Abstract
As a new type of anti-tumor immunotherapy, immune checkpoint inhibitors (ICIs) have improved the prognosis of multiple malignancies. However, renal complications are becoming more frequent. Nephrotoxicity often manifests as acute kidney injury (AKI), and the most common histopathological type is acute tubulointerstitial nephritis (ATIN). Based on previous studies of the incidence and potential risk factors for nephrotoxicity, in this review, we describe the mechanism of AKI after ICIs treatment, summarize the incidence, risk factors, and outcomes of AKI, and discuss the diagnosis and management of immune checkpoint inhibitors-associated acute kidney injury (ICI-AKI). In addition, we review the current status of ICIs rechallenge and the therapeutic strategies of ICIs applied in kidney transplant recipients. Finally, we emphasize the importance of collaboration between nephrologists and oncologists to guide the treatment of ICIs and the management of renal complications.
Keywords: acute kidney injury, nephrotoxicity, immune checkpoint inhibitors, immunotherapy, immune-related adverse events, malignancies
1. Introduction
Immune checkpoint inhibitors (ICIs), a kind of monoclonal antibodies, have been confirmed to significantly improve the overall prognosis of a wide range of malignancies (1–3). However, ICIs also lead to the loss of peripheral tolerance of autoantigens, which leads to autoimmune reactions, called immune-related adverse events (irAEs). The most commonly involved organs and systems include gastrointestinal tract, endocrine, skin, etc (4, 5). Similarly, the nephrotoxicity of ICIs has attracted more and more attention. It usually presents as acute kidney injury (AKI), which can cause irreversible loss of renal function in severe cases (6), and needs to be paid attention to.
In this review, we describe the therapeutic mechanism of ICIs and the possible mechanism leading to AKI, summarize the incidence, risk factors, other clinical characteristics and outcomes of AKI after ICIs treatment, discuss the diagnosis and management of immune checkpoint inhibitor–associated AKI, and focus on the application of ICIs for anti-tumor therapy in kidney transplant recipients.
2. Development of immune-related adverse events
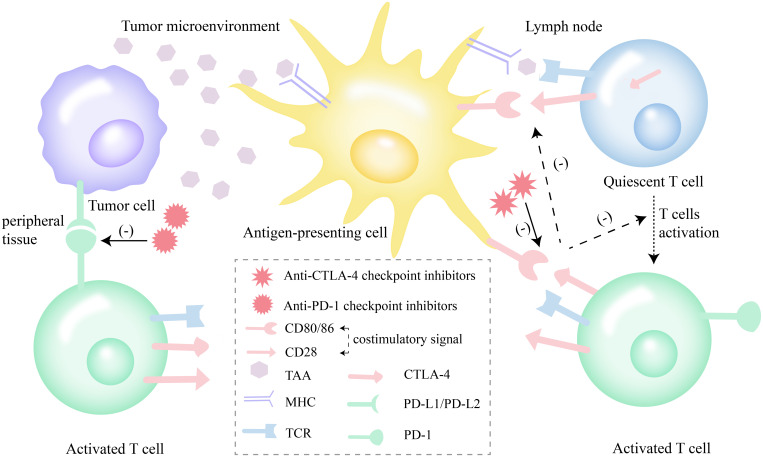
Immune checkpoint inhibitors can not only improve the activity of immune system and enhance the anti-tumor immune response of the body ( Figure 1 ), but also cause the loss of peripheral tolerance of autoantigens, resulting in autoimmune reactions, which are recognized as immune-related adverse events (irAEs). The pathogenesis of irAEs includes inflammatory response caused by cytokines, cross-reaction of similar antigens and complement-mediated direct damage (7). The occurrence of irAEs is affected by many factors. For example, autoimmune tendency, body mass index (BMI), and the treatment regimen of ICIs are related to the incidence of irAEs (7). and tumor types is related to the type of irAEs (7–9).
Figure 1.
Effect of ICIs (anti-CTLA-4 and anti-PD-1) on T-cell function. Anti-CTLA-4 checkpoint inhibitors enhance anti-tumor response by promoting the activation of quiescent T cells in lymph nodes. Anti-PD-1 checkpoint inhibitors impede the exhaustion of activated T cells by blocking of the PD-1 axis. ICIs, immune checkpoint inhibitors; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; PD-L2, programmed cell death ligand-2; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; TAA, tumor-associated antigens; MHC, major histocompatibility complex; TCR, T cell receptor.
Multiple different systems have been reported to develop irAEs, involving endocrine, gastrointestinal, skin, musculoskeletal, urinary and other systems (7, 10). In particular, attention should be paid to the neurological and fatal irAEs. timely detection and intervention to prevent further deterioration and irreversible damage (11). Currently, treatment strategies for irAEs include screening for autoantibodies in patients who are predisposed to autoimmunity before ICIs are administered, grading treatment and deciding whether to discontinue ICIs according to the severity of irAEs (7, 12). The treatment of irAEs includes corticosteroids, immunosuppressive agents, intravenous immunoglobulin, monoclonal antibodies, and plasma exchange (7). It should be added that treatment of irAEs with immunosuppressive agents does not appear to affect the antitumor activity of ICIs (13).
3. Development of immune checkpoint inhibitors-associated acute kidney injury
3.1. Definition, grading, classification of AKI and ICI-AKI definition
Renal adverse effects caused by ICIs usually manifested as AKI (6). In the current studies (14–16), AKI was defined as a 1.5-fold increase in serum creatinine (SCr) from baseline or an increase of ≥0.3 mg/dL (26.5 µmol/L) after starting ICIs. The Kidney Disease Improving Global Outcomes (KDIGO) criteria were used to grade AKI according to the relative change in SCr (16–18). Similarly, the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAEs) defines and grades AKI by comparing SCr with an “upper limit of normal” cutoff parameters (19). However, this approach often underestimates the incidence of AKI because it ignores those AKI that increase in the normal range of creatinine (mostly low-grade AKI) (20).
Previous studies have divided AKI into four categories based on its etiology, including ICI-AKI, hemodynamic AKI/acute tubular necrosis (ATN), obstructive AKI and AKI of undetermined cause. Nephrologists attribute the cause of ICI-AKI directly to ICIs. Hemodynamic AKI/ATN refers to AKI that occurs in the context of dehydration (such as circulatory failure, diarrhea, vomiting, etc.), tumor lysis syndrome, sepsis, or ischemic ATN (14, 18, 21).
3.2. Possible mechanisms of ICI-AKI
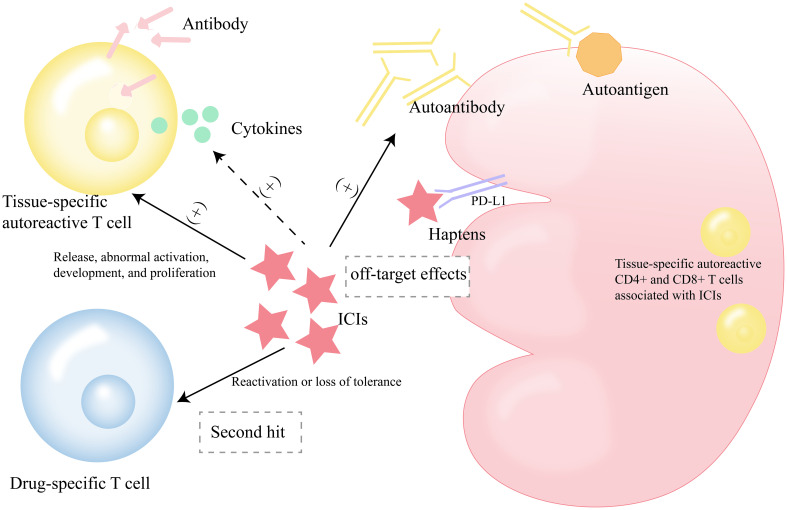
Different studies have hypothesized the mechanism of ICI-AKI ( Figure 2 ). First, ICIs, including anti-CTLA4 and anti-PD-1, may favor the generation of autoantibodies against autoantigens on renal tubular epithelial cells (TECs), mesangial cells, and podocytes, such as anti-dsDNA and antinuclear antigen antibodies (22–25). They engage in an autoimmune reaction with specific renal autoantigens. Some studies have found that the treatment and interruption of ICIs and corticosteroids treatment affect the levels of some autoantibodies in the serum circulation, such as lupus-like glomerulopathy and anti-dsDNA and anti-nuclear antigen antibodies, which are very similar to the phenotype of autoimmune lupus nephritis (22–24, 26).
Figure 2.
Possible mechanisms of ICI-AKI. ICIs, immune checkpoint inhibitors; ICI-AKI, immune checkpoint inhibitors-associated acute kidney injury.
Second, another mechanism may be that ICIs themselves or bind to kidney-expressed checkpoint receptors to form haptens, known as “off-target effects” (25), which are recognized by local dendritic cells (DCs) and trigger immune responses after acquiring immunogenicity when they are metabolized by renal tubular cells or when they bind to carrier proteins to form antigen-antibody complexes (27, 28). This “reprogramming” of the immune system results in the loss of peripheral tolerance to renal endogenous antigens (29, 30). It has been found that PD-L1 is expressed in renal TECs (31). In a murine model of nephrotoxic nephritis, Neumann et al. found that Foxp3+ regulatory T cells (Tregs) infiltrating the kidney express PD-L1. After inhibition of PD-L1 signaling in the murine model (knockout or blockade of PD-L1), Tregs are increased in the kidney, ultimately exacerbating nephrotoxic nephritis. It should be noted that the gene expression profile of Tregs is also altered by the lack of PD-L1 in renal inflammation. Furthermore, Tregs isolated from the PD-L1-depleted murine model were found to have impaired suppressive capacity in vitro and no protective effect on nephrotoxic nephritis in vivo, indirectly suggesting a protective role of PD-L1 in renal injury (32). In addition, the study by Shim et al. found that PD-1/PD-L1 interaction modulated T cell infiltration and lipocalin 2 expression in kidney transplant murine (33).
