Abstract
Background
The incidence of all periprosthetic fractures (PPF), which require complex surgical treatment associated with high morbidity and mortality, is predicted to increase. The evolving surgical management has created a knowledge gap regarding its impact on immediate outcomes. This study aimed to describe current management strategies for PPF and their repercussions for in-hospital outcomes as well as to evaluate their implications for the community.
Methods
PIPPAS (Peri-Implant PeriProsthetic Survival Analysis) was a prospective multicentre observational study of 1387 PPF performed during 2021. Descriptive statistics summarized the epidemiology, fracture characteristics, management, and immediate outcomes. A mixed-effects logistic regression model was employed to evaluate potential predictors of in-hospital mortality, complications, discharge status, and weight-bearing restrictions.
Results
The study encompassed 32 (2.3%) shoulder, 4 (0.3%) elbow, 751 (54.1%) hip, 590 (42.5%) knee, and 10 (0.7%) ankle PPF. Patients were older (median 84 years, IQR 77–89), frail [median clinical frailty scale (CFS) 5, IQR 3–6], presented at least one comorbidity [median Charlson comorbidity index (CCI) 5, IQR 4–7], were community dwelling (81.8%), and had outdoor ambulation ability (65.6%). Femoral knee PPF were most frequently associated with uncemented femoral components, while femoral hip PPF occurred equally in cemented and uncemented stems. Patients were managed surgically (82%), with co-management (73.9%), through open approaches (85.9%) after almost 4 days (IQR, 51.9–153.6 h), with prosthesis revision performed in 33.8% of femoral hip PPF and 6.5% of femoral knee PPF. For half of the patients, the discharge instructions mandated weight-bearing restrictions. In-hospital mortality rates were 5.2% for all PPF and 6.2% for femoral hip PPF. Frailty, age > 84 years, mild cognitive impairment, CFS > 3, CCI > 3, and non-geriatric involvement were candidate predictors for in-hospital mortality, medical complications, and discharge to a nursing care facility. Management involving revision arthroplasty by experienced surgeons favoured full weight-bearing, while an open surgical approach favoured weight-bearing restrictions.
Conclusions
Current arthroplasty fixation check and revision rates deviate from established guidelines, yet full weight-bearing is favoured. A surgical delay of over 100 h and a lack of geriatric co-management were related to in-hospital mortality and medical complications. This study recommends judicious hypoaggressive approaches. Addressing complications and individualizing the surgical strategy can lead to enhanced functional outcomes, alleviating the economic and social burdens upon hospital discharge.
Level of Evidence Level IV case series.
Trial registration: registered at ClinicalTrials.gov (NCT04663893), protocol ID: PI 20-2041.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s10195-024-00746-6.
Keywords: Periprosthetic fracture, Outcome, Mortality, Replacement, Fracture fixation, Geriatric co-management, Incidence, Epidemiology, Management, Frailty
Introduction
Surgical treatment of periprosthetic fractures (PPF) presents challenges due to factors such as the prosthesis, bone defects, remodelling, and osteoporosis, which may hinder reduction and fixation [1–3]. Operative strategies aim to maintain joint/prosthesis functionality and typically involve either revising the joint with a new prosthesis or utilizing specifically adapted fixation devices [4]. When treating PPF, an assessment of the stability of the prosthesis is recommended; if it is loose, revision should be considered [5]. For a well-fixed prosthesis, fixation is preferred. However, an ongoing debate persists regarding whether to revise a loose prosthesis or retain it and fix the fracture, especially in very frail patients [2, 6–9]. Surgical technique and perioperative care significatively impact mortality and outcomes in PPF cases [1, 10]. Most of the studies available on this topic are retrospective, based on small single-hospital cohorts, cover extended time periods, and primarily focus on femoral PPF [1, 11], with limited data available on other locations.
Published studies encompass a wide range of surgical options for treating these fractures, which have evolved substantially in recent years. Retrospective research with outdated data may lack relevance and pertinence. Systematic reviews and meta-analysis highlight the need for prospective research, including registries and trials evaluating the outcomes of these divergent treatment strategies [7, 8, 10, 11]. Consequently, determining the optimal management approach, especially for frail patients, is often challenging. Tools for decision-making are essential to mitigate clinical complications, enhance functional outcomes, preserve quality of life, and reduce mortality.
PPF constitute a highly heterogeneous group of relatively rare fractures, making it difficult to report outcomes and study enough patients to draw robust conclusions. The PIPPAS study (Peri-Implant PeriProsthetic Survival Analysis) sought to prospectively enrol a substantial number of patients with PPF and peri-implant fractures (PIF) over a specified timeframe in Spain. The primary objective of this study was to provide insights into current trends in the management strategies for shoulder, elbow, hip, knee, or ankle PPF. Additionally, it aimed to investigate their impact on complications and mortality in the acute setting and their immediate consequences for the community. Furthermore, the study described the epidemiology, incidence, and characteristics of PPF in the Spanish population. This contemporary information on PPF management and its influence on immediate outcomes will be invaluable for addressing factors related to poorer outcomes.
Material and methods
The PIPPAS study was a collaborative multicentre prospective observational case series study that evaluated PPF and PIF in 59 hospitals, covering 37.5% of the Spanish population (17,779,904 individuals). PPF management was the standard of care for each participating site. Patients were recruited consecutively from January 1 to December 31, 2021. Eligible patients were ≥ 18 years old and admitted with a shoulder, elbow, hip, knee, or ankle PPF. Patients with pathologic or intraoperative fractures, failed fixation without a new fracture line, or pregnancy were excluded. All patients or their relatives provided consent for their inclusion. PPF were defined as fractures occurring in a bone sustaining one component of a joint replacement. PPF were classified using the Unified Classification System (UCS) [12]. For their analysis, type D PPF were allocated to the arthroplasty group (i.e. shoulder or elbow, hip or knee, knee or ankle) that most conditioned surgical management.
The study aimed to provide valuable information about current trends in management strategies for PPF and PIF, their influence on complications and mortality in the acute setting, and their immediate impact on the community. The study also aimed to describe the epidemiology, incidence, and PPF characteristics in the Spanish population.
We enrolled 1387 patients. We collected information on patient demographics, baseline status, treatment, and hospital care based on the variables proposed in the Fragility Fracture Network (FFN) Minimum Common Dataset for hip fracture registries, which were adapted to the specific nature of PPF (Additional file 1 in Appendix S1).
The incidence of PPF was obtained from the mean annual number of elective joint replacements performed during 2019 and 2021 by all participating hospitals. Signs of X-ray loosening or a painful prosthesis prior to the PPF helped with the differentiation of B1 from B2 fractures if no intraoperative stability tests were done. Candidate predictors of in-hospital mortality, in-hospital medical complications (present or absent), weight-bearing restrictions (allowed or forbidden; only for lower limb PPF, LLPPF), and destination at hospital discharge (own home or nursing care facility) were analysed. Candidate predictors were selected according to clinical relevance. Quantitative variables were categorized based on their median values, except for the Charlson comorbidity index (CCI), clinical frailty scale (CFS), and Pfeiffer, where a significant cut-off value was used (p < 0.05).
The manuscript was adapted to the STROBE statement. The study coordination centre and each participating hospital obtained institutional review board approval. This study was performed following the ethical standards laid down in the 1964 Helsinki Declaration and is registered at ClinicalTrials.gov (NCT04663893).
Descriptive statistics summarized the epidemiologic data, fracture characteristics, management aspects, and in-hospital outcomes. Continuous variables were summarized as the mean and standard deviation (SD) or the median and interquartile range (IQR) as appropriate (p < 0.05, Shapiro–Wilk test). Categorical variables were summarized as the absolute frequencies and percentages. Relative risk was calculated with the chi-square test. Candidate predictors were analysed using a mixed-effects logistic regression model, and the results were shown as forest plots. Statistical analysis was conducted using RStudio (v.4.1.0; R Foundation for Statistical Computing, Vienna, Austria). Data were collated centrally using the REDCap data entry system (Vanderbilt University, USA) housed on secure servers at the Instituto de Estudio de Ciencias de la Salud de Castilla y León, Spain.
