deleted in individuals with CDH, but to our knowledge no specific gene mutations have been described in them directly linking them to CDH.
Human genetic disorders indirectly linking retinoid acid signaling and CDH
Genes that have been linked with CDH in humans that are indirectly involved in RA signaling are numerous and can be broadly categorized as genes whose protein products interact with RAR function or they are RA target genes.
As discussed above, there is significant evidence linking genes encoding proteins that interact with RAR function to CDH in animal models, including Wt1, Coup-tf2, Gata4, and Fog2. Interestingly, all these genes are also associated with CDH in humans. Mutations in WT1 (OMIM: 607102) are associated with several syndromes that include diaphragm defects within their phenotypic spectrum, including Denys-Drash syndrome, Frasier syndrome, and Meacham syndrome.128 Amongst several other genes, COUP-TF2 (OMIM: 107773) is located within the 15q26.1–26.2 cytogenetic hotspot for syndromic CDH.129 The occurrence of CDH in mice with a conditional deletion of Coup-tf2 and the later discovery of specific mutations in this gene in individuals with CDH support its importance in diaphragm development.91,130 GATA4 (OMIM: 600576) is found within the CDH critical region 8p23.1,127 with subsequent reports of GATA4 variants in humans causing CDH.131 Similarly, FOG2 (OMIM: 603693) was originally linked to CDH as it is in the critical region 8q22-8q23,127 with later studies showing specific mutations/deletions of this gene were recurrently associated with CDH.98,132 WT1, COUP-TF2, GATA4, and FOG2 are all expressed in the developing diaphragm, and all are strongly linked to CDH via both mouse and human mutations. All four genes have also been linked to RA signaling, providing supporting evidence for the importance of this pathway in normal diaphragm development.
Interestingly, COUP-TF2, GATA4, and FOG2 are all linked to RA signaling via interactions with the RARs. While the evidence is less strong, there are other genes that can be linked to RA signaling in this way. Predicted pathogenic variants in NSD1 have been identified in a cohort of fetuses with CDH.133 This gene is also associated with Beckwith-Wiedemann syndrome that infrequently includes diaphragm defects.133–135 Moreover, NSD1 is another protein that can interact with nuclear receptors, including the RARs.136 Similarly, while there has only been one known report linking it with CDH, SIN3A encodes a transcriptional corepressor that interacts with RARs.26,137 Last, while its exact interaction with RAR signaling remains unclear, KIF7 and RA signaling have been linked to the development of diaphragm defects in mice and three cases of CDH.138–140
In addition to genes that may interact with RA signaling at the level of the receptor, diaphragm defects have been described in association with mutations in numerous RA target genes. In our past analysis of CDH-associated genes, we identified an inclusive list of 218 genes linked with CDH, 44 of which were RA target genes.21 This category includes genes regulated by RA that have direct roles in RA signaling (e.g., STRA6, RALDH2, and RARB), and indirect interactions with RA signaling (e.g., WT1, COUP-TF2, GATA4, and FOG2). Thus, it is possible to envisage how aberrant RA signaling in the developing diaphragm could impact the expression of multiple genes linked with diaphragm development and precipitate CDH.
As we learn increasingly more about the genetics of CDH, a common pathway that has emerged is RA signaling, providing support for the Retinoid Hypothesis in human CDH.21 As discussed above, many CDH-associated genes can be linked with RA signaling, the challenge for the future will be to determine how these genes contribute to diaphragm development and whether it is possible to construct a gene regulatory network to show the relationship between these genes and how aberrant RA signaling can lead to abnormal diaphragm development.
Epidemiological studies and the Retinoid Hypothesis
Population-based studies have provided insight into the link between CDH and the Retinoid Hypothesis, primarily in the context of adequate maternal dietary vitamin A intake. One of the first epidemiological studies to link vitamin A intake and CDH leveraged maternal nutrient intake data between 1997 and 2003 from the US National Birth Defects Prevention Study.141 Vitamin A intake below the 10th percentile was associated with isolated CDH in women who did not use periconceptional vitamin supplements (retinol intake, odds ratio [95% CI] = 2.1 [1.1, 3.9]), and in women who did use periconceptional vitamin supplements (total vitamin A intake, odds ratio [95% CI] = 1.7 [1.2, 2.6]). The authors concluded that this data supported the Retinoid Hypothesis, but it is important to note that an expanded analysis of the US National Birth Defects Prevention Study including maternal nutrient intake data from 1997 to 2011 did not find a significant association between vitamin A intake and CDH.142 Despite this lack of agreement, two other studies from The Netherlands and Japan have shown that low maternal vitamin A intake confers an increased risk of CDH.64,65 In a Dutch population, Beurskens et al. showed that maternal dietary vitamin A intake below the recommended daily intake (<800 µg vitamin A per day) in normal weight mothers was significantly associated with an increased risk of CDH (odds ratio [95% CI] = 7.2 [1.5, 34.4]). In a Japanese population, Michikawa et al., showed that in a similar group of normal weight mothers, high total maternal dietary vitamin A intake was associated with a reduced risk of CDH (odds ratio [95% CI] = 0.6 [0.3, 1.2]). Taken together, there is collective epidemiological evidence that low maternal dietary vitamin A intake is a risk factor for CDH, although this concept requires further exploration.
While there is evidence to suggest that maternal dietary vitamin A intake is an important factor in the etiology of CDH, it has been observed that there is no evidence to suggest an increased incidence of CDH in countries with high rates of vitamin A deficiency.104 A counterpoint to this argument is that birth defect registries in these countries are inadequate, and data regarding the global incidence of CDH is incomplete.3 While considering maternal dietary vitamin A intake as a risk factor for CDH it is important to highlight that even in developed countries inadequate dietary intake can be prevalent in the general population and has been reported in 15.5% of pregnancies in the USA (n = 1003), and 10% in Poland (n = 1764).143,144 In a separate study, ~7% of women of childbearing age in the USA had low serum retinol concentrations, a factor that was linked to lower socioeconomic status.145 While overt vitamin A deficiency might not be a major contributor to the occurrence of CDH, we believe inadequate dietary vitamin A intake is a risk factor that may combine with other factors (genetic or environmental) to cause CDH.57 Indeed, we echo the recent remarks made by Gilbert and Gleghorn,103 supporting adequate maternal vitamin A intake during pregnancy at the population level to help prevent CDH, and possibly other birth defects.