It is reasonable to hypothesize that ICIs promote the release, uncontrolled aberrant activation, development, and proliferation of quiescent tissue-specific autoreactive T cells (34, 35). These CD4+ and CD8+ T cells associated with ICIs can be found in different tissues such as myocardium, skeletal muscle, kidney and tumor, and cross-react with these normal tissues (25, 36). They even produce antibodies to mobilize the typical antibody-induced hypersensitivity (37, 38). In addition, activated T cells infiltrate the renal parenchyma and release cytokines to produce an inflammatory response (39). At present, it has been found that ICIs can promote the increase of proinflammatory cytokines/chemokines such as IL-1Ra, CXCL10, TNF-α, IL-6 (25, 40). It has been found that the expression of PD-L1 at both mRNA and protein levels in the proximal tubules in normal kidney tissue is low enough to cause organ-specific immunity (31).
An alternative hypothesis is that ICIs reactivate drug-specific T cells or lose tolerance to them (41). These T cells are produced when an immune response is triggered by prior treatment with drugs known to cause acute interstitial nephritis (AIN) (nonsteroidal anti-inflammatory drugs, proton pump inhibitors, etc.) or other nephrotoxic drugs (antibiotics, etc.) (25, 34, 35, 37). This mechanism can be called the “second hit” (14). To a certain extent, these drugs act as exogenous antigens or haptens as potentiators for the occurrence of ICIs-related irAEs.
3.3. Clinical features of ICI-AKI
3.3.1. Incidence for ICI-AKI
Nephrotoxicity associated with ICIs is uncommon compared with other common irAEs, such as dermatitis, enterocolitis, and thyroiditis (42, 43). A review of irAEs with ipilimumab summarized rates of skin toxicity from 47% to 68%, diarrhea from 44%, hypophysitis from 1% to 6%, and hepatotoxicity from 3% to 9% (44). A review of endocrine dysfunction published by Barroso-Sousa et al. (45) concluded that in combination therapy with nivolumab plus ipilimumab, the incidence of hypothyroidism, hyperthyroidism, hypophysitis, and autoimmune adrenalitis was 13.2%, 8.0%, 8.0%, and 4.2%, respectively. In a review of management of endocrine and metabolic toxicity published by Spagnolo et al. (46), it was found that the incidence of thyroid disorders was as high as 15-20% and pituitary disorders as high as 10% with the combination of ICIs. In a retrospective study of 740 melanoma patients, 7.3% had serious infections, most of which were bacterial (85%) (47). In a study of 167 patients with malignancies treated with nivolumab, 19.2% developed infectious disease, the most prevalent type of infection was pneumonia, and 78.1% were bacterial (48, 49). However, the estimated incidence of AKI in patients treated with ICIs was found to be 9.9% to 29% and may be rising (25, 34, 50). The different inclusion criteria for AKI in different studies contributed to the differences in incidence. In a retrospective study of 252 patients with malignancies treated with ICIs, the incidence of AKI was 17.9% (51). In a meta-analysis of eighteen articles comprising 12,111 patients receiving ICIs, the incidence of AKI was 16% (6). In a meta-analysis of 48 clinical trials receiving anti-PD-1, the incidence of AKI was 2.2% (52). In a single-center study of 1016 patients with malignancies, the incidence of sustained AKI that lasted at least three consecutive days was 8% (14).
A summary of previous studies found that the incidence of ICI-AKI ranged from 2.2% to 7.1%, which was lower than the incidence of AKI (14, 16, 52–54). However, delayed onset of AKI after initiation of ICIs or the presence of mild AKI that can be easily attributed to other etiologies may lead to an underestimation of the true incidence of ICI-AKI (55). In a study of 239 anti-PD-1 treated patients with advanced melanoma, AKI was mostly pre-renal (49%). This may be related to gastrointestinal irAEs (diarrhea and colitis) and the use of renin-angiotensin-aldosterone system inhibitors (RAASi), whereas ICI-AKI accounted for only 3.3% (18). In addition, AKI in patients treated with ICIs, including ICI-AKI, was mostly low-grade (14, 18, 54, 55). This is consistent with the conclusion that the incidence of grade 3-4 irAEs is lower than that of low-grade irAEs after treatment with ICIs alone or in combination (56). In the study of 676 patients treated with ICIs, the incidence of stage 1 AKI was 76%, and the incidence of stage 1 ICI-AKI was 81.3% (55). However, in a case-control study, only 77 (17.9%) of 429 patients with ICI-AKI had stage 1, while 48.5% had stage 3, which may be related to the diagnosis and inclusion criteria of ICI-AKI (57).
After establishing that ICIs treatment is associated with a higher risk of AKI compared with non-nephrotoxic drug treatment (52, 58), we also found that the incidence of AKI varies according to ICIs treatment. The risk for ICI-AKI was found to be lower with anti-PD-L1 than with anti-PD-1 (59), and sustained AKI occurs more frequently with anti-CTLA-4 therapy (14). In a study of the comparative risk of AKI after ICIs treatment published by Liu et al. (59), it was proposed that the risk of AKI was higher with anti-CTLA-4 monotherapy than with anti-PD-1, for example, the risk of AKI grades 1-5 and 3-5 was higher with ipilimumab than with durvalumab. This may be because CTLA-4 signals play a role in the early stage of T cell activation, while the effect of PD-1 pathway in limiting T cell activity occurs later (60), and this upstream action of anti-CTLA-4 and its low specificity make it more toxic than anti-PD-1 (59). In addition, it also suggested that the risk of AKI is higher with combination therapy (anti-CTLA-4 plus anti-PD-1) than with ICIs alone, and ICIs are time-dependent and dose-dependent, that is, the higher the target concentration, the more side effects. These conclusions were confirmed in other studies (29, 61–64). Based on these findings, it seems reasonable that 1 mg/kg nivolumab plus 3 mg/kg ipilimumab (N1I3), ipilimumab (anti-CTLA-4), and tremelimumab (anti-CTLA-4) are the three highest risk regimens (59). Finally, this study also illustrates that RCC and urothelial carcinoma have a significantly higher risk of AKI compared with other tumors (59). One study has given the explanation that RCC has a higher presence of immune cells infiltration compared with other tumors, and therefore has a stronger immune adverse reaction after ICIs treatment (65).
3.3.2. Latency period between ICIs initiation and ICI-AKI
Compared with extrarenal irAEs, there is a longer latency between ICIs initiation and ICI-AKI development. As examples, the common dermatitis usually develops within 4 weeks (44, 66, 67) and colitis within 6 weeks after ICIs treatment (43). Neurological symptoms of immune-related hypophysitis appeared as early as 6 weeks after ICIs treatment, whereas cutaneous toxicity was observed after an average of 3.6 weeks (44). The aforementioned infections developed over an average of 135 days, with 79.6% of infections occurring within the first 6 months after ICIs treatment (47). In contrast, ICI-AKI usually occurs later and the median time to onset varies widely among studies. In this review, we summarized the median time to onset of ICI-AKI ranging from 1 month to 10 months or even longer (34, 53, 68, 69). It may be related to the long half-life of ICIs (70) and the delay of clinical recognition due to the insensitivity of SCr as a marker of kidney injury (62). From the clinical pharmacokinetics analysis, it was found that the prolonged half-life of ICIs was due to their binding to the protective neonatal Fc receptors to avoid lysosomal degradation (70, 71). It should be noted that the type of ICIs also affected the development of ICI-AKI. Some studies have found that ICI-AKI occurs later after anti-PD-1 treatment than anti-CTLA-4 treatment (34). In an analysis of 1918 reports of ICI-related AKI events, durvalumab had the longest time to onset, followed by nivolumab and avelumab (72).
3.3.3. Risk factors for development of ICI-AKI
Previous studies have found that combination therapy with anti-CTLA-4 and anti-PD-1/anti-PD-L1 is associated with a higher risk of ICI-AKI, which seems plausible (25, 68, 73, 74). In the discussion of a case report, it is proposed that combination therapy with ICIs may play a greater role in enhancing antigen recognition and T-cell proliferation in lymph nodes, as well as an unconstrained cytotoxic T-cell effect in peripheral normal tissues, that is, synergism between different ICIs (75). Secondly, a study has found that the addition of chemotherapy to ICIs treatment increased the risk of AKI (59). Interestingly, cumulative dose of anti-PD-1 was found to be an independent risk factor for the development of AKI in a study of anti-PD-1 in advanced melanoma (18). In particular, a systematic review and meta-analysis of 27 studies and a systematic review of 127 studies both found that older age increased the risk of AKI after ICIs treatment (74, 76). Alternatively, in the study by Qu et al. (77), males were found to be at high risk for ICI-related nephrotoxicity. This may be due to male hormones, which increased oxidative stress, activated the renin-angiotensin system, and worsened fibrosis in damaged kidneys (78). In the study of 676 patients treated with ICIs, a gynecologic malignancy was also found to be independently associated with AKI (55).
