Abstract
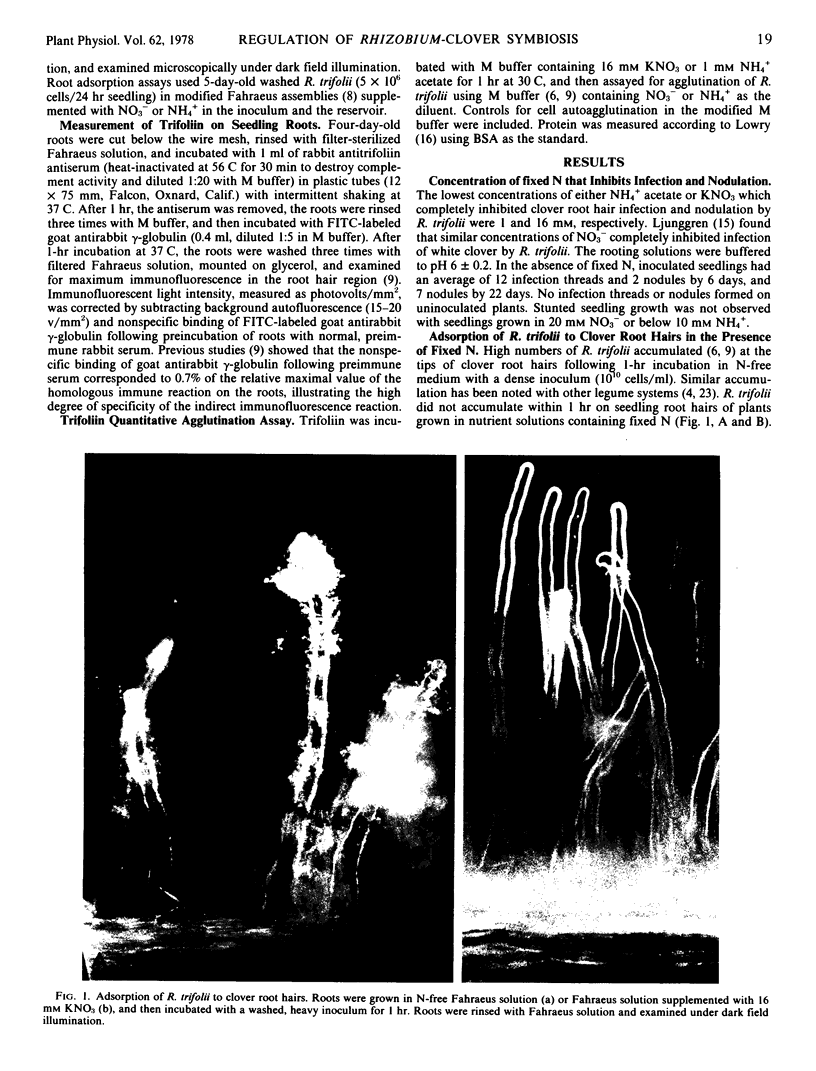
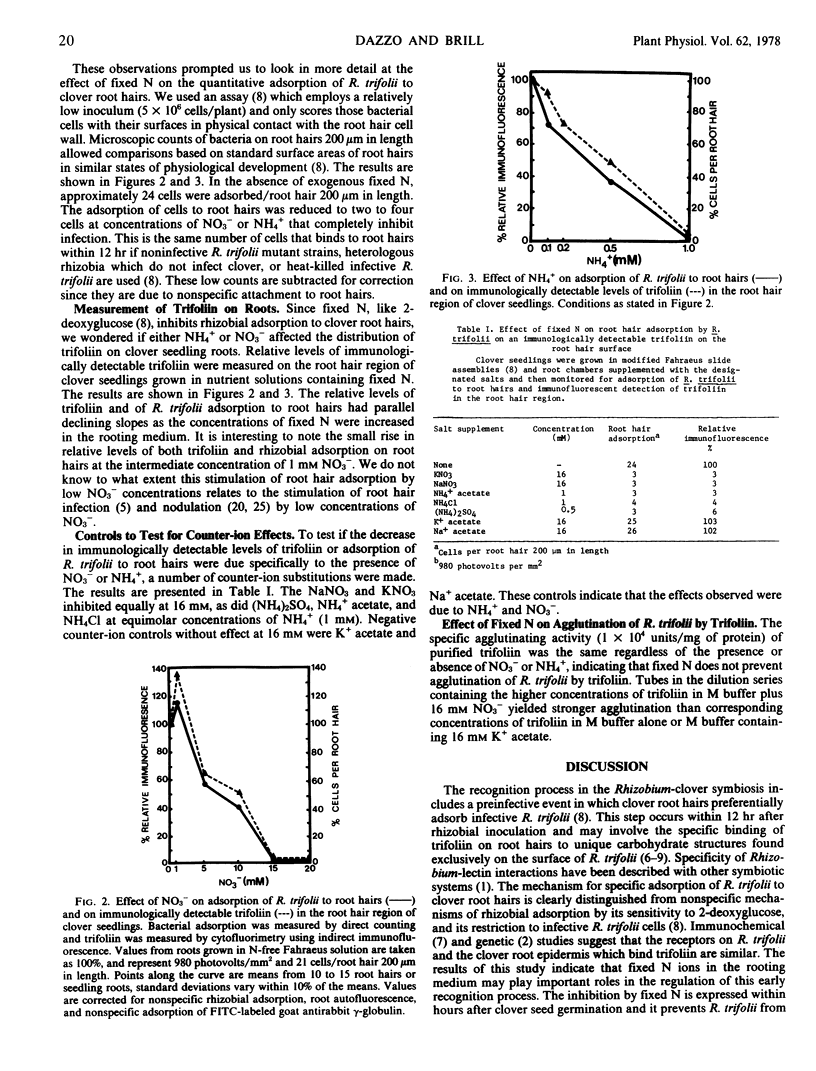
Either NO3− (16 millimolar) or NH4+ (1 millimolar) completely inhibited infection and nodulation of white clover seedlings (Trifoliin repens) inoculated with Rhizobium trifolii. The binding of R. trifolii to root hairs and the immunologically detectable levels of the plant lectin, trifoliin, on the root hair surface had parallel declining slopes as the concentration of either NO3− or NH4+ was increased in the rooting medium. This supports the role of trifoliin in binding R. trifolii to clover root hairs. Agglutination of R. trifolii by trifoliin from seeds was not inhibited by these levels of NO3− or NH4+. The results suggest that these fixed N ions may play important roles in regulating an early recognition process in the Rhizobium-clover symbiosis, namely the accumulation of high numbers of infective R. trifolii cells on clover root hairs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. D. Lectins as determinants of specificity in legume-Rhizobium symbiosis. Basic Life Sci. 1977;9:283–297. doi: 10.1007/978-1-4684-0880-5_18. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Dazzo F. B., Appelbaum E. R., Maier R. J., Brill W. J. Intergeneric transfer of genes involved in the Rhizobium-legume symbiosis. Science. 1977 Dec 2;198(4320):938–940. doi: 10.1126/science.929179. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Receptor site on clover and alfalfa roots for Rhizobium. Appl Environ Microbiol. 1977 Jan;33(1):132–136. doi: 10.1128/aem.33.1.132-136.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