Regarding other environmental risk factors that may intersect with the Retinoid Hypothesis, it is interesting to note that others have drawn links between maternal alcohol use and cigarette smoking and altered retinoid signaling.146 While these links are speculative and require further study, there is experimental evidence that links both alcohol exposure and cigarette smoke exposure to alterations in vitamin A metabolism.146–148
Future perspectives
Looking forward, it is important to consider the limitations of the Retinoid Hypothesis. There are many gaps in our knowledge regarding the hypothesis and here we pose several questions aimed at guiding future studies in the field.
How does the Retinoid Hypothesis explain abnormal diaphragm development at a cellular level?
There is an incomplete understanding of the cellular pathogenesis of CDH. It is thought that the non-muscular mesenchymal cells of the PPF are important in the development of CDH,56,122,149 but how RA signaling works in these cells, and the fate of these cells when RA signaling is disrupted, remains unexplored. Future studies using animal models of CDH can address these gaps, supplemented by studies in PPF-derived cell cultures, and patient-derived fibroblasts.150,151
Does the Retinoid Hypothesis explain all types of diaphragm defects?
The evidence supporting the Retinoid Hypothesis primarily comes from models recapitulating Bochdalek CDH. Moreover, past analysis of CDH-associated genes identified a link between genes involved in retinoid signaling and Bochdalek CDH, but not other types of diaphragm defects.21 Thus, while the Retinoid Hypothesis helps explain the etiology of Bochdalek CDH, there is less evidence linking it to diaphragm eventration, central tendon defects, and Morgagni hernias. This gap in understanding the etiology of rare subtypes of CDH should be the focus of future research.
Does the Retinoid Hypothesis explain all cases of CDH?
The percentage of CDH cases caused by abnormal retinoid signaling is unclear and difficult to estimate. In terms of CDH genetics, it is clear from relatively large cohort studies that genetic mutations directly linked to RA signaling are uncommon.152,153 In our previous analysis of 218 CDH-associated genes, only 11 were directly related to RA signaling.21 Interestingly, 52 of these genes could be indirectly linked as modulators of RA signaling or as RA target genes. If we consider population-based studies associating low maternal dietary vitamin A intake and CDH,64,65 then gene-nutrient interactions impacting RA signaling may emerge as a major contributor to the etiology of CDH. Nevertheless, as discussed elsewhere, alternate explanations for the etiology of CDH exist.
What are the alternative hypotheses to explain the etiology of CDH?
Previous links between CDH and altered thyroid hormone signaling and one-carbon metabolism, have been suggested but not supported by further analyses.58,141,142,154 Other alternative hypotheses that remain to be fully explored include a possible role for maternal dietary vitamin D intake,155,156 as well as an emerging interest in extracellular vesicles and micro RNAs.5,157
Does the Retinoid Hypothesis link abnormal diaphragm and lung development?
While beyond the scope of the current review, it is important to acknowledge that RA signaling is important in lung organogenesis.136–138 In 2000, Keijzer et al. proposed the Dual-hit Hypothesis to explain pulmonary hypoplasia in CDH, which included a direct effect on the lung (first hit) and indirect effects via physical compression secondary to herniation (second hit).139 Given RA’s role in lung development, and its emerging role in the diaphragm, the Retinoid Hypothesis is consistent with a dual-hit model of lung damage in CDH. Here, perturbed RA signaling could contribute to abnormal lung development (first hit), which would be compounded by the effects of perturbed RA signaling on diaphragm formation leading to abdominal organ herniation and further damage to the lungs (second hit), as explored by others.140–142 Whether altered RA signaling contributes to both abnormal lung and diaphragm development in CDH requires further study.
How does the Retinoid Hypothesis relate to CDH in humans?
The strongest evidence for the Retinoid Hypothesis comes from animal models. To strengthen the link with human CDH there should be a continued emphasis on studying the importance of maternal dietary vitamin A intake as a risk factor for CDH, more attention paid to markers of maternal and fetal vitamin A status in CDH, and a continued exploration of interactions between RA signaling and CDH-associated genes.
How do we leverage our understanding of the Retinoid Hypothesis to lessen the impact of CDH?
Zani et al. recently emphasized that by improving our understanding of CDH’s etiology we may improve its diagnosis and allow earlier interventions to improve CDH outcomes.5 Retinoid administration is already being studied as a tool to improve lung development in preclinical models of CDH,158–160 which may be optimized by improving our understanding of underlying defects in RA signaling. Furthermore, if early genetic testing revealed mutations in genes linked with RA signaling, it might be possible to intervene with retinoids to help ameliorate damage to the developing diaphragm/lungs, lessening the impact of CDH. At the population level, we and others believe that attaining optimal maternal dietary vitamin A intake may help lessen the impact of CDH.57,103 This would require efforts highlighting the importance of adequate periconceptional vitamin A intake in women, and their potential benefits with respect to CDH.
Concluding remarks
In the 20 years since the Retinoid Hypothesis was first proposed many studies across multiple disciplines have further supported the relationship between RA signaling and the formation of CDH.31 While the Retinoid Hypothesis has a strong evidence base derived from animal models and growing support from clinical studies, it does have limitations and gaps in our knowledge remain that should guide future research in the field, hopefully translating to improved outcomes for newborns with CDH.
Acknowledgements
This work was funded by the Canadian Institutes of Health Research (CIHR) as well as an Innovation Grant from the Women and Children’s Health Research Institute (WCHRI).