In addition, the history of some comorbidities in cancer patients is also associated with the development of ICI-AKI. In this review, we summarized that hypertension (53), diabetes (51), hypoproteinemia (serum albumin <30g/L) (21, 51), anemia (21), autoimmune diseases, extrarenal irAEs (18, 57, 74, 79), and chronic kidney disease (CKD) (18, 51, 74) are all associated with the higher risk of ICI-AKI. Theoretically, patients with concurrent extrarenal irAEs are more likely to develop renal adverse effects. Because these patients are more likely to produce overactivated T cells to fight against autoantigens and induce autoimmune responses (6, 68). What’s interesting is that lower baseline estimated glomerular filtration rate (eGFR) is also associated with a higher risk of AKI in some studies (57, 68), while the opposite conclusion has been shown in others studies (14, 53, 80, 81). One explanation is that baseline eGFR may be influenced by other confounding factors, such as age and cardiovascular disease (82). An alternative explanation is that patients with lower baseline eGFR have poorer renal reserve, which would raise the SCr threshold rather than increase the risk of immune damage to ICIs (68).
The combined use of nonsteroidal anti-inflammatory drugs (NSAIDs) (14, 74), antibiotics (83), vitamin K antagonist fluindione (54, 74), RAASi (18, 51) and diuretics (55, 74) in cancer patients will increase the risk of ICI-AKI (21). In addition, acid inhibitors, including proton pump inhibitors (PPIs) (14, 57, 76) and histamine H2-receptor antagonists (H2RAs), reduce the therapeutic effect of ICIs and increase the risk of AKI (84). In the study by Chen et al., although the use of PPIs and NSAIDs were both risk factors for all-cause AKI and ICI-AKI, the odds ratio (OR) was higher for ICI-AKI than for all-cause AKI (1.77 vs. 2.42, 1.77 vs. 2.57, respectively) (85). As mentioned above, studies found that retreatment with ICIs after exposure to drugs known to cause AIN, including PPIs, NSAIDs, and antibiotics, activated drug-specific T cells, leading to loss of tolerance (14, 57). Studies have found that PPIs reduce the efficacy of ICIs by inducing changes in gut microbiota (86) and pH of tissues around tumors, and even directly acting on the immune system in the vicinity of tumor tissues (87, 88). In a case report published by Koda et al. (30), a NSCLC patient treated with lansoprazole developed acute tubulointerstitial nephritis (ATIN) after nivolumab treatment. It is possible that nivolumab altered the long-term tolerability of lansoprazole. This illustrates the importance of stopping those drugs known to induce ATIN, such as lansoprazole, during ICIs treatment. For a better understanding, we summarized the current information on risk factors for incident ICI-AKI in Table 1 .
Table 1.
Risk factors for incident ICI-AKI.
| Risk factors | Author (reference) | N= | OR/HR a | 95% CI a |
|---|---|---|---|---|
| combination ICIs therapy | Cortazar et al. (68) | 138 (ICI-AKI) 276 (control) |
3.88 | 2.21 to 6.81 |
| Abdelrahim et al. (73) | 1164 | 3.725, 6.305; 5.101, 9.041. | 1.144 to 12.134, 2.436 to 16.318; 1.554 to 16.745, 3.246 to 25.177. | |
| Caihong Liu et al. (74) | 27 studies | 2.45 | 1.40 to 4.31 | |
| cumulated doses of anti-PD-1 | Stein et al. (18) | 239 | NA | NA |
| ipilimumab | Abdelrahim et al. (73) | 1164 | 3.281; 4.096 | 1.213 to 8.873; 1.415 to 11.856 |
| Caihong Liu et al. (74) | 27 studies | 2.66 | 1.42 to 4.98 | |
| the addition of chemotherapy | Fei Liu et al. (59) | 85 randomized trials | NA | NA |
| older age | Caihong Liu et al. (74) | 27 studies | 1.01 | 1.00 to 1.03 |
| Xu et al. (76) | 127 studies | 1.055 | 1.016 to 1.097 | |
| Comorbidities | ||||
| hypertension | Meraz-Muñoz et al. (53) | 309 | 2.96 | 1.33 to 6.59 |
| diabetes | Guven et al. (51) | 252 | 2.042 | 0.923 to 4.518 |
| hypoproteinemia | 2.848 | 1.225 to 6.621 | ||
| Ji et al. (21) | 1615 | 1.62 | 1.17 to 2.23 | |
| anemia | 1.95 | 1.16 to 3.28 | ||
| CKD | Caihong Liu et al. (74) | 27 studies | 2.90 | 1.65 to 5.11 |
| Stein et al. (18) | 239 | NA | NA | |
| Guven et al. (51) | 252 | 3.385 | 1.510 to 7.588 | |
| Cancer stype | ||||
| RCC and urothelial carcinoma | Fei Liu et al. (59) | 85 randomized trials | NA | NA |
| a gynecologic malignancy | Koks et al. (55) | 676 | 3.91 | 1.55 to 9.85 |
| Concomitant drugs | ||||
| PPIs | Cortazar et al. (68) | 138 (ICI-AKI) 276 (control) |
2.85 | 1.81 to 4.48 |
| Abdelrahim et al. (73) | 1164 | 2.387; 2.355 | 1.328 to 4.291; 1.393 to 3.983 | |
| Caihong Liu et al. (74) | 27 studies | 2.23 | 1.88 to 2.64 | |
| Okamoto et al. (84) | 11 papers | 2.10 | 1.74 to 2.53 | |
| H2RAs | ||||
| NSAIDs | Caihong Liu et al. (74) | 27 studies | 2.61 | 1.90 to 3.57 |
| vitamin K antagonist fluindione | 6.48 | 2.72 to 15.46 | ||
| Espi Liu et al. (54) | 352 | 6.40 | 1.42 to 26.08 | |
| diuretic | Caihong Liu et al. (74) | 27 studies | 1.78 | 1.32 to 2.40 |
| Koks et al. (55) | 676 | 2.61 | 1.21 to 5.60 | |
| RAASi (ACEIs/ARBs) | Caihong Liu et al. (74) | 27 studies | 1.76 | 1.15 to 2.68 |
| Guven et al. (51) | 252 | 2.236 | 1.017 to 4.919 | |
| antibiotics | Seethapathy et al. (83) | 599 | NA | NA |
| lower baseline eGFR | Cortazar et al. (68) | 138 (ICI-AKI) 276 (control) |
1.99 | 1.43 to 2.76 |
| Gupta et al. (57) | 429(ICI-AKI) 429 (control) |
2.23; 2.62 | 1.35 to 3.68; 1.47 to 4.65. |
|
| extrarenal irAEs | Caihong Liu et al. (74) | 27 studies | 2.34 | 1.53 to 3.59 |
| Gupta et al. (57) | 429(ICI-AKI) 429 (control) |
2.07 | 1.53 to 2.78 | |
When the study type is original research, the value is the result of multivariable analysis.
OR, Odds Ratio; HR, Hazard Ratio; CI, Confidence Interval; ICI-AKI, immune checkpoint inhibitors-associated acute kidney injury; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death receptor-1; NA, not available; CKD, chronic kidney disease; RCC, renal cell carcinoma; PPIs, proton pump inhibitors; H2RAs, histamine H2-receptor antagonists; NSAIDs, nonsteroidal anti-inflammatory drugs; RAASi, renin-angiotensin-aldosterone system inhibitors; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin-receptor blockers; eGFR, estimated glomerular filtration rate; irAEs, immune-related adverse events.
3.4. Outcomes of ICI-AKI
Previous studies have found that more than half of patients with ICI-AKI develop renal recovery, which may be due to the low stage of most ICI-AKI (55, 57). Renal recovery of ICI-AKI after corticosteroid therapy is up to more than 90%. In 5 of 6 case reports of anti-PD-1 associated AIN, renal function returned to baseline after corticosteroid therapy (89). Renal recovery was associated with early corticosteroid use and initial steroid dose. In the study by Manohar et al. (90), from the results of the first month of treatment, in 12 cases of biopsy-proven or clinically suspected ICI-associated AIN (ICI-AIN) treated with steroids, patients with complete recovery received a higher steroid dose than those with partial recovery (median 2.79 [1.45 to 3.2] mg/kg per month versus 1.74 [0.8 to 3.2] mg/kg per month). However, delayed treatment with steroids is associated with worse renal prognosis (57, 91, 92). Correspondingly, higher baseline eGFR (57), higher ICI-AKI grade (stage 2 or 3) (21, 57), and extrarenal irAEs (68) were associated with lower odds of renal recovery.
In contrast, for all-cause AKI, studies found that the mortality rate was as high as 93.2% (21), and the median time to death in patients who developed sustained AKI after ICIs treatment was as short as 22 days (14). However, some studies have demonstrated that AKI is not associated with overall survival (OS) (18, 51, 73) and an increased risk of death (53, 55). This may be related to more renal recovery due to lower AKI grade. On the other hand, tumor progression or related complications such as infections also lead to increased mortality (55). It should be noted that, similar to the risk factors for AKI, some studies have found that prophylactic use of antibiotics (16), use of PPIs (18), lower baseline eGFR (57) and hypoproteinemia (51) are also associated with poor OS. Therefore, the relationship among these risk factors, AKI and OS needs to be further explored in more studies.