Results
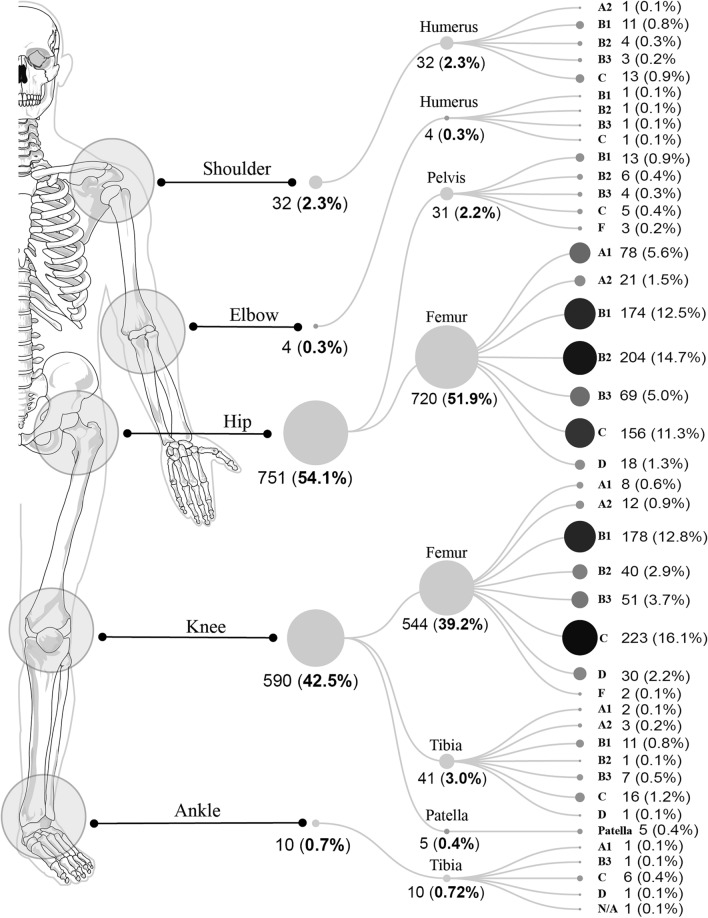
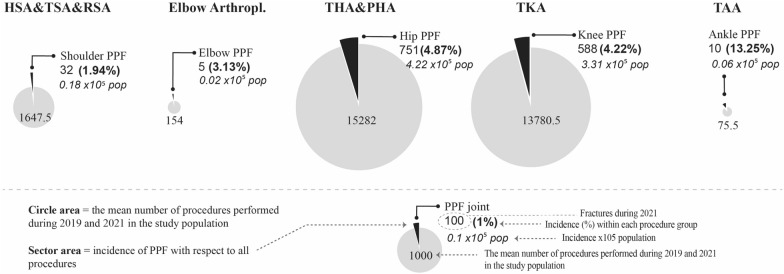
The study included 32 (2.3%) shoulder, 4 (0.3%) elbow, 751 (54.1%) hip, 590 (42.5%) knee, and 10 (0.7%) ankle PPF. The PPF type distribution according to the UCS is detailed in Fig. 1. The overall incidence of PPF during 2021 was 7.80/105 individuals. The estimated incidence of PPF at each location is presented in Fig. 2.
Fig. 1.
Distribution and types of PPF. The number and percentage (with respect to the total number of PPF) of fractures of each type according to the Unified Classification System (UCS) are shown. The size of each circle is proportional to the number of fractures
Fig. 2.
Incidence rates of shoulder PPF, elbow PPF, hip PPF, knee PPF, and ankle PPF in the Spanish population. HSA hemishoulderarthroplasty, TSA total shoulder arthroplasty, RSA reverse shoulder arthroplasty, THA total hip arthroplasty, PHA partial hip arthroplasty, TKA total knee arthroplasty, TAA total ankle arthroplasty.
Patients were older (median 84 years, IQR 77–89), female (n = 1041; 75.1%), frail (median CFS 5, IQR 3–6), American Society of Anesthesiologists classification (ASA) III (52.5%), mildly cognitively impaired (median Pfeiffer 3, IQR 0–6), had at least one comorbidity (median CCI 5, IQR 4–7), were community dwellers (n = 1135;81.8%), and could walk outdoors (n = 1212; 65.6%). The patients′ demographics and baseline data are presented in Table 1. PPF diagnostic features are detailed in Table 2. Femoral components were cemented in 34% of the hip PPF and 73.6% of the knee PPF and were diagnosed as loose in 19.4% and 3.3% of the hip PPF and knee PPF patients, respectively. Femoral knee PPF most commonly occurred with uncemented femoral components (p < 0.01). There were no differences in the incidence of femoral hip PPF between cemented and uncemented stems (p = 0.06).
Table 1.
Demographic and baseline data for patients presenting any periprosthetic fracture (PPF), humeral shoulder PPF, pelvic hip PPF, femoral hip PPF, femoral knee PPF, tibial knee PPF, and tibial ankle PPF
| PPF | Humeral shoulder | Pelvic hip | Femoral hip | Femoral knee | Tibial knee | Tibial ankle | |
|---|---|---|---|---|---|---|---|
| N = 1387 | N = 32 | N = 31 | N = 720 | N = 544 | N = 41 | N = 10 | |
| Age—years | |||||||
| Median (IQR) | 84 (77–89) | 78 (71.75–81) | 81 (72.5–85) | 85 (78–90) | 85 (78–90) | 77 (70–84) | 77 (73.25–79) |
| Gender—no. (%) | |||||||
| Female | 1041 (75.1) | 26 (81.2) | 21 (67.7) | 477 (66.2) | 466 (85.7) | 36 (87.8) | 7 (70) |
| Male | 346 (24.9) | 6 (18.8) | 10 (32.3) | 243 (33.8) | 78 (14.3) | 5 (12.2) | 3 (30) |
| Place of residency—no. (%) | |||||||
| Own home | 1135 (81.8) | 31 (96.9) | 28 (90.3) | 586 (81.4) | 436 (80.1) | 35 (85.4) | 10(100) |
| Nursing home | 229 (16.5) | 0 (0) | 3 (9.7) | 124 (17.2) | 96 (17.6) | 6 (14.6) | 0 (0) |
| Hospital | 7 (0.5) | 0 (0) | 0 (0) | 2 (0.3) | 5 (0.9) | 0 (0) | 0 (0) |
| N/A | 16 (1.2) | 1 (3.1) | 0 (0) | 8 (1.1) | 7 (1.3) | 0 (0) | 0 (0) |
| Pre-fracture mobility*—no. (%) | |||||||
| 1 | 385 (27.8) | 25 (78.5) | 13 (41.9) | 178 (24.7) | 147 (27) | 11 (26.8) | 5 (50) |
| 2 | 299 (21.6) | 3 (9.4) | 9 (29) | 164 (22.8) | 113 (20.8) | 6 (14.6) | 2 (20) |
| 3 | 225 (16.2) | 1 (3.1) | 2 (6.5) | 141 (19.6) | 71 (13.1) | 8 (19.5) | 2 (20) |
| 4 | 287 (20.7) | 0 (0) | 3 (9.7) | 151 (21) | 120 (22.1) | 12 (29.3) | 0 (0) |
| 5 | 177 (12.8) | 2 (6.2) | 4 (12.9) | 78 (10.8) | 89 (16.4) | 4 (9.8) | 0 (0) |
| N/A | 14 (1) | 1 (3.1) | 0 (0) | 8 (1.1) | 4 (0.7) | 0 (0) | 1 (10) |
| Pfeiffer’s SPMSQ—no. | |||||||
| Median (IQR) | 3 (0–6) | 1 (0–1.5) | 2 (0–4) | 3 (1–6) | 3 (0–7) | 0.5 (0–3) | 0 (0–1) |