Author contributions
J.G.R. and R.D.C. both made substantial contributions to the concept of the article, literature review, and drafting of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGivern MR, et al. Epidemiology of congenital diaphragmatic hernia in Europe: a register-based study. Arch. Dis. Child Fetal Neonatal Ed. 2015;100:F137. doi: 10.1136/archdischild-2014-306174. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugam H, Brunelli L, Botto LD, Krikov S, Feldkamp ML. Epidemiology and prognosis of congenital diaphragmatic hernia: a population-based cohort study in Utah. Birth Defects Res. 2017;109:1451–1459. doi: 10.1002/bdr2.1106. [DOI] [PubMed] [Google Scholar]
- 3.Paoletti M, et al. Prevalence and risk factors for congenital diaphragmatic hernia: a global view. J. Pediatr. Surg. 2020;55:2297–2307. doi: 10.1016/j.jpedsurg.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Wagner R, Montalva L, Zani A, Keijzer R. Basic and translational science advances in congenital diaphragmatic hernia. Semin. Perinatol. 2020;44:151170. doi: 10.1053/j.semperi.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Zani A, et al. Congenital diaphragmatic hernia. Nat. Rev. Dis. Prim. 2022;8:37. doi: 10.1038/s41572-022-00362-w. [DOI] [PubMed] [Google Scholar]
- 6.Wright NJ, et al. Mortality from gastrointestinal congenital anomalies at 264 hospitals in 74 low-income, middle-income, and high-income countries: a multicentre, international, prospective cohort study. Lancet. 2021;398:325–339. doi: 10.1016/S0140-6736(21)00767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn J, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J. Pediatr. 2013;163:114–119.e1. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harting MT, Lally KP. The Congenital Diaphragmatic Hernia Study Group registry update. Semin Fetal Neonatal Med. 2014;19:370–375. doi: 10.1016/j.siny.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Cameron DB, et al. Quantifying the burden of interhospital cost variation in pediatric surgery: implications for the prioritization of comparative effectiveness research. JAMA Pediatr. 2017;171:e163926–e163926. doi: 10.1001/jamapediatrics.2016.3926. [DOI] [PubMed] [Google Scholar]
- 10.Raval MV, Wang X, Reynolds M, Fischer AC. Costs of congenital diaphragmatic hernia repair in the United States—extracorporeal membrane oxygenation foots the bill. J. Pediatr. Surg. 2011;46:617–624. doi: 10.1016/j.jpedsurg.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 11.Lewit RA, Jancelewicz T. Sources of regional and center-level variability in survival and cost of care for congenital diaphragmatic hernia (CDH) J. Pediatr. Surg. 2021;56:130–135. doi: 10.1016/j.jpedsurg.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Wong M, et al. Pulmonary hypertension in congenital diaphragmatic hernia patients: prognostic markers and long-term outcomes. J. Pediatr. Surg. 2018;53:918–924. doi: 10.1016/j.jpedsurg.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Mills J, Safavi A, Skarsgard ED. Chylothorax after congenital diaphragmatic hernia repair: a population-based study. J. Pediatr. Surg. 2012;47:842–846. doi: 10.1016/j.jpedsurg.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Nagata, K. et al. Risk factors for the recurrence of the congenital diaphragmatic hernia—report from the long-term follow-up study of Japanese CDH study group. Eur. J. Pediatr. Surg.25, 9–14 (2014). [DOI] [PubMed]
- 15.Jancelewicz T, Chiang M, Oliveira C, Chiu PP. Late surgical outcomes among congenital diaphragmatic hernia (CDH) patients: Why long-term follow-up with surgeons is recommended. J. Pediatr. Surg. 2013;48:935–941. doi: 10.1016/j.jpedsurg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Burns, N. G. & Kardon, G. Chapter Four—The role of genes and environment in the etiology of congenital diaphragmatic hernias. in Current Topics in Developmental Biology (eds Lipinski, R. J. & Krauss, R. S.) vol. 152 115–138 (Academic Press, 2023). [DOI] [PMC free article] [PubMed]
- 17.Pober B. Genetic aspects of human congenital diaphragmatic hernia. Clin. Genet. 2008;74:1–15. doi: 10.1111/j.1399-0004.2008.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peppa M, et al. Congenital diaphragmatic hernia subtypes: Comparing birth prevalence, occurrence by maternal age, and mortality in a national birth cohort. Paediatr. Perinat. Epidemiol. 2023;37:143–153. doi: 10.1111/ppe.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman KG, et al. Congenital diaphragmatic defects: proposal for a new classification based on observations in 234 patients. Pediatr. Dev. Pathol. 2012;15:265–274. doi: 10.2350/11-05-1041-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kardon G, et al. Congenital diaphragmatic hernias: from genes to mechanisms to therapies. Dis. Model Mech. 2017;10:955–970. doi: 10.1242/dmm.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalmer TRA, Clugston RD. Gene ontology enrichment analysis of congenital diaphragmatic hernia-associated genes. Pediatr. Res. 2019;85:13–19. doi: 10.1038/s41390-018-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Salem AH. Congenital hernia of Morgagni in infants and children. J. Pediatr. Surg. 2007;42:1539–1543. doi: 10.1016/j.jpedsurg.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Russell MK, et al. Congenital diaphragmatic hernia candidate genes derived from embryonic transcriptomes. Proc. Natl Acad. Sci. 2012;109:2978–2983. doi: 10.1073/pnas.1121621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veenma DCM, de Klein A, Tibboel D. Developmental and genetic aspects of congenital diaphragmatic hernia. Pediatr. Pulmonol. 2012;47:534–545. doi: 10.1002/ppul.22553. [DOI] [PubMed] [Google Scholar]