3.5. Diagnosis of ICI-AKI
3.5.1. Clinical work-up of ICI-AKI
The clinical and laboratory features of ICI-AKI are similar to AKI of other causes and are not sensitive and specific. They include pyuria in about half of patients, microscopic haematuria and subnephrotic proteinuria in a variable proportion of patients, and eosinophilia in a minority of patients (16, 28, 29, 68, 69). In a study of 676 cancer patients treated with ICIs by Koks et al. (55), of 54 AKI patients who underwent urinalysis, 37% had leucocyturia and 33.3% had microscopic hematuria, and of 14 patients with ICI-AKI who underwent urinalysis, they accounted for 42.9% and 21.4%, respectively. In a study by Oleas et al. (93), which included 826 cancer patients treated with ICIs, of the 8 AKI patients who underwent urinalysis, seven patients (87%) had subnephrotic proteinuria, two patients (25%) had microscopic isomorphic haematuria, five patients (62%) had eosinophiluria, and of these 8 patients, one patient (12%) had eosinophilia. In addition, a low fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUrea) suggests prerenal renal injury and may help rule out intrinsic renal injury (94). The difference is that, in contrast to FeNa, FeUrea is not affected by diuretics and thus appears to be more accurate in distinguishing between prerenal and renal AKI (95, 96). Based on the above findings, in addition to regular SCr examination, regular urinalysis and even 24-hour urinary protein quantification may be considered in patients with risk factors for ICI-AKI. Imaging procedures may be an alternative way to exclude other possible causes of AKI, such as an ultrasound to rule out urinary tract obstruction, and A CT scan may be considered after sufficient vigilance for further impairment of the patient’s renal function with intravenous contrast (95, 97). After excluding other causes of AKI, in patients for whom renal biopsy is contraindicated, positron emission tomography-computed tomography (PET-CT) may be useful to further identify ICI-AIN (98). In the study by Qualls et al., PET-CT of patients diagnosed with ICI-AIN showed an increased uptake of 18F-flourodeoxyglucose (FDG) in the renal cortices bilaterally. In contrast, for those with non-AIN AKI, including prerenal azotemia or cardiorenal syndrome, FDG uptake after AKI was unchanged or slightly decreased from baseline before AKI. However, FDG uptake is subject to various factors (e.g., PET acquisition protocols, reconstruction algorithms, and differences in the timing and dose of FDG injection). The comparison of FDG uptake after AKI and baseline (before AKI) may be more beneficial to improve the diagnostic clarity (99).
3.5.2. Histopathologic features of ICI-AKI
In the present case reports and series, the most common histopathology of ICI-AKI is ATIN (57, 68, 100). However, glomerular or tubular pathologies have been described in several studies, including thrombotic microangiopathy (TMA) (29, 101), pauci-immune glomerulonephritis (102), membranous glomerulonephritis (MN) (53), minimal change disease (MCD) (103, 104), C3 glomerulonephritis, immunoglobulin A (IgA) nephropathy (105), lupus nephropathy, focal segmental glomerular sclerosis (106), granulomatous formations with multinucleated giant cells, renal tubular acidosis (RTA) (107, 108), and acute tubular injury (ATI) (69). In a study of 16 patients who underwent renal biopsy after ICIs treatment, Mamlouk et al. found that 14 patients had histopathological ATIN, alone or in combination with interstitial inflammation associated with glomerular pathologies, including pauci-immune glomerulonephritis, MN, C3 glomerulonephritis, IgA nephropathy, and amyloid A (AA) amyloidosis (28). In a recent prospective study, 10 patients with ICI-AKI all had AIN, of whom 9 had ATI. However, 4 patients with AKI caused by other causes showed ATI and renal thrombotic microangiopathy, with mild to severe interstitial fibrosis or tubular atrophy (15). In particular, these lesions are often accompanied by infiltration of predominantly CD3+ and CD4+ T lymphocytes, varying degrees of mononuclear cells, eosinophils, and plasma cells, interstitial edema, and granuloma (29, 90).
At present, clinical manifestations and laboratory tests are often not reliable in predicting renal lesions and non-AIN lesions have a poor response to empirical corticosteroids treatment (28). However, Renal biopsy is helpful to confirm the diagnosis, guide the withdrawal of ICIs and the treatment of corticosteroids, and assess the rechallenge of ICIs treatment. Therefore, after joint evaluation by nephrologists and oncologists to rule out contraindications, we recommend renal biopsy in patients with stage 2 or higher AKI (28).
3.5.3. Biomarkers for AKI development after ICIs treatment
With the wide application of ICIs in anti-tumor therapy, according to the existing literature, many biomarkers, including circulating blood cell count, cytokines, autoantibodies, etc., can be used to assist in judging the risk and development of irAEs and evaluating the prognosis of patients. However, the specific biomarkers for AKI development are still limited. At present, some studies have found that IFN-α–induced transcript can be used to distinguish T cell-mediated rejection from ICI-AIN after ICIs treatment (109). Lower soluble CD25 and higher soluble CD163 are associated with higher irAEs and urinary soluble CD163 can also be used to identify the etiology of AKI. To be specific, urinary soluble CD163 levels were significantly higher in glomerulopathy injuries than in interstitial or tubular injuries (110, 111). Furthermore, a study also found urinary soluble CD163 is correlated with histological features of ANCA-associated glomerulonephritis and can be used to recognize relapsing ANCA-associated glomerulonephritis (112). Urine retinol binding protein/urine creatinine (uRBP/Cr) is useful in distinguishing ICI-AKI from other causes of AKI (62). In a cohort study of biomarkers for ICI-AKI, serum C-reactive protein (CRP) and uRBP/Cr measures were higher in patients with ICI-AKI than in patients with non-ICI-AKI (113). Therefore, in our opinion, an increase in these two markers may be considered as an indication for renal biopsy when infectious causes have been ruled out.
It should be noted that studies have shown that IL-17, IL-6 are associated with irAEs. And the importance of anti-IL-17 and anti-IL-6 in enhancing the efficacy of ICIs and controlling the development of irAEs has been confirmed (114, 115). Similarly, there are evidences that modulation of the gut microbiota enhances the antitumor effects of ICIs (116, 117). However, at present, studies on the association of these cytokines and gut microbes with ICI-AKI are very limited. In the future, the biomarkers for AKI development need to be further studied.
3.6. Management of ICI-AKI
3.6.1. Treatment of ICI-AKI
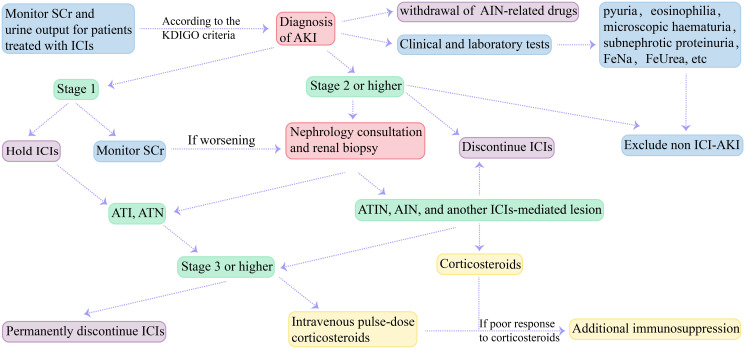
Treatment of ICI-AKI includes withdrawal of ICIs and AIN-related drugs (e.g., PPIs and NSAIDs), and administration of medications such as corticosteroids on the basis of renal biopsy. Previous studies suggested that ICIs should be discontinued in patients with stage 2 or higher ICI-AKI, especially in those without pre-renal AKI or evidence of urinary obstruction (18, 35, 50). Patients with persistent stage 1 or more than stage 2 AKI (including stage 2) should undergo nephrology consultation and consider renal biopsy (118–120). It is recommended to discontinue ICIs when the result is ATIN, AIN, or another immune-mediated lesion, whereas patients with renal biopsy showing ATI or ATN should not discontinue ICIs (121). The Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher nephrotoxicity should permanently discontinue ICIs therapy (119, 120). Some studies have found that systemic antibiotics, PPIs and anti-PD-1/anti-PD-L1 combination therapy have poor clinical outcomes (122). Therefore, AIN-related drugs (e.g., PPIs and NSAIDs) should be discontinued in patients with ICI-AKI. Earlier corticosteroid treatment was found to be associated with a better prognosis (57, 91, 92). Therefore, empirical corticosteroids can be used in patients whose causes other than ICIs have been ruled out and who have contraindications to renal biopsy (68). Patients with mild ICI-AKI can only receive oral prednisone (1mg/kg), and patients with stage 3 ICI-AKI or refractory renal dysfunction can receive intravenous pulse-dose corticosteroids (123, 124). In addition, additional immunosuppression including mycophenolate mofetil, infliximab, rituximab, and cyclophosphamide can be used for patients with refractory cases who do not respond well to high-dose corticosteroids (35). A case of mycophenolate mofetil for the treatment of nephrotic syndrome was previously reported (106). Similarly, Lin et al. studied the use of infliximab in 10 patients with ICI-ATIN and found that infliximab was effective in patients who had severe side effects or had a poor response to corticosteroids therapy (125). We summarized recommendations for the diagnosis and treatment of ICI-AKI in Figure 3 . The recommendations for corticosteroids treatment vary from one guideline to another. The clinical practice guidelines for the management of ICIs-associated nephrotoxicity were summarized in Table 2 (118–120, 126, 127).
Figure 3.
Diagnosis and treatment recommendations for ICI-AKI. ICIs, immune checkpoint inhibitors; SCr, serum creatinine; KDIGO, Kidney Disease Improving Global Outcomes; AKI, acute kidney injury; AIN, acute interstitial nephritis; FeNa, fractional excretion of sodium; FeUrea, fractional excretion of urea; ICI-AKI, immune checkpoint inhibitors-associated acute kidney injury; ATI, acute tubular injury; ATN, acute tubular nephritis; ATIN, acute tubulointerstitial nephritis.
Table 2.
