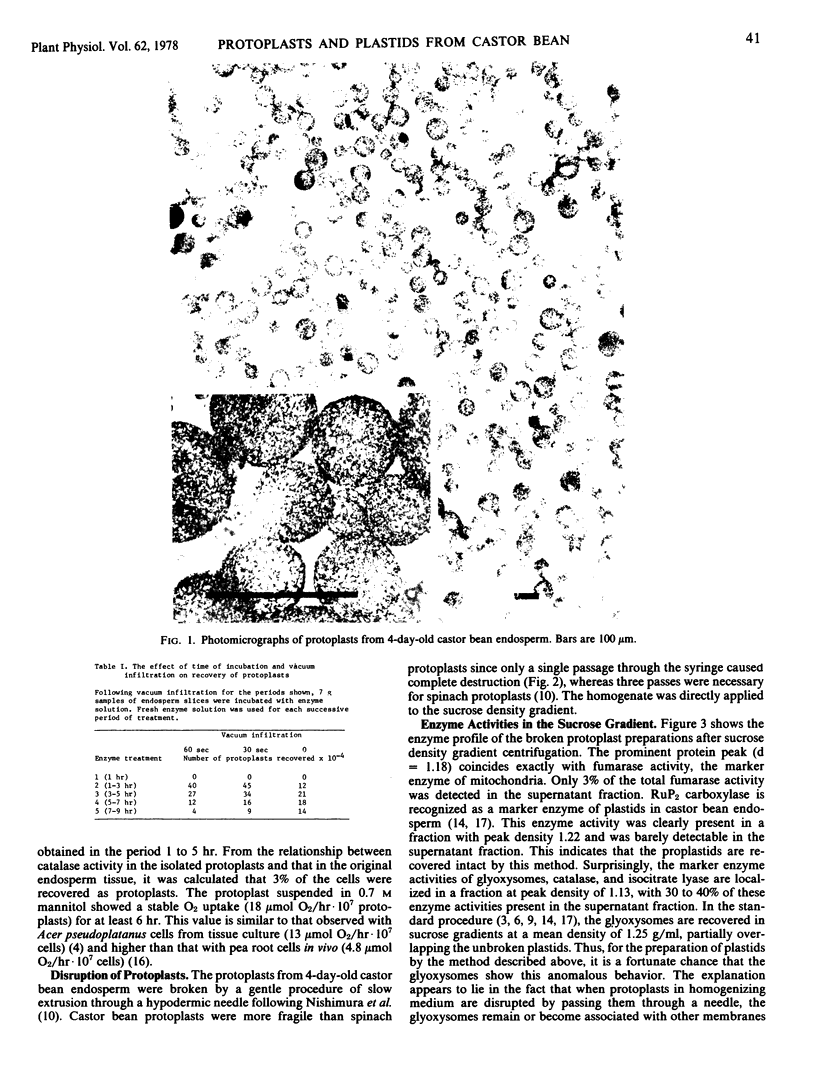
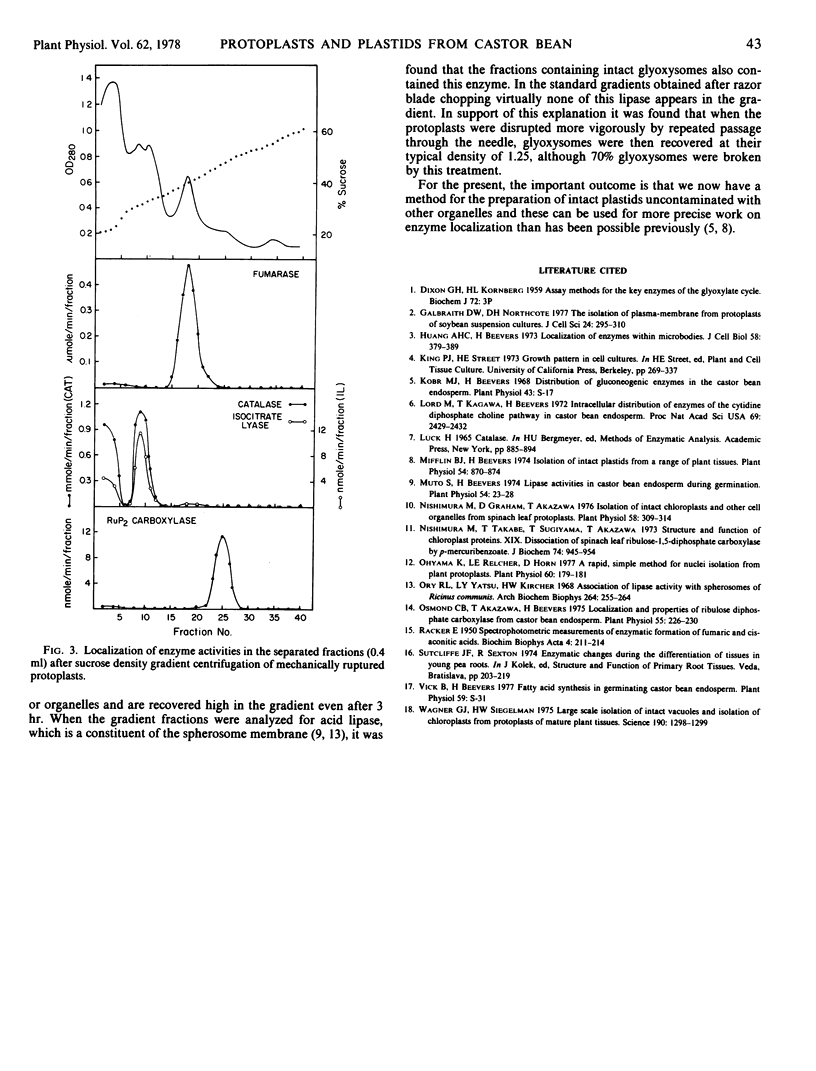
Abstract
Protoplasts were prepared from castor bean (Ricinus communis) endosperm by treatment with a mixture of the commercial enzymes Macerozyme R-10 and Cellulose “Onozuka” R-10. The protoplasts were gently ruptured by forcing the suspension through a hypodermic needle and the homogenate centrifuged on a linear sucrose gradient. From such a homogenate the mitochondria are recovered at their typical isopycnic density of 1.18 g/ml, but the glyoxysomes are retained, with other membranes, at a density of 1.13. The plastids reach their typical density of 1.22 on the gradient and are thus clearly separated from other organelles. Moreover, since essentially all of the ribulose bisphosphate carboxylase activity on the gradient is present in this fraction it can be concluded that the plastids are intact and have been recovered in high yield.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 1976 Sep;58(3):309–314. doi: 10.1104/pp.58.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Takabe T., Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. XIX. Dissociation of spinach leaf ribulose-1,5-diphosphate carboxylase by p-mercuribenzoate. J Biochem. 1973 Nov;74(5):945–954. [PubMed] [Google Scholar]
- Ohyama K., Pelcher L. E., Horn D. A rapid, simple method for nuclei isolation from plant protoplasts. Plant Physiol. 1977 Aug;60(2):179–181. doi: 10.1104/pp.60.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]