- 25.Longoni M, et al. Genome-wide enrichment of damaging de novo variants in patients with isolated and complex congenital diaphragmatic hernia. Hum. Genet. 2017;136:679–691. doi: 10.1007/s00439-017-1774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, et al. Increased burden of de novo predicted deleterious variants in complex congenital diaphragmatic hernia. Hum. Mol. Genet. 2015;24:4764–4773. doi: 10.1093/hmg/ddv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAteer JP, Hecht A, De Roos AJ, Goldin AB. Maternal medical and behavioral risk factors for congenital diaphragmatic hernia. J. Pediatr. Surg. 2014;49:34–38. doi: 10.1016/j.jpedsurg.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Schulz F, et al. Parental risk factors for congenital diaphragmatic hernia—a large German case-control study. BMC Pediatr. 2021;21:278. doi: 10.1186/s12887-021-02748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García AM, Machicado S, Gracia G, Zarante IM. Risk factors for congenital diaphragmatic hernia in the Bogota birth defects surveillance and follow-up program, Colombia. Pediatr. Surg. Int. 2016;32:227–234. doi: 10.1007/s00383-015-3832-7. [DOI] [PubMed] [Google Scholar]
- 30.Felix JF, et al. Environmental factors in the etiology of esophageal atresia and congenital diaphragmatic hernia: Results of a case-control study. Birth Defects Res. A Clin. Mol. Teratol. 2008;82:98–105. doi: 10.1002/bdra.20423. [DOI] [PubMed] [Google Scholar]
- 31.Greer JJ, Babiuk RP, Thebaud B. Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr. Res. 2003;53:726–730. doi: 10.1203/01.PDR.0000062660.12769.E6. [DOI] [PubMed] [Google Scholar]
- 32.Giguère V, Evans RM. Chronicle of a discovery: the retinoic acid receptor. J. Mol. Endocrinol. 2022;69:T1–T11. doi: 10.1530/JME-22-0117. [DOI] [PubMed] [Google Scholar]
- 33.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects: thematic review series: fat-soluble vitamins: vitamin A. J. Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duester G. Retinoid signaling in control of progenitor cell differentiation during mouse development. Semin Cell Dev. Biol. 2013;24:694–700. doi: 10.1016/j.semcdb.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor, C., Varshosaz, P. & Moise, A. R. Mechanisms of feedback regulation of vitamin A metabolism. Nutrients14, 1312 (2022). [DOI] [PMC free article] [PubMed]
- 37.Ghyselinck NB, Duester G. Retinoic acid signaling pathways. Development. 2019;146:dev167502. doi: 10.1242/dev.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson DH. @article{Anderson1949, author = {D.H. Anderson}, journal = {Am. J. Dis. Child.}, pages = {888-889}, title = {Incidence of congenital diaphragmatic hernia in the young of rats bred on a diet deficient in vitamin. Am. J. Dis. Child. 1941;62:888–889. [Google Scholar]
- 39.Andersen DH. Effect of diet during pregnancy upon the incidence of congenital hereditary diaphragmatic hernia in the rat; failure to produce cystic fibrosis of the pancreas by maternal vitamin A deficiency. Am. J. Pathol. 1949;25:163–185. [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin a deficiency. Effects of restoration of vitamin a at various times during gestation. Am. J. Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 41.Andersen DH. Incidence of congenital diaphragmatic hernia in the young of rats bred on a diet deficient in vitamin A. Am. J. Dis. Child. 1941;62:888–889. [Google Scholar]
- 42.Mendelsohn C, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 43.Ghyselinck NB, et al. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- 44.Mey J, Babiuk RP, Clugston R, Zhang W, Greer JJ. Retinal dehydrogenase-2 is inhibited by compounds that induce congenital diaphragmatic hernias in rodents. Am. J. Pathol. 2003;162:673–679. doi: 10.1016/s0002-9440(10)63861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M-h, MacGowan A, Ward S, Bavik C, Greer JJ. The activation of the retinoic acid response element is inhibited in an animal model of congenital diaphragmatic hernia. Neonatology. 2003;83:157–161. doi: 10.1159/000068932. [DOI] [PubMed] [Google Scholar]
- 46.Thébaud B, et al. Vitamin A decreases the incidence and severity of nitrofen-induced congenital diaphragmatic hernia in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277:L423–L429. doi: 10.1152/ajplung.1999.277.2.L423. [DOI] [PubMed] [Google Scholar]
- 47.Major, D. et al. Retinol status of newborn infants with congenital diaphragmatic hernia. Pediatr. Surg. Int.13, 547–549 (1998). [DOI] [PubMed]
- 48.Allan DW, Greer JJ. Pathogenesis of nitrofen-induced congenital diaphragmatic hernia in fetal rats. J. Appl Physiol. 1997;83:338–347. doi: 10.1152/jappl.1997.83.2.338. [DOI] [PubMed] [Google Scholar]
- 49.Clugston RD, Greer JJ. Diaphragm development and congenital diaphragmatic hernia. Semin Pediatr. Surg. 2007;16:94–100. doi: 10.1053/j.sempedsurg.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Clugston RD, Zhang W, Álvarez S, de Lera AR, Greer JJ. Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. Am. J. Respir. Cell Mol. Biol. 2010;42:276–285. doi: 10.1165/rcmb.2009-0076OC. [DOI] [PubMed] [Google Scholar]
- 51.Carmona R, et al. Conditional deletion of WT1 in the septum transversum mesenchyme causes congenital diaphragmatic hernia in mice. Elife. 2016;5:e16009. doi: 10.7554/eLife.16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edel, G. et al. Cellular Origin(s) of Congenital Diaphragmatic Hernia. Front. Pediatr.9, 804496 (2021). [DOI] [PMC free article] [PubMed]
- 53.Kluth D, et al. Nitrofen-induced diaphragmatic hernias in rats: an animal model. J. Pediatr. Surg. 1990;25:850–854. doi: 10.1016/0022-3468(90)90190-k. [DOI] [PubMed] [Google Scholar]