ASCO, NCCN, SITC, and ESMO clinical practice guidelines for the management of ICIs-associated nephrotoxicity.
| Treatment | Stage/Grade a | Guidelines | |||
|---|---|---|---|---|---|
| ASCO | NCCN | SITC | ESMO | ||
| ICIs interruption | 1 | NO | NO | NO | NO |
| 2 | NO, but if worsening, YES. | YES | |||
| 3 | YES | ||||
| 4 | |||||
| Corticosteroids (prednisone) or additional immunosuppression | 1 | NO | The first-line treatment for ICI-TIN is glucocorticoids. If ineffectiveness, infliximab or mycophenolate mofetil. |
NO | |
| 2 | Initial dose of 0.5-1 mg/kg/d, if worsening, increase to 1-2 mg/kg/d. | If worsening, initial oral dose of 0.5-1 mg/kg. | |||
| 3 | Initial dose of 1-2 mg/kg/d, if worsening, consider additional immunosuppression. | If worsening, initiate intra-venous methyl-prednisolone 1-2 mg/kg. | |||
| 4 | |||||
| ICIs rechallenge | 1 | YES | If improved to ≤Grade 1, YES. | YES | If improved to ≤Grade 1, YES |
| 2 | NA | ||||
| 3 | NO | NA | |||
| 4 | |||||
| Others | 1 | SCr monitoring | SCr and urine protein monitoring | • Concomitant medications known to cause ICI-ATIN interruption • Nephrology consultation • If the lack of specific clinical features of ICI-AKI, renal biopsy. |
• Other causes assessment • Other nephrotoxic drugs interruption • Personalized renal biopsy |
| 2 | • Nephrology consultation • Other causes assessment |
Nephrology consultation | |||
| 3 | Renal biopsy | ||||
| 4 | |||||
Nephrotoxicity can be graded using the CTCAE scale and can also be graded with the KDIGO) criteria.
ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; SITC, Society for Immunotherapy of Cancer; ESMO, European Society for Medical Oncology; ICIs, immune checkpoint inhibitors; ICI-TIN, immune checkpoint inhibitor-associated acute tubulointerstitial nephritis; ICI-AKI, immune checkpoint inhibitor-associated acute kidney injury; NA, not available; SCr, serum creatinine.
3.6.2. ICIs rechallenge after ICI-AKI
Another unsettled question is whether ICIs rechallenge after improvement of renal function in ICI-AKI patients, which is reported by the available literature documenting. In the study by Gupta et al. (57), 28.2% of 429 ICI-AKI patients underwent ICIs rechallenge; of these patients, 76.9% had renal recovery and 16.5% had recurrent ICI-AKI. Fortunately, survival was similar between patients who underwent rechallenge and those who did not. Studies have shown that 16% to 23% of patients with ICI-AKI who receive ICIs rechallenge have a relatively low risk for recurrence (62). Therefore, for the rechallenge of ICIs in patients with ICI-AKI, studies considered that for patients with no ATIN or other ICIs caused lesions on renal biopsy, patients with ATI on renal biopsy, or patients with AIN who have a good response to corticosteroid therapy, when the ICI-AKI has recovered below stage 1 and there is no recurrence, ICIs can be considered. Concomitant low-dose prednisone therapy may be used to reduce the risk of renal irAEs (90, 100, 124).
Finally, studies have shown that ICIs can be safely used in patients with liver or kidney impairment, even in patients with CKD undergoing dialysis treatment, because ICIs are not cleared by the liver or kidney (128–130). The efficacy and safety of ICIs are not affected by age (131). Alternatively, preexisting autoimmune disease is not an absolute contraindication to ICIs treatment (132). Based on the available data and the lifesaving nature of ICIs, it seems to us that the recurrence rate of ICI-AKI after ICIs rechallenge is currently relatively low. For patients with ICIs as the only effective anti-tumor therapy, ICIs rechallenge can be performed after nephrologists and oncologists discuss its benefits and risks together. At the same time, SCr, urine and electrolytes should be monitored, and RRT should be performed when necessary.
4. Kidney transplant and ICIs therapy
The treatment of ICIs increases the risk of allograft rejection in graft recipients. This acute rejection is due to the activation of graft-specific T cells (133). In a study of 39 solid organ transplantation recipients treated with ICIs at MD Anderson Cancer Center, 41% had allograft rejection, 81% had graft loss, and 46% died (134). Similarly, in a retrospective cohort study of 69 kidney transplant recipients treated with ICIs, 42% experienced acute rejection, of which 19 experienced graft loss. This rejection was 50% mediated by pure T cells and 50% by a mixture of acute T cells and antibodies (135). In a recent review, it was summarized that 10% to 65% of solid organ transplant recipients treated with ICIs are at risk for acute allograft rejection, with a median onset time of 21 days after treatment initiation, and 24% to 81% of solid organ transplant recipients may lose their allografts (136). The difference is that anti-PD-1/PD-L1 seems to be more likely to cause rejection in kidney transplant recipients than anti-CTLA-4 (34, 137, 138).
However, for kidney transplant recipients in whom ICIs are the only effective antitumor therapy, previous studies have suggested strategies to mitigate the risk of rejection and increase the efficacy of ICIs. First, baseline immunosuppression was maintained in kidney-transplant recipients before treatment with immune checkpoint inhibitors (139). Second, to increase allograft tolerance, mini-pulse steroids may be used concomitantly at the initiation of ICIs (138). Most importantly, switching calcineurin inhibitors (CNI) to mammalian target of rapamycin inhibitors (mTORi) prior to ICIs treatment can improve OS of cancer patients and grafts (140, 141). In turn, dual immunosuppression regimen combining mTORi and corticosteroids or CNI also help maintain immunosuppression (136). In conclusion, ICIs can be used in kidney transplant recipients after weighing the benefits and risks of antineoplastic therapy and graft loss when no other similarly effective treatment options are available. However, possible rejection after ICIs initiation must be alert and prevented as much as possible.
5. Electrolyte abnormalities associated with ICIs
Anti-tumor therapy with ICIs can cause a variety of electrolyte abnormalities. Hyponatremia was the most common, and others included hypokalemia, hyperkalemia, hypophosphatemia, and hypocalcemia. In a retrospective observational study of 2458 patients treated with ICIs, hyponatremia occurred in 62%. In terms of severe electrolyte abnormalities, the proportions of hypophosphatemia, hypokalemia and hyponatremia were higher (17%, 6% and 6%, respectively). Hypocalcemia and hyperkalemia accounted for 0.2% and 0.6%, respectively (142). In addition, in a meta-analysis of patients with advanced NSCLC who received ICIs, the incidence of hyponatremia was 8.7% in the study groups of six randomized controlled trials; in 7 randomized controlled trials, the incidence of hypokalemia in the study group was 10.4% (143). The causes of electrolyte abnormalities are diverse. Immune-endocrine disease is an important cause of electrolyte abnormalities, such as hypophysitis, adrenal insufficiency, primary hyperparathyroidism and hypothyroidism (144–146). In the study by Patel et al., the mechanisms of hyponatremia include hypovolemic hyponatremia due to hemodynamic disorders caused by volume depletion, hypervolemic hyponatremia due to CHF or nephrosis, syndrome of inappropriate antidiuretic hormone secretion, and endocrine diseases (147). In addition, Izzedine et al. summarized the etiology of hypercalcemia, including endocrine disorders, sarcoid-like granuloma, humoral hypercalcemia due to parathyroid related hormone and pseudo- or hyperprogressive disease during ICIs therapy (148).
Of note, studies have reported concurrent renal complications in patients who developed electrolyte abnormalities after treatment with ICIs. Balakrishna et al. reported a case of hypokalemia after treatment with nivolumab, with increased SCr and 1+ protein on urinalysis, suggesting concurrent AKI (149). In addition, Herrmann et al. reported three cases of patients treated with ICIs who developed electrolyte disturbances secondary to renal tubular acidosis, one of which was accompanied by hypokalemia, and renal biopsy showed chronic active tubulointerstitial nephritis with moderate arteriosclerosis (108). Similarly, Rai et al. reported a case of nivolumab induced adrenal insufficiency with specific presentation of hypotension and hyponatremia, and interestingly, AKI developed in this patient (150). Although studies on electrolyte abnormalities and nephrotoxicity caused by ICIs are limited, an increasing number of case reports have aroused our attention in this regard. In our view, the monitoring and treatment of ICIs nephrotoxicity also requires more attention to electrolytes, and vice versa. But more research is needed on their relationship.
6. Conclusion
With the application of ICIs in clinical anti-tumor therapy, its nephrotoxicity is becoming more and more common, often manifested as AKI. The etiology and histopathology of AKI need to be further clarified. Renal biopsy is essential. However, non-invasive diagnostic methods such as biomarkers and imaging are also being developed. More research is also needed to improve the treatment methods, including AKI graded corticosteroids therapy and additional immunosuppression benefits.
Furthermore, the rechallenge of ICIs is also worthy of attention. Based on the clinical benefits of ICIs and relatively controllable renal complications, for cancer patients for whom ICIs is the only effective treatment, the rechallenge of ICIs with concomitant the management of nephrotoxicity may be a more beneficial way after the collaborative discussion between nephrologists and oncologists. However, as with the use of ICIs in the treatment of kidney transplant recipients, kidney damage should be taken seriously, and further studies are needed to refine treatment strategies. At present, studies on electrolyte abnormalities associated with ICIs are limited, and the association with nephrotoxicity needs to be further studied, which is of great significance for the prevention and treatment of nephrotoxicity.
Finally, with the continued benefits of ICIs in anti-tumor therapy, more attention should be paid to its adverse events in the future researches, especially irAEs with poor prognosis and high mortality. In our view, for each of these irAEs, further studies are needed to clarify their possible mechanisms and identify specific biomarkers to aid diagnosis and standardize effective treatment.
Author contributions
PZ: Conceptualization, Writing – original draft, Writing – review & editing. YG: Conceptualization, Writing – review & editing. ZK: Conceptualization, Writing – review & editing. JW: Writing – review & editing. SS: Writing – review & editing. WH: Writing – review & editing. JL: Writing – review & editing. ZL: Writing – original draft, Writing – review & editing. RW: Writing – original draft, Writing – review & editing.
Acknowledgments
Thanks to all the scientists working in this field and those public research funds that fund our research about this topic.