| N/A | 74 (5,3) | 1 (3.1) | 2 (6.5) | 35 (4.9) | 31 (5,7) | 3 (7.3) | 1 (10) |
| CFS—no. | |||||||
| Median (IQR) | 5 (3–6) | 3 (2.75–5) | 4 (3–5.5) | 5 (4–6) | 5 (3–7) | 5 (3–6) | 3 (2.25–3) |
| N/A | 30 (2.2) | 0 (0) | 0 (0) | 17 (2.4) | 11 (2) | 1 (2.4) | 0 (0) |
| ASA—no. (%) | |||||||
| 1 | 11 (0.8) | 1 (3.1) | 0 (0) | 7 (1) | 3 (0.6) | 0 (0) | 0 (0) |
| 2 | 368 (26.5) | 17 (53.1) | 9 (29) | 170 (23.6) | 148 (27.2) | 12 (29.3) | 6 (60) |
| 3 | 728 (52.5) | 11 (34.4) | 12 (38.7) | 391 (54.3) | 292 (53.7) | 17 (41.5) | 4 (40) |
| 4 | 154 (11.1) | 1 (3.1) | 2 (6.5) | 77 (10.7) | 72 (13.2) | 2 (4.9) | 0 (0) |
| 5 | 3 (0.2) | 0 (0) | 0 (0) | 1 (0.1) | 2 (0.4) | 0 (0) | 0 (0) |
| N/A | 123 (8.9) | 2 (6.2) | 8 (25.8) | 74 (10.3) | 27 (5) | 10 (24.4) | 0 (0) |
| Charlson comorbidity index—no. | |||||||
| Median (IQR) | 5 (4–7) | 4 (3–5) | 5 (4– 6.5) | 5 (4–7) | 5 (4–7) | 5 (3–7) | 3.5 (3–4) |
| Osteoprotective treatment—no. (%) | |||||||
| No treatment | 930 (67.1) | 22 (68.8) | 17 (54.8) | 492 (68.3) | 359 (66) | 29 (70.7) | 6 (60) |
| Osteoprotective treatment^ | 457 (32.9) | 10 (31.2) | 14 (45.2) | 228 (31.7) | 185 (34) | 12 (29.3) | 4 (40) |
| Anti-resorptive | 102 (22.3) | 2 (20) | 3 (21.4) | 54 (23.7) | 36 (19.5) | 5 (41.7) | 1 (25) |
| Bone-forming | 19 (4.2) | 0 (0) | 1 (7.1) | 9 (3.9) | 8 (4.3) | 1 (8.3) | 0 (0) |
| Calcium | 262 (57.3) | 4 (40) | 7 (50) | 135 (59.2) | 106 (57.3) | 5 (41.7) | 2 (50) |
| Vitamin D | 379 (82.9) | 8 (80) | 13 (92.9) | 194 (85.1) | 146 (78.9) | 11 (91.7) | 4 (100) |
| Antiaggregant or anticoagulant medication—no. (%) | |||||||
| None | 864 (62.3) | 29 (90.6) | 22 (71) | 431 (59.9) | 339 (62.3) | 29 (70.7) | 9 (90) |
| Acenocumarol or NOAC or PAA | 494 (35.6) | 3 (9.4) | 7 (22.6) | 274 (38.1) | 194 (35.7) | 11 (26.8) | 1 (10) |
| Double | 19 (1.4) | 0 (0) | 2 (6.5) | 12 (1.7) | 4 (0.7) | 1 (2.4) | 0 (0) |
| N/A | 10 (0.7) | 0 (0) | 0 (0) | 3 (0.4) | 7 (1.3) | 0 (0) | 0 (0) |
| Hb at admission (g/dL)—no. | |||||||
| Median (IQR) | 12.2 (10.9–13.4) | 12.9 (11.4–13.8) | 12.7 10.9–13.2) | 12.2 (11–13.4) | 12.1 (10.8–13.35) | 12.4 (10.8–13.6) | 12.3 (11.7–13.5) |
| N/A | 19 (1.4) | 0 (0) | 2 (6.5) | 13 (1.8) | 1 (0.2) | 2 (4.9) | 1 (10) |
| Time between last prosthesis and PPF (months)—no. (%) | |||||||
| < 1 month | 36 (2.6) | 2 (6.3) | 1 (3.2) | 25 (3.5) | 6 (1.1) | 1 (2.4) | 1 (10) |
| From 1 to < 6 months | 62 (4.5) | 1 (3.1) | 3 (9.7) | 34 (4,7) | 17 (3.1) | 5 (12.2) | 1 (10) |
| From 6 to < 12 months | 33 (2.4) | 0 (0) | 2 (6.5) | 17 (2.4) | 11 (2) | 2 (4.9) | 1 (10) |
| From 1 to < 5 years | 217 (15.6) | 16 (50) | 5 (16.1) | 101 (14) | 86 (15.8) | 5 (12.2) | 1 (10) |
| ≥ 5 years | 993 (71.6) | 11 (34.4) | 20 (65.4) | 518 (71.9) | 407 (74.8) | 27 (65.9) | 5 (50) |
| N/A | 46 (3.3) | 2 (6.3) | 0 (0) | 25 (3.5) | 17 (3.1) | 1 (2.4) | 1 (10) |
Humeral elbow and patellar PPF are not detailed (n = 4 and n = 5)
IQR interquartile range, N/A not available, Pfeiffer′s SPMSQ Pfeiffer′s Short Portable Mental Status Questionnaire, CFS clinical frailty scale, ASA American Society of Anesthesiologists (ASA) physical status classification system, NOAC new oral anti-coagulant, PAA platelet anti-aggregant, Hb haemoglobin
*Pre-fracture mobility scale: 1, completely independent gait; 2, independent gait outdoors with one technical aid; 3, independent gait outdoors with two technical aids; 4, independent gait indoors only, with or without aids; 5, no mobility at all or only with the help of two other people
^ Osteoprotective treatment: the percentage for each individual treatment was calculated with respect to the total number of patients receiving osteoprotective treatment
Table 2.