- 54.Tenbrinck R, et al. Experimentally induced congenital diaphragmatic hernia in rats. J. Pediatr. Surg. 1990;25:426–429. doi: 10.1016/0022-3468(90)90386-n. [DOI] [PubMed] [Google Scholar]
- 55.Chiu, P. P. L. New insights into congenital diaphragmatic hernia—a surgeon’s introduction to CDH animal models. Front. Pediatr. 2, 36 (2014). [DOI] [PMC free article] [PubMed]
- 56.Babiuk RP, Greer JJ. Diaphragm defects occur in a CDH hernia model independently of myogenesis and lung formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L1310–L1314. doi: 10.1152/ajplung.00257.2002. [DOI] [PubMed] [Google Scholar]
- 57.Rocke AW, et al. Low maternal vitamin A intake increases the incidence of teratogen induced congenital diaphragmatic hernia in mice. Pediatr. Res. 2022;91:83–91. doi: 10.1038/s41390-021-01409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble BR, et al. Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1079–L1087. doi: 10.1152/ajplung.00286.2007. [DOI] [PubMed] [Google Scholar]
- 59.Kling DE, et al. Nitrofen induces apoptosis independently of retinaldehyde dehydrogenase (RALDH) inhibition. Birth Defects Res. B Dev. Reprod. Toxicol. 2010;89:223–232. doi: 10.1002/bdrb.20247. [DOI] [PubMed] [Google Scholar]
- 60.Kutasy B, Pes L, Friedmacher F, Paradisi F, Puri P. Nitrofen increases total retinol levels in placenta during lung morphogenesis in the nitrofen model of congenital diaphragmatic hernia. Pediatr. Surg. Int. 2014;30:1017–1022. doi: 10.1007/s00383-014-3525-7. [DOI] [PubMed] [Google Scholar]
- 61.Baptista MJ, et al. Antenatal vitamin A administration attenuates lung hypoplasia by interfering with early instead of late determinants of lung underdevelopment in congenital diaphragmatic hernia. J. Pediatr. Surg. 2005;40:658–665. doi: 10.1016/j.jpedsurg.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 62.Oshiro T, Asato Y, Sakanashi M, Ohta T, Sugahara K. Differential effects of vitamin A on fetal lung growth and diaphragmatic formation in nitrofen-induced rat model. Pulm. Pharm. Ther. 2005;18:155–164. doi: 10.1016/j.pupt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Babiuk RP, Thébaud B, Greer JJ. Reductions in the incidence of nitrofen-induced diaphragmatic hernia by vitamin A and retinoic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L970–L973. doi: 10.1152/ajplung.00403.2003. [DOI] [PubMed] [Google Scholar]
- 64.Beurskens LWJE, et al. Dietary vitamin A intake below the recommended daily intake during pregnancy and the risk of congenital diaphragmatic hernia in the offspring. Birth Defects Res. A Clin. Mol. Teratol. 2013;97:60–66. doi: 10.1002/bdra.23093. [DOI] [PubMed] [Google Scholar]
- 65.Michikawa T, et al. Maternal dietary intake of vitamin A during pregnancy was inversely associated with congenital diaphragmatic hernia: the Japan Environment and Children’s Study. Br. J. Nutr. 2019;122:1295–1302. doi: 10.1017/S0007114519002204. [DOI] [PubMed] [Google Scholar]
- 66.Kool HM, et al. Inhibition of retinoic acid signaling induces aberrant pericyte coverage and differentiation resulting in vascular defects in congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317:L317–L331. doi: 10.1152/ajplung.00104.2018. [DOI] [PubMed] [Google Scholar]
- 67.Cipollone, D., Cozzi, D. A., Businaro, R. & Marino, B. Congenital diaphragmatic hernia after exposure to a triple retinoic acid antagonist during pregnancy. J. Cardiovasc. Med.18, 389–392 (2017). [DOI] [PubMed]
- 68.Petkovich M, Chambon P. Retinoic acid receptors at 35 years. J. Mol. Endocrinol. 2022;69:T13–T24. doi: 10.1530/JME-22-0097. [DOI] [PubMed] [Google Scholar]
- 69.Mascrez B, et al. The RXRα ligand-dependent activation function 2 (AF-2) is important for mouse development. Development. 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- 70.O’Byrne SM, et al. Retinoid absorption and storage is impaired in mice lacking lecithin: retinol acyltransferase (LRAT)*. J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quadro L, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghyselinck NB, et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Billings SE, et al. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013;27:4877–4889. doi: 10.1096/fj.13-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abu-Abed S, et al. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 76.Yu L, Hernan RR, Wynn J, Chung WK. The influence of genetics in congenital diaphragmatic hernia. Semin Perinatol. 2020;44:151169. doi: 10.1053/j.semperi.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cleal L, et al. Resolving the heterogeneity of diaphragmatic mesenchyme: a novel mouse model of congenital diaphragmatic hernia. Dis. Model Mech. 2021;14:dmm046797. doi: 10.1242/dmm.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilm, B. & Muñoz-Chapuli, R. The role of WT1 in embryonic development and normal organ homeostasis. in The Wilms’ Tumor (WT1) Gene: Methods and Protocols (ed Hastie, N.) 23–39 (Springer New York, 2016). 10.1007/978-1-4939-4023-3_3. [DOI] [PubMed]
- 79.Paris ND, Coles GL, Ackerman KG. Wt1 and β-catenin cooperatively regulate diaphragm development in the mouse. Dev. Biol. 2015;407:40–56. doi: 10.1016/j.ydbio.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clugston RD, et al. Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am. J. Pathol. 2006;169:1541–1549. doi: 10.2353/ajpath.2006.060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreidberg JA, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 82.Hastie ND. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development. 2017;144:2862–2872. doi: 10.1242/dev.153163. [DOI] [PubMed] [Google Scholar]