Glossary
| ICIs | immune checkpoint inhibitors |
| AKI | acute kidney injury |
| ATIN | acute tubulointerstitial nephritis |
| ICI-AKI | immune checkpoint inhibitors-associated acute kidney injury |
| PD-1 | programmed cell death receptor-1 |
| PD-L1 | programmed cell death ligand-1 |
| CTLA-4 | cytotoxic T lymphocyte-associated antigen-4 |
| RCC | renal cell carcinoma |
| NSCLC | non-small cell lung cancer |
| irAEs | immune-related adverse events |
| TAAs | tumor-associated antigens |
| MHC | major histocompatibility complex |
| APCs | antigen-presenting cells |
| TCR | T cell receptor |
| PFS | progression-free survival |
| OS | overall survival |
| BMI | body mass index |
| SCr | serum creatinine |
| KDIGO | Kidney Disease Improving Global Outcomes |
| RRT | renal replacement therapy |
| NCI-CTCAEs | National Cancer Institute’s Common Terminology Criteria for Adverse Events |
| ATN | acute tubular nephritis |
| TECs | tubular epithelial cells |
| dsDNA | double-stranded DNA |
| DCs | dendritic cells |
| Tregs | regulatory T cells |
| IL-1Ra | interleukin-1 receptor antagonist |
| CXCL | C-X-C motif chemokine ligand |
| TNF-α | tumor necrosis factor-α |
| IL | interleukin |
| mRNA | messenger RNA |
| AIN | acute interstitial nephritis |
| RAASi | renin-angiotensin-aldosterone system inhibitors |
| ACEIs | angiotensin-converting enzyme inhibitors |
| ARBs | angiotensin-receptor blockers |
| CKD | chronic kidney disease |
| eGFR | estimated glomerular filtration rate |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PPIs | proton pump inhibitors |
| H2RAs | histamine H2-receptor antagonists |
| OR | odds ratio |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| ICI-AIN | immune checkpoint inhibitors-associated acute interstitial nephritis |
| LDH | lactate dehydrogenase |
| FeNa | fractional excretion of sodium |
| FeUrea | fractional excretion of urea |
| CT | computed tomography |
| PET-CT | positron emission tomography-computed tomography |
| FDG | 18F-flourodeoxyglucose |
| TMA | thrombotic microangiopathy |
| MN | membranous glomerulonephritis |
| MCD | minimal change disease |
| IgA | immunoglobulin A |
| RTA | renal tubular acidosis |
| ATI | acute tubular injury |
| AA | amyloid A |
| PLR | platelet/lymphocyte ratio |
| NLR | neutrophil/lymphocyte ratio |
| CRP | C-reactive protein |
| uRBP/Cr | urine retinol binding protein/urine creatinine |
| IFN-α | interferon-α |
| TGF-β1 | transforming growth factor-β1 |
| LIPI | Lung Immune Prognostic Index |
| ICI-ATIN | immune checkpoint inhibitors-associated acute tubulointerstitial nephritis |
| ASCO | American Society of Clinical Oncology |
| CNI | calcineurin inhibitors |
| mTORi | mammalian target of rapamycin inhibitors |
| NCCN | National Comprehensive Cancer Network |
| SITC | Society for Immunotherapy of Cancer |
| ESMO | European Society for Medical Oncology |
| NA | not available. |
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Natural Science Foundation of China (Grant: 81873615, 82070744, 81770723, 81400732), Academic promotion programme of Shandong First Medical University (NO: 2019QL022) and Taishan Scholars Program (NO: ts201712090, tsqn201812138)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. (2020) 11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 6. Xie W, Xiao S, Li X, Huang J, Li G, Zhang Z. Incidence, mortality, and risk factors of acute kidney injury after immune checkpoint inhibitors: Systematic review and meta-analysis of real-world evidence. Eur J Intern Med. (2023) 115:88–95. doi: 10.1016/j.ejim.2023.05.034. [DOI] [PubMed] [Google Scholar]
- 7. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 9. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: A meta-analysis. Front Pharmacol. (2018) 9:1430. doi: 10.3389/fphar.2018.01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 11. Roth P, Winklhofer S, Muller AMS, Dummer R, Mair MJ, Gramatzki D, et al. Neurological complications of cancer immunotherapy. Cancer Treat Rev. (2021) 97:102189. doi: 10.1016/j.ctrv.2021.102189. [DOI] [PubMed] [Google Scholar]
- 12. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. (2021) 92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 13. Pico de Coana Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. (2015) 21:482–91. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 14. Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. (2019) 14:1692–700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farooqui N, Zaidi M, Vaughan L, McKee TD, Ahsan E, Pavelko KD, et al. Cytokines and immune cell phenotype in acute kidney injury associated with immune checkpoint inhibitors. Kidney Int Rep. (2023) 8:628–41. doi: 10.1016/j.ekir.2022.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu X, Wu R, Ji Y, Huang M, Feng Z. Identifying Patients at Risk of Acute Kidney Injury among Patients Receiving Immune Checkpoint Inhibitors: A Machine Learning Approach. Diagnostics (Basel). (2022) 12(12):3157. doi: 10.3390/diagnostics12123157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 18. Stein C, Burtey S, Mancini J, Pelletier M, Sallee M, Brunet P, et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: a real-life study in a single-centre cohort. Nephrol Dial Transplant. (2021) 36:1664–74. doi: 10.1093/ndt/gfaa137. [DOI] [PubMed] [Google Scholar]
- 19. Ishitsuka R, Miyazaki J, Ichioka D, Inoue T, Kageyama S, Sugimoto M, et al. Impact of acute kidney injury defined by CTCAE v4.0 during first course of cisplatin-based chemotherapy on treatment outcomes in advanced urothelial cancer patients. Clin Exp Nephrol. (2017) 21:732–40. doi: 10.1007/s10157-016-1327-z. [DOI] [PubMed] [Google Scholar]
- 20. Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. (2020) 97:62–74. doi: 10.1016/j.kint.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 21. Ji MS, Wu R, Feng Z, Wang YD, Wang Y, Zhang L, et al. Incidence, risk factors and prognosis of acute kidney injury in patients treated with immune checkpoint inhibitors: a retrospective study. Sci Rep. (2022) 12:18752. doi: 10.1038/s41598-022-21912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PloS One. (2016) 11:e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izzedine H, Gueutin V, Gharbi C, Mateus C, Robert C, Routier E, et al. Kidney injuries related to ipilimumab. Invest New Drugs. (2014) 32:769–73. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 24. Benfaremo D, Manfredi L, Luchetti MM, Gabrielli A. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: A review of the literature. Curr Drug Saf. (2018) 13:150–64. doi: 10.2174/1574886313666180508122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. (2018) 29:2039–52. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. (2009) 361:211–2. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 27. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 28. Mamlouk O, Selamet U, MaChado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. (2019) 7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. (2016) 90:638–47. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koda R, Watanabe H, Tsuchida M, Iino N, Suzuki K, Hasegawa G, et al. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. (2018) 19:48. doi: 10.1186/s12882-018-0848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. (2005) 115:184–91. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32. Neumann K, Ostmann A, Breda PC, Ochel A, Tacke F, Paust H-J, et al. The co-inhibitory molecule PD-L1 contributes to regulatory T cell-mediated protection in murine crescentic glomerulonephritis. Sci Rep. (2019) 9(1):2038. doi: 10.1038/s41598-018-38432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shim YJ, Khedraki R, Dhar J, Fan R, Dvorina N, Valujskikh A, et al. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int. (2020) 98:897–905. doi: 10.1016/j.kint.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol. (2017) 45:160–9. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 35. Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. (2017) 32:936–42. doi: 10.1093/ndt/gfw382. [DOI] [PubMed] [Google Scholar]
- 36. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marco T, Anna P, Annalisa T, Francesco M, Stefania SL, Stella D, et al. The mechanisms of acute interstitial nephritis in the era of immune checkpoint inhibitors in melanoma. Ther Adv Med Oncol. (2019) 11:1758835919875549. doi: 10.1177/1758835919885202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. (2020) 38:326–33. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 40. Murakami N, Motwani S, Riella LV. Renal complications of immune checkpoint blockade. Curr Probl Cancer. (2017) 41:100–10. doi: 10.1016/j.currproblcancer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, et al. Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol. (2006) 17:2919–27. doi: 10.1681/ASN.2006050418. [DOI] [PubMed] [Google Scholar]
- 42. Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez-Almazor ME, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. (2018) 36:638–46. doi: 10.1007/s10637-017-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. (2013) 119:1675–82. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 44. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. (2012) 30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 45. Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: Practical recommendations for diagnosis and clinical management. Cancer. (2018) 124:1111–21. doi: 10.1002/cncr.31200. [DOI] [PubMed] [Google Scholar]
- 46. Spagnolo CC, Giuffrida G, Cannavo S, FranChina T, Silvestris N, Ruggeri RM, et al. Management of endocrine and metabolic toxicities of immune-checkpoint inhibitors: from clinical studies to a real-life scenario. Cancers (Basel). (2022) 15(1):246. doi: 10.3390/cancers15010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. (2016) 63:1490–3. doi: 10.1093/cid/ciw539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamashima R, Uchino J, Morimoto Y, Iwasaku M, Kaneko Y, Yamada T, et al. Association of immune checkpoint inhibitors with respiratory infections: A review. Cancer Treat Rev. (2020) 90:102109. doi: 10.1016/j.ctrv.2020.102109. [DOI] [PubMed] [Google Scholar]