Diagnostic features of periprosthetic fractures (PPF)
| Joint | Shoulder 32 (2.3) |
Elbow 4 (0.3) |
Hip 751 (54.1) |
Knee 590 (42.5) |
Ankle 10 (0.7) |
|||
|---|---|---|---|---|---|---|---|---|
| Bone n (%) |
Humerus 32 (100) |
Humerus 4 (100) |
Pelvis 31 (4.1) |
Femur 720 (95.9) |
Femur 544 (92.2) |
Tibia 41 (6.9) |
Tibia 10 (100) |
|
| Method of fixation | ||||||||
| Uncemented | 16 (50) | 1 (25) | 495 (65.9) | 154 (26.1) | 1 (10) | |||
| Cemented | 16 (50) | 3 (75) | 255 (34) | 434 (73.6) | 9 (90) | |||
| N/A | 1 (0.1) | 2 (0.3) | ||||||
| Presence of stem | ||||||||
| Stemless | 0 (0) | 1 (25) | 5 (16.1) | 64 (8.9) | 449 (82.5) | 24 (58.5) | 2 (20) | |
| Stem | 32 (100) | 3 (75) | 26 (83.9) | 655 (91) | 94 (17.3) | 17 (41.5) | 8 (80) | |
| N/A | 1 (0.1) | 1 (0.2) | ||||||
| Infection | ||||||||
| Negative | 29 (90.6) | 4 (100) | 31 (100) | 694 (96.4) | 526 (96.7) | 37 (90.2) | 8 (80) | |
| Positive | 3 (9.4) | 0 (0) | 0 (0) | 25 (3.5) | 18 (3.3) | 4 (9.8) | 2 (20) | |
| N/A | 1 (0.1) | |||||||
| Loose prosthesis | ||||||||
| Negative | 26 (81.2) | 2 (50) | 25 (80.6) | 578 (80.3) | 511 (93.9) | 36 (87.8) | 10 (100) | |
| Positive | 6 (18.8) | 2 (50) | 6 (19.4) | 140 (19.4) | 33 (6.1) | 4 (9.8) | 0(0) | |
| N/A | 2 (0.3) | 1 (2.4) | ||||||
| X-ray signs of loose prosthesis | ||||||||
| Negative | 25 (78.1) | 1 (25) | 30 (96.8) | 610 (84.7) | 506 (93) | 37 (90.2) | 9 (90) | |
| Positive | 7 (21.9) | 3 (75) | 1 (3.2) | 109 (15.1) | 37 (6.8) | 4 (9.8) | 1 (10) | |
| N/A | 1 (0.1) | 1 (0.2) | ||||||
| Painful prosthesis | ||||||||
| Negative | 22 (68.8) | 3 (75) | 26 (83.9) | 631 (87.6) | 455 (83.6) | 27 (65.9) | 8 (80) | |
| Positive | 10 (31.2) | 1 (25) | 5 (16.1) | 88 (12.2) | 88 (16.2) | 14 (34.1) | 2 (20) | |
| N/A | 1 (0.1) | 1 (0.2) | ||||||
| Time from arthroplasty to PPF (months)—no. (%) | ||||||||
| < 1 month | 2 (6.3) | 0 (0) | 1 (3.2) | 25 (3.5) | 6 (1.1) | 1 (2.4) | 1 (10) | |
| From 1 to < 6 months | 1 (3.1) | 0 (0) | 3 (9.7) | 34 (4.7) | 17 (3.1) | 5 (12.2) | 1 (10) | |
| From 6 to < 12 months | 0 (0) | 0 (0) | 2 (6.5) | 17 (2.4) | 11 (2) | 2 (4.9) | 1 (10) | |
| From 1 to < 5 years | 16 (50) | 1 (25) | 5 (16.1) | 101 (14) | 86 (15.8) | 5 (12.2) | 1 (10) | |
| ≥ 5 years | 11 (34.4) | 3 (75) | 20 (64.5) | 518 (71.9) | 407 (74.8) | 27 (65.9) | 5 (50) | |
| N/A | 2 (6.3) | 25 (3.5) | 17 (3.1) | 1 (2.4) | 1 (10) | |||
N/A not available
Most patients were managed surgically (82%) under spinal anaesthesia (69%) after almost 4 days of surgical delay (92.5 IQR, 51.9–153.6 h) and through an open approach (85.9%). Femoral knee PPF were the most likely to be treated operatively (90.8%), and pelvic hip PPF were the most likely to be non-operatively managed (45.2%). The stability of prosthetic fixation was not checked in 44.1% of the patients: two-thirds of the femoral hip PPF and less than half of the femoral knee PPF cases. Prosthetic revision was performed in 33.3% of patients with femoral hip PPF, while 93.5% of femoral knee PPF received fixation. Among multiple fixation techniques, the most frequently used was a single plate (56.1%). Patients with lower limb PPF (LLPPF) managed only with fixation had a higher relative risk of being managed with restricted weight-bearing than those having their prosthesis revised (p < 0.01). Table 3 describes the management and surgical techniques for all PPF.
Table 3.
Management of all periprosthetic fractures (PPF), humeral shoulder PPF, humeral elbow PPF, pelvic hip PPF, femoral hip PPF, femoral knee PPF, tibial knee PPF, and tibial ankle PPF (humeral elbow PPF and patellar PPF are not detailed; n = 4 and n = 5, respectively)
| PPF | Humeral shoulder PPF | Pelvic hip PPF | Femoral hip PPF | Femoral knee PPF | Tibial knee PPF | Tibial ankle PPF | |
|---|---|---|---|---|---|---|---|
| N = 1387 | N = 32 | N = 31 | N = 720 | N = 544 | N = 41 | N = 10 | |
| Treatment—no. (%) | |||||||
| Operative | 1137 (82) | 29 (90.6) | 17 (54.8) | 555 (77.1) | 494 (90.8) | 26 (63.4) | 9 (90) |
| Non-operative | 248 (17.9) | 3 (9.4) | 14 (45.2) | 165 (22.9) | 49 (9) | 14 (34.1) | 1 (10) |
| N/A | 2 (0.1) | 1 (0.2) | 1 (2.4) | ||||
| Surgical delay (h) | |||||||
| Median (IQR) |
92.5 (51.9–153.6) |
152.5 (82.6–208) | 141.1 (65.3–210) | 97.9 (56.4–157) | 86.3 (48–136.4) | 203.7 (96.4–288) | 71.2 (45.25–120) |
| N/A | 8 (0.7) | 4 (12.5) | 14 (45.2) | 2 (0.3) | 3 (0.6) | 16 (39) | 1 (10) |
| Type of anaesthesia | |||||||
| General | 251 (22.1) | 26 (89.7) | 8 (47.1) | 139 (25) | 68 (13.8) | 6 (23.1) | 1 (10) |
| Spinal | 784 (69) | 2 (6.9) | 10 (58.8) | 371 (66.8) | 374 (75.7) | 17 (65.4) | 6 (60) |
| Regional | 173 (15.2) | 6 (20.7) | 0 (0) | 77 (13.9) | 82 (16.6) | 4 (15.4) | 3 (30) |
| Surgical approach | |||||||
| Open | 745 (65.4) | 27 (93.1) | 15 (88.2) | 437 (78.7) | 238 (48.1) | 16 (59.3) | 6 (66.7) |
| MIS | 233 (20.5) | 2 (6.9) | 1 (5.9) | 69 (12.4) | 155 (31.3) | 3 (11.1) | 2 (22.2) |
| PC | 152 (13.3) | 0 (0) | 1 (5.9) | 44 (7.9) | 100 (20.2) | 6 (22.2) | 1 (11.1) |
| N/A | 9 (0.8) | 0 (0) | 0 (0) | 5 (0.9) | 2 (0.4) | 1 (3.7) | 0 (0) |
| Direct stability check | |||||||
| No | 501 (44.1) | 9 (31) | 3 (17.6) | 191 (34.4) | 276 (55.9) | 13 (50) | 7 (77.8) |
| Yes, from the joint | 266 (23.4) | 6 (20.7) | 13 (76.5) | 153 (27.6) | 85 (17.2) | 6 (23.1) | 0 (0) |
| Yes, from the fracture site | 364 (32) | 14 (48.3) | 1 (5.9) | 206 (37.1) | 133 (26.9) | 6 (23.1) | 2 (22.2) |