- 83.Guadix JA, et al. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development. 2011;138:1093–1097. doi: 10.1242/dev.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 85.Pereira FA, Qiu Y, Zhou G, Tsai M-J, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CT, et al. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol. Cell Biol. 2004;24:10835–10843. doi: 10.1128/MCB.24.24.10835-10843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doi T, Sugimoto K, Puri P. Prenatal retinoic acid up-regulates pulmonary gene expression of COUP-TFII, FOG2, and GATA4 in pulmonary hypoplasia. J. Pediatr. Surg. 2009;44:1933–1937. doi: 10.1016/j.jpedsurg.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 88.Qiu Y, Krishnan V, Pereira FA, Tsai SY, Tsai MJ. Chicken ovalbumin upstream promoter-transcription factors and their regulation. J. Steroid Biochem. Mol. Biol. 1996;56:81–85. doi: 10.1016/0960-0760(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 89.Jonk LJC, et al. Cloning and expression during development of three murine members of the COUP family of nuclear orphan receptors. Mech. Dev. 1994;47:81–97. doi: 10.1016/0925-4773(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 90.Tsai SY, Tsai M-J. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age*. Endocr. Rev. 1997;18:229–240. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- 91.You L-R, et al. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc. Natl Acad. Sci. USA. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perrino C, Rockman HA. GATA4 and the two sides of gene expression reprogramming. Circ. Res. 2006;98:715–716. doi: 10.1161/01.RES.0000217593.07196.af. [DOI] [PubMed] [Google Scholar]
- 93.Zhou, P., He, A. & Pu, W. T. Chapter five—regulation of GATA4 transcriptional activity in cardiovascular development and disease. in Current Topics in Developmental Biology (ed. Bruneau, B. G.) vol. 100 143–169 (Academic Press, 2012). [DOI] [PubMed]
- 94.Su D, Gudas LJ. Retinoic acid receptor γ activates receptor tyrosine kinase Tie1 gene transcription through transcription factor GATA4 in F9 stem cells. Exp. Hematol. 2008;36:624–641. doi: 10.1016/j.exphem.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 95.Jay PY, et al. Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev. Biol. 2007;301:602–614. doi: 10.1016/j.ydbio.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merrell AJ, et al. Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat. Genet. 2015;47:496–504. doi: 10.1038/ng.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu JR, et al. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol. Cell Biol. 1999;19:4495–4502. doi: 10.1128/mcb.19.6.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ackerman KG, et al. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:e10. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang Y, Drysdale TA, Evans T. A role for GATA-4/5/6 in the regulation of Nkx2.5 expression with implications for patterning of the precardiac field. Dev. Biol. 1999;216:57–71. doi: 10.1006/dbio.1999.9469. [DOI] [PubMed] [Google Scholar]
- 101.Clabby ML, et al. Receptor α represses GATA-4-mediated Transcription via a Retinoid-dependent Interaction with the cardiac-enriched repressor FOG-2*. J. Biol. Chem. 2003;278:5760–5767. doi: 10.1074/jbc.M208173200. [DOI] [PubMed] [Google Scholar]
- 102.Huggins GS, Bacani CJ, Boltax J, Aikawa R, Leiden JM. Friend of GATA 2 physically interacts with chicken ovalbumin upstream promoter-TF2 (COUP-TF2) and COUP-TF3 and represses COUP-TF2-dependent activation of the atrial natriuretic factor promoter*. J. Biol. Chem. 2001;276:28029–28036. doi: 10.1074/jbc.M103577200. [DOI] [PubMed] [Google Scholar]
- 103.Gilbert, R. M. & Gleghorn, J. P. Connecting clinical, environmental, and genetic factors point to an essential role for vitamin A signaling in the pathogenesis of congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell. Mol. Physiol.10.1152/ajplung.00349.2022 (2023). [DOI] [PMC free article] [PubMed]
- 104.Beurskens LWJE, et al. Retinol status of newborn infants is associated with congenital diaphragmatic hernia. Pediatrics. 2010;126:712–720. doi: 10.1542/peds.2010-0521. [DOI] [PubMed] [Google Scholar]
- 105.Loo CKC, et al. Lung and liver growth and retinoic acid status in human fetuses with congenital diaphragmatic hernia. Early Hum. Dev. 2018;116:17–23. doi: 10.1016/j.earlhumdev.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Rocke AW, Clugston RD. Comment on “Lung and Liver growth and retinoic acid status in human fetuses with congenital diaphragmatic hernia”. Early Hum. Dev. 2018;116:93. doi: 10.1016/j.earlhumdev.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Czuba, L. C. et al. Plasma retinoid concentrations are altered in pregnant women. Nutrients14, 1365 (2022). [DOI] [PMC free article] [PubMed]
- 108.Jeong H, et al. Temporal changes in the systemic concentrations of retinoids in pregnant and postpartum women. PLoS One. 2023;18:e0280424. doi: 10.1371/journal.pone.0280424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bates CJ. Vitamin A in pregnancy and lactation. Proc. Nutr. Soc. 1983;42:65–79. doi: 10.1079/pns19830008. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi YI, Smith JE, Goodman DS. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am. J. Physiol. Endocrinol. Metab. 1977;233:E263. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- 111.Satre MA, Ugen KE, Kochhar DM. Developmental changes in endogenous retinoids during pregnancy and embryogenesis in the mouse. Biol. Reprod. 1992;46:802–810. doi: 10.1095/biolreprod46.5.802. [DOI] [PubMed] [Google Scholar]