- 49. Fujita K, Kim YH, Kanai O, Yoshida H, Mio T, Hirai T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir Med. (2019) 146:66–70. doi: 10.1016/j.rmed.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 50. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guven DC, Ozbek DA, Sahin TK, Kavgaci G, Aksun MS, Erul E, et al. The incidence and risk factors for acute kidney injury in patients treated with immune checkpoint inhibitors. Anticancer Drugs. (2023) 34:783–90. doi: 10.1097/CAD.0000000000001463. [DOI] [PubMed] [Google Scholar]
- 52. Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. (2019) 34:108–17. doi: 10.1093/ndt/gfy105. [DOI] [PubMed] [Google Scholar]
- 53. Meraz-Munoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. (2020) 8(1):e000467. doi: 10.1136/jitc-2019-000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Espi M, Teuma C, Novel-Catin E, Maillet D, Souquet PJ, Dalle S, et al. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur J Cancer. (2021) 147:29–39. doi: 10.1016/j.ejca.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 55. Koks MS, Ocak G, Suelmann BBM, Hulsbergen-Veelken CAR, Haitjema S, Vianen ME, et al. Immune checkpoint inhibitor-associated acute kidney injury and mortality: An observational study. PloS One. (2021) 16:e0252978. doi: 10.1371/journal.pone.0252978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. (2021) 9(10):e003467. doi: 10.1136/jitc-2021-003467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen G, Qin Y, Fan QQ, Zhao B, Mei D, Li XM. Renal adverse effects following the use of different immune checkpoint inhibitor regimens: A real-world pharmacoepidemiology study of post-marketing surveillance data. Cancer Med. (2020) 9:6576–85. doi: 10.1002/cam4.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu F, Wang Z, Li X, Zhang Z, Yang Y, Chen J, et al. Comparative risk of acute kidney injury among cancer patients treated with immune checkpoint inhibitors. Cancer Commun (Lond). (2023) 43:214–24. doi: 10.1002/cac2.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. (2008) 224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 61. Liu K, Qin Z, Xu X, Li T, Ge Y, Mao H, et al. Comparative risk of renal adverse events in patients receiving immune checkpoint inhibitors: A bayesian network meta-analysis. Front Oncol. (2021) 11:662731. doi: 10.3389/fonc.2021.662731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Q, Moledina DG, Sise ME. Immune checkpoint inhibitors and kidney disease. Curr Opin Nephrol Hypertens. (2022) 31:449–55. doi: 10.1097/MNH.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 63. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. (2010) 11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 64. De Giglio A, Grandinetti V, Aprile M, Borelli G, Campus A, Croci Chiocchini AL, et al. Patterns of renal toxicity from the combination of pemetrexed and pembrolizumab for advanced nonsquamous non-small-cell lung cancer (NSCLC): A single-center experience. Lung Cancer. (2022) 174:91–6. doi: 10.1016/j.lungcan.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 65. Lee JS, Ruppin E. Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 ligand 1. JAMA Oncol. (2019) 5:1614–8. doi: 10.1001/jamaoncol.2019.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. (2017) 377:1824–35. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 67. Weber JS, Hodi FS, Wolchok JD, Topalian SL, SChadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 68. Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J Am Soc Nephrol. (2020) 31:435–46. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. (2019) 12:81–8. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Centanni M, Moes D, Troconiz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. (2019) 58:835–57. doi: 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. (2010) 49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72. Zhu J, Wu J, Chen P, You K, Su J, Gao Z, et al. Acute kidney injury associated with immune checkpoint inhibitors: A pharmacovigilance study. Int Immunopharmacol. (2022) 113:109350. doi: 10.1016/j.intimp.2022.109350. [DOI] [PubMed] [Google Scholar]
- 73. Abdelrahim M, Mamlouk O, Lin H, Lin J, Page V, Abdel-Wahab N, et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: a 10-year single-institution analysis. Oncoimmunology. (2021) 10:1927313. doi: 10.1080/2162402X.2021.1927313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu C, Wei W, Yang L, Li J, Yi C, Pu Y, et al. Incidence and risk factors of acute kidney injury in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1173952. doi: 10.3389/fimmu.2023.1173952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Murakami N, Borges TJ, Yamashita M, Riella LV. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J. (2016) 9:411–7. doi: 10.1093/ckj/sfw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu L-Y, Zhao H-Y, Yu X-J, Wang J-W, Zheng X-Z, Jiang L, et al. Clinicopathological features of kidney injury related to immune checkpoint inhibitors: A systematic review. J Clin Med. (2023) 12(4):1349. doi: 10.3390/jcm12041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qu J, Ding Y, Jiang K, Hao J, Li Y, Zhang A, et al. Nephrotoxicity of immune checkpoint inhibitors: A disproportionality analysis from 2013 to 2020. Tohoku J Exp Med. (2021) 254:275–82. doi: 10.1620/tjem.254.275. [DOI] [PubMed] [Google Scholar]
- 78. Valdivielso JM, Jacobs-Cacha C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. (2019) 28:1–9. doi: 10.1097/MNH.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 79. Sorah JD, Rose TL, Radhakrishna R, Derebail VK, Milowsky MI. Incidence and prediction of immune checkpoint inhibitor-related nephrotoxicity. J Immunother. (2021) 44:127–31. doi: 10.1097/CJI.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yan H, Tang M, Zhu W, Yang Y. Immune checkpoint inhibitor-associated acute kidney injury in patients with cancer: a systematic review and meta-analysis of risk factors. Clin Exp Nephrol. (2023) 27:603–12. doi: 10.1007/s10157-023-02344-y. [DOI] [PubMed] [Google Scholar]
- 81. Qin Z, Liu K, Xu X, Li T, Ge Y, Wu B, et al. Incidence, predictors and 6-month overall outcome of acute kidney injury in Chinese patients receiving PD-1 inhibitors. Future Oncol. (2022) 18:1951–62. doi: 10.2217/fon-2021-1004. [DOI] [PubMed] [Google Scholar]
- 82. Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. (2010) 5:1690–5. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 83. Seethapathy H, Zhao S, Strohbehn IA, Lee M, Chute DF, Bates H, et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int Rep. (2020) 5:1700–5. doi: 10.1016/j.ekir.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Okamoto K, Saito Y, Yamaguchi A, Takekuma Y, Sugawara M. Acid suppressants reduce the therapeutic effect of immune checkpoint inhibitors and increase the risk of acute kidney injury: a meta-analysis. Int J Clin Oncol. (2023) 28:1343–53. doi: 10.1007/s10147-023-02385-z. [DOI] [PubMed] [Google Scholar]
- 85. Chen J-J, Lee T-H, Kuo G, Yen C-L, Lee C-C, Chang C-H, et al. All-cause and immune checkpoint inhibitor–associated acute kidney injury in immune checkpoint inhibitor users: a meta-analysis of occurrence rate, risk factors and mortality. Clin Kidney J. (2024) 17(1):sfad292. doi: 10.1093/ckj/sfad292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. (2016) 65:740–8. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. (2013) 2:e22058. doi: 10.4161/onci.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Giordan Q, Salleron J, Vallance C, Moriana C, Clement-Duchene C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front Immunol. (2021) 12:716317. doi: 10.3389/fimmu.2021.716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. (2016) 68:287–91. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 90. Manohar S, Ghamrawi R, Chengappa M, Goksu BNB, KottsChade L, Finnes H, et al. Acute interstitial nephritis and checkpoint inhibitor therapy: single center experience of management and drug rechallenge. Kidney360. (2020) 1:16–24. doi: 10.34067/KID.0000152019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fernandez-Juarez G, Perez JV, Caravaca-Fontan F, Quintana L, Shabaka A, Rodriguez E, et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. (2018) 13:1851–8. doi: 10.2215/CJN.01390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gonzalez E, Gutierrez E, Galeano C, Chevia C, de Sequera P, Bernis C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. (2008) 73:940–6. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 93. Oleas D, Bolufer M, Agraz I, Felip E, Munoz E, Gabaldon A, et al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-centre experience. Clin Kidney J. (2021) 14:1364–70. doi: 10.1093/ckj/sfaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. (2012) 7:167–74. doi: 10.2215/CJN.09490911. [DOI] [PubMed] [Google Scholar]