| N/A | 8 (0.7) | 0 (0) | 0 (0) | 5 (0.9) | 1 (0.2) | 1 (3.8) | 0 (0) |
| Cerclage for reduction | |||||||
| No | 611 (53.7) | 13 (44.8) | 12 (70.6) | 156 (28.1) | 397 (80.4) | 23 (88.5) | 8 (88.9) |
| Yes | 520 (45.7) | 16 (55.2) | 5 (29.4) | 394 (71) | 97 (19.6) | 2 (7.7) | 1 (11.1) |
| N/A | 8 (0.7) | 0 (0) | 0 (0) | 5 (0.9) | 1 (0.2) | 1 (3.8) | 0 (0) |
| Replacement | |||||||
| No | 887 (78) | 25 (86.2) | 5 (29.4) | 362 (65.2) | 462 (93.5) | 19 (73.1) | 8 (88.9) |
| Yes (cementless) | 156 (13.7) | 0 (0) | 9 (52.9) | 139 (25) | 5 (1) | 2 (7.7) | 0 (0) |
| Yes (cemented) | 88 (7.7) | 4 (13.8) | 3 (17.6) | 49 (8.8) | 27 (5.5) | 4 (15.4) | 1 (11.1) |
| N/A | 8 (0.7) | 0 (0) | 0 (0) | 5 (0.9) | 1 (0.2) | 1 (3.8) | 0 (0) |
| Type of fixation | |||||||
| 1 plate | 638 (56.1) | 19 (65.5) | 4 (23.5) | 327 (58.9) | 270 (54.7) | 13 (50) | 4 (44.4) |
| 2 plates | 46 (4) | 4 (13.8) | 1 (5.9) | 6 (1.1) | 27 (5.5) | 4 (15.4) | 3 (33.3) |
| Nail | 194 (17.1) | 1 (3.4) | 0 (0) | 58 (10.5) | 135 (27.3) | 0 (0) | 0 (0) |
| Ex fix | 4 (0.4) | 0 (0) | 0 (0) | 0 (0) | 3 (0.6) | 1 (3.8) | 0 (0) |
| Cerclage | 300 (26.4) | 7 (24.1) | 1 (5.9) | 240 (43.2) | 46 (9.3) | 2 (7.7) | 0 (0) |
| Isolated screws | 19 (1.7) | 0 (0) | 2 (11.8) | 2 (0.4) | 9 (1.8) | 3 (11.5) | 2 (22.2) |
| Bone graft | |||||||
| No | 1070(77.1) | 24 (25) | 14 (45.2) | 515 (71.5) | 479 (88.1) | 25 (61) | 8 (80) |
| Yes: | 317 (22.9) | 8 (75) | 17 (54.8) | 205 (28.5) | 65 (11.9) | 16 (39) | 2 (20) |
| Strut | 33 (2.9) | 4 (13.8) | 0 (0) | 23 (4.1) | 4 (0.8) | 0 (0) | 0 (0) |
| N/A | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Overlap in mm | |||||||
| Yes | 653 (57.4) | 20 (69) | 2 (11.8) | 322 (58) | 290 (58.7) | 12 (46.2) | 4 (44.4) |
| Median (IQR) | 87 (4 –142) | 77 (53–93.5) | 135 (127.5–142.5) | 130 (90–161) | 50 (37–75.8) | 42 (20–73.25) | 61.5 (59–79.25) |
| Gap in mm | |||||||
| Yes | 118 (10.4) | 3 (10.3) | 0 (0) | 41 (7.4) | 64 (13) | 6 (23.1) | 4 (44.4) |
| Median (IQR) | 140 (33–200) | 42 (22.5–57.5) | 140 (67–200) | 130.5 (23.8–189.8) | 135 (75–207) | 225 (186–262.5) | |
| Kissing implants | |||||||
| Yes | 31 (2.7) | 0 (0) | 0 (0) | 6 (1.1) | 25 (5.1) | 0 (0) | 0 (0) |
| Surgeon experience | |||||||
| > 20 replacements | 316 (27.8) | 7 (24.1) | 10 (58.8) | 206 (37.1) | 84 (17) | 5 (19.2) | 2 (22.2) |
| > 20 MIPO | 324 (28.5) | 10 (34.5) | 6 (35.3) | 124 (22.3) | 172 (34.8) | 8 (30.8) | 3 (33.3) |
| < 20 replacements & MIPO | 533 (46.9) | 14 (48.3) | 3 (17.6) | 247 (44.5) | 248 (50.2) | 12 (46.2) | 5 (55.6) |
Except for “treatment”, categorical variables are summarized as the absolute frequency and percentage with respect to the number of patients surgically managed in each group.
IQR interquartile range, MIS minimally invasive surgery, PC percutaneous, ex fix external fixator, mm millimeters, MIPO minimally invasive plating ostheosynthesis, N/A not available
The overall in-hospital mortality was 5.1%. At least one medical complication appeared in 42% of the patients: the most common complications were delirium and renal and pulmonary complications. Clinical co-management with geriatricians, internal medicine, or specialties other than anaesthesia was available for 73.9% of patients, and 78.9% required transfusion of at least one unit of packed red blood cells (cut-off level for transfusion: 7.5–8 g/dL). Regardless of the type of fracture, 77.8% of patients were initially mobilized out of bed within 24–48 h after surgery. Full weight-bearing was allowed for 34.4% of the patients with LLPPF, and 37.2% of patients went to a nursing home after discharge. Table 4 summarizes postoperative care and outcomes until hospital discharge.
Table 4.
Postoperative care data for grouped periprosthetic fractures (PPF), humeral shoulder PPF, humeral elbow PPF, pelvic hip PPF, femoral Hip PPF, femoral knee PPF, tibial knee PPF, and tibial ankle PPF [humeral elbow (n = 4), patellar (n = 5), and tibial ankle (n = 10) PPF are not included]
| PPF | Humeral shoulder PPF | Pelvic hip PPF | Femoral hip PPF | Femoral knee PPF | Tibial knee PPF | |
|---|---|---|---|---|---|---|
| N = 1387 | N = 32 | N = 31 | N = 720 | N = 544 | N = 41 | |
| In-hospital mortality | ||||||
| Alive | 1311 (94.5) | 32 (100) | 30 (96.8) | 673 (93.5) | 518 (95.2) | 40 (97.6) |
| Died before surgery | 24 (1.7) | 0 (0) | 0 (0) | 19 (2.6) | 5 (0.9) | 0 (0) |
| Died in surgery | 2 (0.1) | 0 (0) | 0 (0) | 0 (0) | 2 (0.4) | 0 (0) |
| Died after surgery | 46 (3.3) | 0 (0) | 1 (3.2) | 26 (3.6) | 18 (3.3) | 0 (0) |
| N/A | 4 (0.3) | 0 (0) | 0 (0) | 2 (0.3) | 1 (0.2) | 1 (2.4) |
| Presence of medical complications during hospital stay | ||||||
| No | 804 (58) | 27 (84.4) | 22 (71) | 410 (56.9) | 303 (55.7) | 27 (65.9) |
| Yes (any) | 583 (42) | 5 (15.6) | 9 (29) | 310 (43.1) | 241 (44.3) | 14 (34.1) |
| Cardiac | 144 (24.7) | 2 (40) | 4 (44.4) | 73 (23.5) | 61 (25.3) | 3 (21.4) |
| Pulmonary | 163 (28) | 2 (40) | 4 (44.4) | 93 (30) | 60 (24.9) | 3 (21.4) |
| Pulmonary thromboembolism | 9 (1.5) | 0 (0) | 0 (0) | 5 (1.6) | 3 (1.2) | 0 (0) |
| Renal | 183 (31.4) | 2 (40) | 3 (33.3) | 99 (31.9) | 76 (31.5) | 2 (14.3) |
| Cerebral | 19 (3.3) | 0 (0) | 0 (0) | 12 (3.9) | 6 (2.5) | 1 (7.1) |
| Gastrointestinal | 100 (17.2) | 0 (0) | 0 (0) | 62 (20) | 36 (14.9) | 2 (14.3) |