- 112.Brosens, E. et al. Unraveling the genetics of congenital diaphragmatic hernia: an ongoing challenge. Front. Pediatr.9, 915 (2022). [DOI] [PMC free article] [PubMed]
- 113.Goumy C, et al. Retinoid pathway and congenital diaphragmatic hernia: hypothesis from the analysis of chromosomal abnormalities. Fetal Diagn. Ther. 2010;28:129–139. doi: 10.1159/000313331. [DOI] [PubMed] [Google Scholar]
- 114.Kawaguchi R, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science (1979) 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 115.Andijani AA, Shajira ES, Abushaheen A, Al-Matary A. Microphthalmia syndrome 9: case report of a newborn baby with pulmonary hypoplasia, diaphragmatic eventration, microphthalmia, cardiac defect and severe primary pulmonary hypertension. Am. J. Case Rep. 2019;20:354–360. doi: 10.12659/AJCR.912873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pasutto F, Flinter F, Rauch A, Reis A. Novel STRA6 null mutations in the original family described with Matthew–Wood syndrome. Am. J. Med. Genet. A. 2018;176:134–138. doi: 10.1002/ajmg.a.38529. [DOI] [PubMed] [Google Scholar]
- 117.Seller MJ, et al. Two sibs with anophthalmia and pulmonary hypoplasia (the Matthew-Wood syndrome) Am. J. Med. Genet. 1996;62:227–229. doi: 10.1002/(SICI)1096-8628(19960329)62:3<227::AID-AJMG5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 118.Marcadier JL, et al. A novel mutation in two Hmong families broadens the range of STRA6-related malformations to include contractures and camptodactyly. Am. J. Med. Genet. A. 2016;170:11–18. doi: 10.1002/ajmg.a.37389. [DOI] [PubMed] [Google Scholar]
- 119.Gudas LJ. Retinoid metabolism: new insights. J. Mol. Endocrinol. 2022;69:T37–T49. doi: 10.1530/JME-22-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beecroft SJ, et al. Biallelic hypomorphic variants in ALDH1A2 cause a novel lethal human multiple congenital anomaly syndrome encompassing diaphragmatic, pulmonary, and cardiovascular defects. Hum. Mutat. 2021;42:506–519. doi: 10.1002/humu.24179. [DOI] [PubMed] [Google Scholar]
- 121.Leon E, Nde C, Ray RS, Preciado D, Zohn IE. ALDH1A2-related disorder: a new genetic syndrome due to alteration of the retinoic acid pathway. Am. J. Med. Genet. A. 2023;191:90–99. doi: 10.1002/ajmg.a.62991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clugston RD, Zhang W, Greer JJ. Gene expression in the developing diaphragm: significance for congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L665–L675. doi: 10.1152/ajplung.00027.2008. [DOI] [PubMed] [Google Scholar]
- 123.Dollé P. Developmental expression of retinoic acid receptors (RARs) Nucl. Recept Signal. 2009;7:nrs.07006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Srour M, et al. Recessive and dominant mutations in retinoic acid receptor beta in cases with microphthalmia and diaphragmatic hernia. Am. J. Hum. Genet. 2013;93:765–772. doi: 10.1016/j.ajhg.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nobile, S., Pisaneschi, E., Novelli, A. & Carnielli, V. P. A rare mutation of retinoic acid receptor-β associated with lethal neonatal Matthew-Wood syndrome. Clin. Dysmorphol.28, 75–77 (2019). [DOI] [PubMed]
- 126.Srour M, et al. Gain-of-function mutations in RARB cause intellectual disability with progressive motor impairment. Hum. Mutat. 2016;37:786–793. doi: 10.1002/humu.23004. [DOI] [PubMed] [Google Scholar]
- 127.Holder AM, et al. Genetic factors in congenital diaphragmatic hernia. Am. J. Hum. Genet. 2007;80:825–845. doi: 10.1086/513442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller-Hodges E, Hohenstein P. WT1 in disease: shifting the epithelial–mesenchymal balance. J. Pathol. 2012;226:229–240. doi: 10.1002/path.2977. [DOI] [PubMed] [Google Scholar]
- 129.Klaassens M, et al. Congenital diaphragmatic hernia and chromosome 15q26: determination of a candidate region by use of fluorescent in situ hybridization and array-based comparative genomic hybridization. Am. J. Hum. Genet. 2005;76:877–882. doi: 10.1086/429842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.High FA, et al. De novo frameshift mutation in COUP-TFII (NR2F2) in human congenital diaphragmatic hernia. Am. J. Med Genet. A. 2016;170:2457–2461. doi: 10.1002/ajmg.a.37830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu L, et al. Variants in GATA4 are a rare cause of familial and sporadic congenital diaphragmatic hernia. Hum. Genet. 2013;132:285–292. doi: 10.1007/s00439-012-1249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Longoni M, et al. Prevalence and penetrance of ZFPM2 mutations and deletions causing congenital diaphragmatic hernia. Clin. Genet. 2015;87:362–367. doi: 10.1111/cge.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kammoun M, et al. Genetic profile of isolated congenital diaphragmatic hernia revealed by targeted next-generation sequencing. Prenat. Diagn. 2018;38:654–663. doi: 10.1002/pd.5327. [DOI] [PubMed] [Google Scholar]
- 134.Francisco T, Gonçalves RM, Borges C, Neto MT. Multiple haemangiomas, diaphragmatic eventration and Beckwith-Wiedemann syndrome: an unusual association. BMJ Case Rep. 2013;2013:bcr2013010077. doi: 10.1136/bcr-2013-010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cohen MM, Jr, Gorlin RJ, Feingold M, ten Bensel RW. The Beckwith-Wiedemann syndrome. Seven new cases. Am. J. Dis. Child. 1971;122:515–519. doi: 10.1001/archpedi.1971.02110060085015. [DOI] [PubMed] [Google Scholar]