- 95. Dumoulin DW, Visser S, Cornelissen R, van Gelder T, Vansteenkiste J, von der Thusen J, et al. Renal toxicity from pemetrexed and pembrolizumab in the era of combination therapy in patients with metastatic nonsquamous cell NSCLC. J Thorac Oncol. (2020) 15:1472–83. doi: 10.1016/j.jtho.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 96. Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. (2002) 62:2223–9. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 97. Fried JG, Morgan MA. Renal imaging: core curriculum 2019. Am J Kidney Dis. (2019) 73:552–65. doi: 10.1053/j.ajkd.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 98. Manohar S, Albright RC., Jr. Interstitial nephritis in immune checkpoint inhibitor therapy Kidney Int. (2019) 96:252 doi: 10.1016/j.kint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 99. Qualls D, Seethapathy H, Bates H, Tajmir S, Heidari P, Endres P, et al. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J Immunother Cancer. (2019) 7:356. doi: 10.1186/s40425-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eijgelsheim M, Sprangers B. Kidney biopsy should be performed to document the cause of immune checkpoint inhibitor-associated acute kidney injury: PRO. Kidney360. (2020) 1:158–61. doi: 10.34067/KID.0001192019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tinawi M, Bastani B. Nephrotoxicity of immune checkpoint inhibitors: acute kidney injury and beyond. Cureus. (2020) 12:e12204. doi: 10.7759/cureus.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gallan AJ, Alexander E, Reid P, Kutuby F, Chang A, Henriksen KJ. Renal vasculitis and pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis. (2019) 74:853–6. doi: 10.1053/j.ajkd.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 103. Gao B, Lin N, Wang S, Wang Y. Minimal change disease associated with anti-PD1 immunotherapy: a case report. BMC Nephrol. (2018) 19:156. doi: 10.1186/s12882-018-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, et al. Nephrotic syndrome with cancer immunotherapies: A report of 2 cases. Am J Kidney Dis. (2017) 70:581–5. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 105. Kishi S, Minato M, Saijo A, Murakami N, Tamaki M, Matsuura M, et al. IgA nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med. (2018) 57:1259–63. doi: 10.2169/internalmedicine.9814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EJ, van Herpen CML, Willemsen A. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: A case report. J Immunother. (2017) 40:345–8. doi: 10.1097/CJI.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 107. El Bitar S, Weerasinghe C, El-Charabaty E, Odaimi M. Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med. (2018) 2018:8408015. doi: 10.1155/2018/8408015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Herrmann SM, Alexander MP, Romero MF, Zand L. Renal tubular acidosis and immune checkpoint inhibitor therapy: an immune-related adverse event of PD-1 inhibitor-A report of 3 cases. Kidney Med. (2020) 2:657–62. doi: 10.1016/j.xkme.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Adam BA, Murakami N, Reid G, Du K, Jasim R, Boils CL, et al. Gene expression profiling in kidney transplants with immune checkpoint inhibitor-associated adverse events. Clin J Am Soc Nephrol. (2021) 16:1376–86. doi: 10.2215/CJN.00920121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cardena-Gutierrez A, Lopez Barahona M. Predictive biomarkers of severe immune-related adverse events with immune checkpoint inhibitors: prevention, underlying causes, intensity, and consequences. Front Med (Lausanne). (2022) 9:908752. doi: 10.3389/fmed.2022.908752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sun PP, Zhou XJ, Su JQ, Wang C, Yu XJ, Su T, et al. Urine macrophages reflect kidney macrophage content during acute tubular interstitial and glomerular injury. Clin Immunol. (2019) 205:65–74. doi: 10.1016/j.clim.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 112. Aendekerk JP, Timmermans SAMEG, Busch MH, Potjewijd J, Heeringa P, Damoiseaux JGMC, et al. Urinary soluble CD163 and disease activity in biopsy-proven ANCA-associated glomerulonephritis. Clin J Am Soc Nephrology. (2020) 15:1740–8. doi: 10.2215/CJN.07210520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Isik B, Alexander MP, Manohar S, Vaughan L, KottsChade L, Markovic S, et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep. (2021) 6:1022–31. doi: 10.1016/j.ekir.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochim Biophys Acta (BBA) - Rev Cancer. (2022) 1877(4):188758. doi: 10.1016/j.bbcan.2022.188758. [DOI] [PubMed] [Google Scholar]
- 115. Holmstroem RB, Nielsen OH, Jacobsen S, Riis LB, Theile S, Bjerrum JT, et al. COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J ImmunoTherapy Cancer. (2022) 10(9):e005111. doi: 10.1136/jitc-2022-005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Simpson RC, Shanahan ER, Scolyer RA, Long GV. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2023) 20:697–715. doi: 10.1038/s41571-023-00803-9. [DOI] [PubMed] [Google Scholar]
- 117. Huang J, Gong C, Zhou A. Modulation of gut microbiota: a novel approach to enhancing the effects of immune checkpoint inhibitors. Ther Adv Med Oncol. (2023) 15:17588359231204854. doi: 10.1177/17588359231204854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–iv42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 119. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1. 2019. J Natl Compr Canc Netw. (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013 [DOI] [PubMed] [Google Scholar]
- 120. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 121. Herrmann SM, Perazella MA. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int Rep. (2020) 5:1139–48. doi: 10.1016/j.ekir.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. (2020) 8(2):e001361. doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. (2016) 17:188. doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Perazella MA, Sprangers B. AKI in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. (2019) 14:1077–9. doi: 10.2215/CJN.02340219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lin JS, Mamlouk O, Selamet U, Tchakarov A, Glass WF, Sheth RA, et al. Infliximab for the treatment of patients with checkpoint inhibitor-associated acute tubular interstitial nephritis. Oncoimmunology. (2021) 10:1877415. doi: 10.1080/2162402X.2021.1877415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9(6):e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 128. Tabei T, Natsume I, Kobayashi K. Successful treatment of metastatic clear cell carcinoma with nivolumab in a patient receiving dialysis treatment. Int J Urol. (2017) 24:708–10. doi: 10.1111/iju.13420. [DOI] [PubMed] [Google Scholar]
- 129. Vitorino M, Santos C. Use of pembrolizumab in end-stage renal disease: A case report with complete response. Case Rep Oncol. (2022) 15:187–90. doi: 10.1159/000521979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tiu BC, Strohbehn IA, Zhao S, Ouyang T, Hanna P, Wang Q, et al. Safety of immune checkpoint inhibitors in patients with advanced chronic kidney disease: A retrospective cohort study. Oncologist. (2023) 28:e379–e90. doi: 10.1093/oncolo/oyad001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 132. Kennedy LC, Bhatia S, Thompson JA, Grivas P. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J Natl Compr Canc Netw. (2019) 17:750–7. doi: 10.6004/jnccn.2019.7310. [DOI] [PubMed] [Google Scholar]
- 133. Wu J, Huang J, Zhu J, He Z, Chen M, Gao S, et al. Immune checkpoint inhibitors increase the risk of kidney transplant rejection: a real-world pharmacovigilance study. Expert Opin Drug Saf. (2023) 22:231–5. doi: 10.1080/14740338.2022.2110234. [DOI] [PubMed] [Google Scholar]
- 134. Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. (2019) 7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. (2021) 100:196–205. doi: 10.1016/j.kint.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ferrandiz-Pulido C, Leiter U, Harwood C, Proby CM, Guthoff M, Scheel CH, et al. Immune checkpoint inhibitors in solid organ transplant recipients with advanced skin cancers-emerging strategies for clinical management. Transplantation. (2023) 107:1452–62. doi: 10.1097/TP.0000000000004459 [DOI] [PubMed] [Google Scholar]
- 137. Delyon J, Zuber J, Dorent R, Poujol-Robert A, Peraldi MN, Anglicheau D, et al. Immune checkpoint inhibitors in transplantation-A case series and comprehensive review of current knowledge. Transplantation. (2021) 105:67–78. doi: 10.1097/TP.0000000000003292. [DOI] [PubMed] [Google Scholar]
- 138. Bermejo S, Bolufer M, Riveiro-Barciela M, Soler MJ. Immunotherapy and the spectrum of kidney disease: should we individualize the treatment? Front Med (Lausanne). (2022) 9:906565. doi: 10.3389/fmed.2022.906565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Carroll RP, Boyer M, Gebski V, Hockley B, Johnston JK, Kireta S, et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol. (2022) 23:1078–86. doi: 10.1016/S1470-2045(22)00368-0. [DOI] [PubMed] [Google Scholar]
- 140. Venkatachalam K, Malone AF, Heady B, Santos RD, Alhamad T. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation. (2020) 104:1041–7. doi: 10.1097/TP.0000000000002914. [DOI] [PubMed] [Google Scholar]
- 141. Rousseau B, Guillemin A, Duvoux C, Neuzillet C, Tlemsani C, Compagnon P, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. (2019) 144:886–96. doi: 10.1002/ijc.31769. [DOI] [PubMed] [Google Scholar]
- 142. Seethapathy H, Rusibamayila N, Chute DF, Lee M, Strohbehn I, Zubiri L, et al. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol Dialysis Transplantation. (2021) 36:2241–7. doi: 10.1093/ndt/gfaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cantini L, Merloni F, Rinaldi S, Lenci E, Marcantognini G, Meletani T, et al. Electrolyte disorders in advanced non-small cell lung cancer patients treated with immune check-point inhibitors: A systematic review and meta-analysis. Crit Rev Oncology/Hematology. (2020) 151:102974. doi: 10.1016/j.critrevonc.2020.102974. [DOI] [PubMed] [Google Scholar]
- 144. Newman C, Kgosidalwa O, Hakami OA, Kennedy C, Grogan L, Agha A. Multiple endocrinopathies, hypercalcaemia and pancreatitis following combined immune checkpoint inhibitor use- case report and review of literature. BMC Endocrine Disord. (2021) 21(1):33. doi: 10.1186/s12902-021-00693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Workeneh BT, Jhaveri KD, Rondon-Berrios H. Hyponatremia in the cancer patient. Kidney Int. (2020) 98:870–82. doi: 10.1016/j.kint.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 146. Zhang Y, Cui Y, Li Y, Cong L. Immune checkpoint inhibitor-induced primary hyperparathyroidism in a small-cell lung cancer patient: A case report. Medicina. (2023) 59(2):215. doi: 10.3390/medicina59020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Patel SS, Roy S, Pokal M, Gayam V, Adapa S, Patel ES. Hyponatremia and fever in a patient on ipilimumab and nivolumab (Immune checkpoint inhibitors): A case report. J Invest Med High Impact Case Rep. (2021) 9:23247096211045249. doi: 10.1177/23247096211045249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Izzedine H, Chazal T, Wanchoo R, Jhaveri KD. Immune checkpoint inhibitor–associated hypercalcaemia. Nephrol Dialysis Transplantation. (2022) 37:1598–608. doi: 10.1093/ndt/gfaa326. [DOI] [PubMed] [Google Scholar]
- 149. Balakrishna P, Villegas A. Hypokalemic paralysis secondary to immune checkpoint inhibitor therapy. Case Rep Oncological Med. (2017) 2017:1–4. doi: 10.1155/2017/5063405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rai M, Go M. Nivolumab induced adrenal insufficiency: rare side-effect of a new anti-cancer therapy - immune-checkpoint inhibitors. Cureus. (2020). 12(4):e7625. doi: 10.7759/cureus.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]