| Urinary tract infection | 122 (20.9) | 1 (20) | 1 (11.1) | 70 (22.6) | 50 (20.7) | 0 (0) |
| Delirium | 223 (38.3) | 2 (40) | 2 (22.2) | 116 (37.4) | 99 (41.1) | 4 (28.6) |
| In-hospital fractures | 11 (1.9) | 0 (0) | 0 (0) | 5 (1.6) | 4 (1.7) | 1 (7.1) |
| Medical staff involved in the patient care (other than trauma and anaesthesia) | ||||||
| No | 362 (26.1) | 23 (71.9) | 9 29) | 178 (24.7) | 120 (22.1) | 19 (46.3) |
| Geriatrician | 403 (29.1) | 1 (3.1) | 8 (25.8) | 213 (29.6) | 169 (31.1) | 10 (24.4) |
| Internal medicine | 393 (28.3) | 5 (15.6) | 5 (16.1) | 205 (28.5) | 172 (31.6) | 4 (9.8) |
| Geriatrician and others | 145 (10.5) | 1 (3.1) | 4 (12.9) | 84 (11.7) | 51 (9.4) | 5 (12.2) |
| Others | 78 (5.6) | 2 (6.2) | 4 (12.99 | 37 (5.1) | 31 (5.7) | 2 (4.9) |
| N/A | 6 (0.4) | 0 (0) | 1 (3.2) | 3 (0.4) | 1 (0.2) | 1 (2.4) |
| Initial postoperative mobilization out of bed | ||||||
| < 24 h | 449 (32.4) | 29 (90.6) | 12 (38.7) | 223 (31) | 154 (28.3) | 17 (41.5) |
| 24–48 h | 546 (39.4) | 3 (9.4) | 10 (32.3) | 273 (37.9) | 247 (45.4) | 10 (24.4) |
| > 48 h | 353 (25.5) | 0 (0) | 8 (25.8) | 200 (27.8) | 132 (24.3) | 11 (26.8) |
| N/A | 39 (2.8) | 0 (0) | 1 (3.2) | 24 (3.3) | 11 (2) | 3 (7.3) |
| Weight-bearing restrictions | ||||||
| No restrictions | 477 (34.4) | 29 (90.6) | 6 (19.4) | 233 (32.4) | 197 (36.2) | 5 (12.2) |
| Only for transfers | 177 (12.8) | 1 (3.1) | 5 (16.1) | 105 (14.6) | 64 (11.8) | 2 (5.3) |
| Not allowed | 696 (50.2) | 2 (6.2) | 19 (61.3) | 361 (50.1) | 272 (50) | 31 (75.6) |
| N/A | 37 (2.7) | 0 (0) | 1 (3.2) | 21 (2.9) | 11 (2) | 3 (7.3) |
| Ability to walk at hospital discharge | ||||||
| Yes | 905 (65.2) | 32 (100) | 24 (77.4) | 485 (67.4) | 355 (65.3) | 30 (73.2) |
| No | 427 (30.8) | 0 (0) | 6 (19.4) | 206 (28.6) | 170 (31.2) | 6 (14.6) |
| N/A | 55 (4) | 0 (0) | 1 (3.2) | 29 (4.1) | 19 (3.5) | 5 (12.2) |
| Destination at hospital discharge | ||||||
| Home | 787 (56.7) | 29 (90.6) | 18 (58.1) | 407 (56.5) | 293 (53.9) | 24 (58.5) |
| Nursing home | 453 (32.7) | 2 (6.) | 11 (35.5) | 239 (33.2) | 187 (34.4) | 12 (29.3) |
| Hospital | 63 (4.5) | 1 (3.1) | 0 (0) | 26 (3.6) | 33 (6.1) | 3 (7.3) |
| N/A | 84 (6) | 0 (0) | 2 (6.5) | 48 (6.7) | 31 (5.7) | 2 (4.9) |
| Osteoprotective treatment at discharge+ | ||||||
| No treatment | 669 (48.2) | 21 (65.6) | 10 (32.3) | 348 (48.3) | 253 (46.5) | 26 (63.4) |
| Osteoprotective treatment | 718 (51.8) | 11 (34.3) | 21 (67.7) | 372 (51.7) | 291 (53.5) | 15 (36.6) |
| Anti-resorptive | 264 (36.8) | 1 (9.1) | 6 (28.6) | 144 (38.7) | 105 (36.1) | 7 (46.7) |
| Bone-forming | 47 (6.5) | 2 (18.2) | 4 (19) | 20 (5.4) | 19 (6.5) | 2 (13.3) |
| Calcium | 428 (59.6) | 5 (45.5) | 10 (47.6) | 215 (42.2) | 184 (63.2) | 8 (53.3) |
| Vitamin D | 560 (78) | 9 (81.8) | 19 (90.5) | 287 (77.2) | 227 (78) | 11 (73.3) |
| Total length of hospital stay (h) | ||||||
| Median (IQR) | 245 (164–370.9) | 198 (129.5–295.3) | 228.7 (117.1–393.7) | 260 (163.5–405.4) | 234.6 (166.8–336.6) | 261.5 (106.5–451.3) |
| N/A | 84 (6.1) | 0 (0) | 2 (6.5) | 51 (7.1) | 28 (5.1) | 2 (4.9) |
| Postoperative length of hospital stay (h) | ||||||
| Median (IQR) | 168 (96–264) | 48 (48–102) | 168 (138–264) | 168 (120–288) | 144 (96–216) | 120 (72–264) |
| N/A | 312 (22.5) | 4 (12.5) | 15 (48.4) | 200 (27.8) | 73 (13.4) | 16 (39) |
| Level of haemoglobin after surgical treatment | ||||||
| Median (IQR) | 9.6 (8.7–10.7) | 10.3 (9.3–11.7) | 10 (8.7–11.7) | 9.7 (8.7–10.9) | 9.4 (8.6–10.4) | 10.3 (9.6–12–2) |
| N/A | 91 (6.6) | 5 (15.6) | 5 (16.1) | 51 (7.1) | 15 (2.8) | 9 (22) |
| Difference in haemoglobin level* | ||||||
| Median (IQR) | 2.3 (0.9–3.6) | 2.4 (0.9–3.3) | 1.5 (0.4–3.2) | 2.2 (0.8–3.7) | 2.6 (1.1–3.7) | 1.7 (0.6–2.3) |
| N/A | 91 (6.6) | 5 (15.6) | 5 (16.1) | 51 (7.1) | 15 (2.8) | 9 (22) |
| Management of the anaemia | ||||||
| No | 487 (35.1) | 27 (84.4) | 13 (41.9) | 252 (35) | 158 (29) | 21 (51.2) |
| Transfusion | 710 (78.9) | 3 (60) | 11 (61.1) | 374 (51.9) | 307 (56.4) | 13 (65) |
| Intravenous iron | 389 (43.2) | 2 (40) | 8 (44.4) | 207 (28.8) | 163 (30) | 8 (40) |
IQR interquartile range; N/A not available
*Difference between haemoglobin level at admission and haemoglobin level after surgical treatment or before hospital discharge if patient was managed non-surgically. +The percentages for the different osteoprotective treatments at discharge were calculated with respect to the total number of patients who were receiving osteoprotective treatment
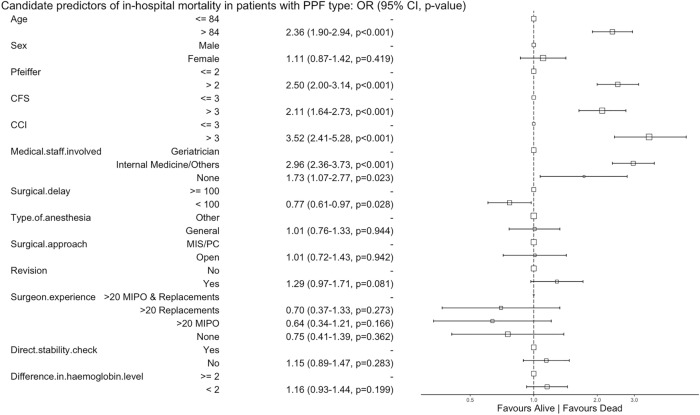
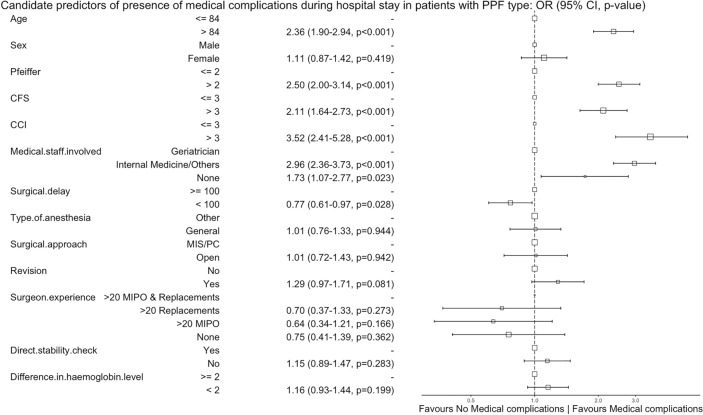
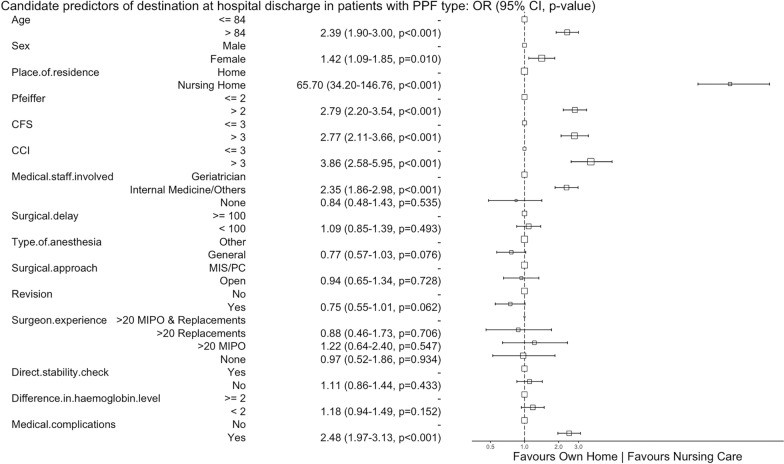
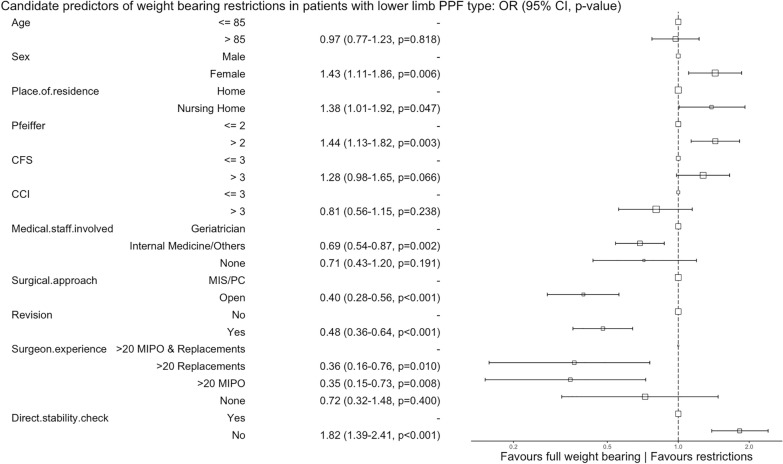
Older age (> 84 years), cognitive impairment (Pfeiffer > 2), frailty (CFS > 3), comorbidity (CCI > 3), and involvement of non-geriatric specialties favoured in-hospital mortality, medical complications, and hospital discharge to a nursing care facility (Figs. 3, 4, and 5). An operative delay of < 100 h was associated with a reduced risk of in-hospital mortality and medical complications. Experienced surgeons, treatment with a revision prosthesis, and surgical treatment favoured full weight-bearing at hospital discharge. Weight-bearing restrictions at discharge were more common following an open approach or when the stability of the prosthesis was not checked directly (Fig. 6).
Fig. 3.