- 136.Huang N, et al. Two distinct nuclear receptor interaction domains in NSD1, a novel SET protein that exhibits characteristics of both corepressors and coactivators. EMBO J. 1998;17:3398–3412. doi: 10.1093/emboj/17.12.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hong S-H, David G, Wong C-W, Dejean A, Privalsky ML. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor α (RARα) and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc. Natl Acad. Sci. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Longoni M, et al. Molecular pathogenesis of congenital diaphragmatic hernia revealed by exome sequencing, developmental data, and bioinformatics. Proc. Natl Acad. Sci. 2014;111:12450–12455. doi: 10.1073/pnas.1412509111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coles GL, Ackerman KG. Kif7 is required for the patterning and differentiation of the diaphragm in a model of syndromic congenital diaphragmatic hernia. Proc. Natl Acad. Sci. 2013;110:E1898–E1905. doi: 10.1073/pnas.1222797110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta N, et al. Nasopharyngeal teratoma, congenital diaphragmatic hernia and Dandy–Walker malformation—a yet uncharacterized syndrome. Clin. Genet. 2016;90:470–471. doi: 10.1111/cge.12830. [DOI] [PubMed] [Google Scholar]
- 141.Yang W, et al. Nutrient intakes in women and congenital diaphragmatic hernia in their offspring. Birth Defects Res. A Clin. Mol. Teratol. 2008;82:131–138. doi: 10.1002/bdra.20436. [DOI] [PubMed] [Google Scholar]
- 142.Carmichael SL, et al. Congenital diaphragmatic hernia and maternal dietary nutrient pathways and diet quality. Birth Defects Res. 2020;112:1475–1483. doi: 10.1002/bdr2.1770. [DOI] [PubMed] [Google Scholar]
- 143.Bailey RL, Pac SG, Fulgoni VL, 3rd, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open. 2019;2:e195967. doi: 10.1001/jamanetworkopen.2019.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jankowska, A. et al. Determinants of the essential elements and vitamins intake and status during pregnancy: a descriptive study in polish mother and child cohort. Nutrients13, 949 (2021). [DOI] [PMC free article] [PubMed]
- 145.Hanson, C., Lyden, E., Abresch, C. & Anderson-Berry, A. Serum retinol concentrations, race, and socioeconomic status in of women of childbearing age in the United States. Nutrients8, 508 (2016). [DOI] [PMC free article] [PubMed]
- 146.Caspers KM, et al. Maternal periconceptional exposure to cigarette smoking and alcohol consumption and congenital diaphragmatic hernia. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:1040–1049. doi: 10.1002/bdra.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Molotkov A, Duester G. Retinol/ethanol drug interaction during acute alcohol intoxication in mice involves inhibition of retinol metabolism to retinoic acid by alcohol dehydrogenase*. J. Biol. Chem. 2002;277:22553–22557. doi: 10.1074/jbc.M201603200. [DOI] [PubMed] [Google Scholar]
- 148.Yılmaz G, et al. The effect of passive smoking and breast feeding on serum antioxidant vitamin (A, C, E) levels in infants. Acta Paediatr. 2009;98:531–536. doi: 10.1111/j.1651-2227.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 149.Sefton EM, Gallardo M, Kardon G. Developmental origin and morphogenesis of the diaphragm, an essential mammalian muscle. Dev. Biol. 2018;440:64–73. doi: 10.1016/j.ydbio.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bogenschutz EL, Sefton EM, Kardon G. Cell culture system to assay candidate genes and molecular pathways implicated in congenital diaphragmatic hernias. Dev. Biol. 2020;467:30–38. doi: 10.1016/j.ydbio.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goumy C, et al. Fetal skin fibroblasts: a cell model for studying the retinoid pathway in congenital diaphragmatic hernia. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:195–200. doi: 10.1002/bdra.20647. [DOI] [PubMed] [Google Scholar]
- 152.Qiao L, et al. Rare and de novo variants in 827 congenital diaphragmatic hernia probands implicate LONP1 as candidate risk gene. Am. J. Hum. Genet. 2021;108:1964–1980. doi: 10.1016/j.ajhg.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qi H, et al. De novo variants in congenital diaphragmatic hernia identify MYRF as a new syndrome and reveal genetic overlaps with other developmental disorders. PLoS Genet. 2018;14:e1007822. doi: 10.1371/journal.pgen.1007822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beurskens LWJE, de Jonge R, Schoonderwaldt EM, Tibboel D, Steegers-Theunissen RPM. Biomarkers of the one-carbon pathway in association with congenital diaphragmatic hernia. Birth Defects Res. A Clin. Mol. Teratol. 2012;94:557–560. doi: 10.1002/bdra.23039. [DOI] [PubMed] [Google Scholar]
- 155.Adrien N, et al. Early pregnancy vitamin D status and risk of select congenital anomalies in the National Birth Defects Prevention Study. Birth Defects Res. 2023;115:290–301. doi: 10.1002/bdr2.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Turkmen GG, et al. Levels of serum vitamin D and calcium in pregnancies complicated with fetal congenital diaphragmatic hernia and normal pregnancies. J. Matern. Fetal Neonatal Med. 2017;30:990–994. doi: 10.1080/14767058.2016.1196662. [DOI] [PubMed] [Google Scholar]
- 157.Antounians L, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci. Transl. Med. 2021;13:eaax5941. doi: 10.1126/scitranslmed.aax5941. [DOI] [PubMed] [Google Scholar]
- 158.Verla MA, Style CC, Olutoye OO. Prenatal intervention for the management of congenital diaphragmatic hernia. Pediatr. Surg. Int. 2018;34:579–587. doi: 10.1007/s00383-018-4270-0. [DOI] [PubMed] [Google Scholar]
- 159.Delabaere A, et al. Retinoic acid and tracheal occlusion for diaphragmatic hernia treatment in rabbit fetuses. Prenat. Diagn. 2018;38:482–492. doi: 10.1002/pd.5256. [DOI] [PubMed] [Google Scholar]
- 160.Kirby E, Keijzer R. Congenital diaphragmatic hernia: current management strategies from antenatal diagnosis to long-term follow-up. Pediatr. Surg. Int. 2020;36:415–429. doi: 10.1007/s00383-020-04625-z. [DOI] [PubMed] [Google Scholar]