Candidate predictors of in-hospital mortality in patients with a periprosthetic fracture (PPF): alive at hospital discharge vs dies in hospital before hospital discharge. The reference category for each variable is the first value. The size of the box is proportional to the number of patients in the category. CI confidence interval, OR odds ratio, CFS clinical frailty scale, CCI Charlson comorbidity index, MIS minimally invasive surgery, PC percutaneous, MIPO minimally invasive plating osteosynthesis
Fig. 4.
Candidate predictors of medical complications during hospital stay in patients with a periprosthetic fracture (PPF): complication vs no complications. The reference category for each variable is the first value. The size of the box is proportional to the number of patients in the category. CI confidence interval, OR odds ratio, CFS clinical frailty scale, CCI Charlson comorbidity index, MIS minimally invasive surgery, PC percutaneous, MIPO minimally invasive plating osteosynthesis
Fig. 5.
Candidate predictors of destination at hospital discharge in patients with a periprosthetic fracture (PPF): own home vs nursing care. The reference category for each variable is the first value. The size of the box is proportional to the number of patients in the category. CI confidence interval, OR odds ratio, CFS clinical frailty scale, CCI Charlson comorbidity index, MIS minimally invasive surgery, PC percutaneous, MIPO minimally invasive plating osteosynthesis
Fig. 6.
Candidate predictors of weight-bearing restrictions in patients with a lower limb [i.e. hip (pelvis and femur), knee (femur and tibia), and ankle (tibia)] periprosthetic fracture (PPF): full weight-bearing vs restrictions. The reference category for each variable is the first value. The size of the box is proportional to the number of patients in the category. CI confidence interval, OR odds ratio, CFS clinical frailty scale, CCI Charlson comorbidity index, MIS minimally invasive surgery, PC percutaneous, MIPO minimally invasive plating osteosynthesis
Discussion
Despite their low incidence, PPF are severe injuries in older persons [1, 6, 10, 11]. Published data are scarce for PPF other than femoral PPF [7, 11, 13, 14]. Large sample sizes were necessary to draw conclusions regarding the current management and early outcomes of these patients [15]. To our knowledge, this is the largest specific dataset for prospectively collected PPF. The 1-year data collection period (2021) offers an up-to-date view of PPF management. The use of the minimum dataset proposed by the FNN for hip fracture registries makes future comparisons and projections possible.
The incidence and distribution of PPF and differences in sex ratios and age reflect joint replacement indications and life expectancy, which vary among cultures and populations [16]. Most series reported a mean age of 64–78 years [2, 14, 17], which was younger than our population. The femur was the most common location (91%) because THA and TKA are the most implanted joint replacements overall [18, 19].
Data related to patients′ pre-fracture health status are usually limited to the ASA scores. Information on CCI, mobility, references to osteoporosis treatment, or place of residence documentation are scarce in previous studies. PIPPAS demonstrated that CFS, CCI, and cognitive impairment were related to poorer immediate outcomes. In frail patients, CFS, CCI, and cognitive status can help complications to be addressed and an adequate surgical strategy to improve functional weight-bearing and to minimize the social burden at hospital discharge to be selected. The percentage of patients with an ASA II score ranges across studies from 24% (in our study) to 54% [11, 20], which is probably influenced by differences in age.
The distribution of femoral hip PPF is similar in other published series: around 15% were type A, 70% type B, and 15% type C [2, 21]. The exception is the Mayo Clinic′s series, where 34.7% of the patients were type A1 [22]. In 24 patients, Liu et al. found a higher percentage of type C tibia knee PPF than we did: 70.8% vs 39% [14]. According to the Mayo Clinic′s series and the review by Carli, postoperative femoral hip PPF were most common with uncemented stems [15, 22]; the same is true for hemiarthroplasty [23], which showed different incidences depending on the stem design [22]. In our study, as in that of Karam et al., femoral hip PPF occurred equally in cemented and uncemented stems (p = 0.06) [24]. We found that femoral knee PPF were more common with uncemented femoral components than with cemented ones (p < 0.01), although Nugent et al. found no difference [25]. Detailed analysis of the implant design and fixation method in each joint and type of PPF are needed to clarify their contributions to the PFF risk.
In-hospital mortality for femoral hip PPF ranges from 2.4% to 8% [22, 27]. This variation may be due to the patients’ age and comorbidities, as age and frailty variables favoured in-hospital mortality in our study. Patients managed by a geriatrician had a lower risk of medical complications and in-hospital mortality and a greater chance of returning to the community at hospital discharge (p < 0.05). Therefore, as suggested [1], multidisciplinary co-management starting at diagnosis should be organized to benefit patients with PPF and address potential complications promptly.
A wide range of surgical strategies can be applied to each PPF type, and this range of strategies depends on the PPF type considered [1, 2, 6, 7, 9, 11, 13, 14, 20]. Surgical management can be grouped into revision arthroplasty or internal fixation. Revision to a long stem is recommended for all type B2 femoral hip PPF, especially transverse patterns [3, 28, 29]. Other series had higher revision rates than seen in the current study: 60.9–86.8% for femoral hip PPF and 19.3% for femoral knee PPF [1, 11, 14]. Recent publications show a trend towards considering internal fixation in Vancouver B2 and B3 fractures [1, 2, 6, 7, 9, 13, 20]. PIPPAS showed that revision arthroplasty favoured full weight-bearing and hospital discharge to the patient’s own home (p < 0.05), but revision hip arthroplasty for PPF is associated with a high risk of mortality (p < 0.05) [13], as revealed in this study. We found that the recommendation for routine intraoperative stem stability tests before fixation was not followed, as the stability of one-third of femoral stems was not checked. However, not checking the stability of the prosthesis favoured weight-bearing restrictions (p < 0.05). The reason for this might be that when the stability is checked, the most common surgical strategy is prosthesis revision, which favours full weight-bearing. When the surgical strategy is fixation, usually with plates, surgeons might not feel confident with full weight-bearing. Compared to revision of the prosthesis, patients managed only with fixation showed a higher relative risk of restricted weight-bearing (p < 0.01). There is a tendency to use double plating techniques to increase stability to allow full weight-bearing, although they are not widely used. Anatomical polyaxial locking plates allow less invasive surgical approaches and the placement of locking screws around the stem, thus providing a certain degree of stability to a loose prosthesis [4]. Further analysis of the influence of full weight-bearing on failure of fixation rates in LLPPF managed only with fixation should follow. Therefore, our suggestion is to individualize every case, taking into consideration how frail the patient is and the surgical strategy options that could be used to achieve the best functional outcome in each scenario.
Open approaches were related to restricted weight-bearing for at least 30 days postoperatively (p < 0.05), but many authors mainly used open approaches for fixation [3, 6, 7, 13, 17], even though hypoaggressive approaches are recommended for PPF fixation [4, 26]. Surgeon experience favoured full weight-bearing (p < 0.05). Competency in the management of PPF could help improve in-hospital outcomes.
The operative delay varies from 6.06 to 4.07 days in published series [6, 11, 21, 31, 32]. Johnson-Lynn found no association between surgical delay and inpatient mortality [5], but we observed that surgery within the first 100 h (4.17 days) favoured survival and reduced the risk of medical complications (p < 0.05). Patients who are fit for surgery may benefit from prompt surgical management, and co-management may improve their medical condition, limiting the influence of comorbidities on survival and complications.
There is limited information regarding the type of anaesthesia in the management of PPF. Haughom et al. reported that 83.4% of patients with femoral hip PPF underwent surgery under general anaesthesia [32], in contrast to 13.8% and 25% of patients with femoral knee and femoral hip PPF in the PIPPAS study. However, the type of anaesthesia did not influence in-hospital outcomes in PPF.
Strut grafts have been widely used in the management of PPF [22], although there is a trend towards limiting their use: 1.4% in COMPOSE [11] and 2.2% in PIPPAS. This may be attributed to the increased use of hypoaggressive approaches and double-plate fixation when additional stability is required.
Non-operative management for femoral PPF is limited to selected cases, with reported rates ranging from 0 to 33% [11, 30]. Non-operative treatment was a candidate predictor of in-hospital mortality and worse outcomes (p < 0.05). It remains unclear whether the indication for non-surgical treatment was driven by the patient′s comorbidities or the fracture pattern. Nevertheless, it is generally accepted that patients with femoral fractures benefit from surgery [4, 33].
Early weight-bearing is a crucial factor in limiting the impact of LLPPF on functional outcomes and the return to the community [34, 35]. Restricted weight-bearing is associated with limited possibility of returning to the community, resulting in economic and social burdens [34, 35]. Frailty variables correlated with weight-bearing restrictions and cannot be modified. However, the operative technique can be improved to allow unrestricted weight-bearing, facilitating early functional recovery and social independence [3].
This study has several limitations, including the following. (1) The heterogeneity of PPF—although multiple surgical strategies were employed, the population, management, and outcomes were quite similar. (2) Specific details of and differences between fracture types and their surgical treatments were not explored, so further analysis should follow. A comprehensive understanding of these fractures can assist readers in organizing their resources. (3) Participating sites were responsible for data accuracy. (4) The stability of prosthesis fixation was always determined by the treating site. (5) Candidate predictors provide useful information about the potential correlation between two variables, but they cannot be used to determine casuality.
Conclusions
PPF patients are frail: CFS > 3, CCI > 3 and mild cognitive impairment (Pfeiffer > 2) are associated with higher morbidity and mortality rates in the acute setting. Hospital discharge to a nursing home and weight-bearing restrictions are common outcomes in such cases. Surgical strategies directly influence these immediate outcomes. However, current arthroplasty fixation check and revision rates do not adhere to established guidelines. Nevertheless, revision arthroplasty and experienced surgeons were associated with fewer weight-bearing restrictions. A surgical delay exceeding 100 h and a lack of co-management with geriatricians were linked to in-hospital mortality and medical complications. Hypoaggressive approaches favoured full weight-bearing and are therefore recommended for fixation. The PIPPAS study provides insights into potential risk factors, which can aid in the development of individualized management strategies for the benefit of patients with PPF.
Supplementary information
Additional file 1. Data collected from patients presenting with a Periprosthetic fracture.
Acknowledgements
We thank Dasha Gorbenko del Blanco (PhD, medical illustrator, dasha.blanco@gmail.com) for her artwork when producing the figures and graphical abstract for the PIPPAS study.
Group authorship: “The PIPPAS Study group”
The PIPPAS Study Group Members
Héctor J. Aguado MD PhD (Hospital Clínico Universitario de Valladolid) (1). Pablo Castillón-Bernal MD PhD (Hospital Universitari Mútua de Terrassa (Barcelona)) (2). Jordi Teixidor-Serra MD, Yaiza García-Sánchez MS (Hospital Universitari Vall d′Hebrón de Barcelona) (3). Josep M. Muñoz-Vives MD (Hospital Fundació Althaia de Manresa (Barcelona)) (4), Pilar Camacho-Carrasco MD PhD, Montsant Jornet-Gibert PhD (Hospital Clínic de Barcelona) (5). Cristina Ojeda-Thies MD PhD (Hospital 12 de Octubre de Madrid) (6). Pablo García-Portabella MD (Hospital de Jove de Gijón (Asturias)) (7). Adela Pereda-Manso MS, Elvira Mateos-Álvarez MD, Javier Manzano-Mozo PhD, Raquel Carrillo-Gómez MPsy MEd, Sergio País-Ortega MD, Virginia García-Virto MD, David Noriega-González MD PhD, Begoña Aránzazu Álvarez-Ramos MD PhD, Abel Ganso-Pérez MD, Carmen Cervera-Díaz MD, María Plata-García MD, Alina Ortega-Briones MD, Juan Berrocal-Cuadrado MD, Diego Criado del Rey-Machimbarrena MD (Hospital Clínico Universitario de Valladolid) (1). Jordi Salvador MD PhD, Laura Rey MD (Hospital Universitari Mútua de Terrassa (Barcelona)) (2). Jordi Tomás-Hernández MD, Jordi Selga-Marsà MD, José Vicente Andrés-Peiró MD PhD (Hospital Universitari Vall d′Hebrón de Barcelona) (3). Jordi Querolt-Coll MD, Guillermo Triana MD (Hospital Fundació Althaia de Manresa (Barcelona)) (4). Marian Vives-Barquiel MD, Marina Renau-Cerrillo MD, Borja Campuzano-Bitterling MD, José M. Hernández MD, Ricardo Ostilla MD, Anna Carreras-Castañer MD, Pere Torner MD PhD (Hospital Clínic de Barcelona (5). Rebeca Díaz Suárez MD, Eliam Ajuria Fernández MD, Carlos Olaya-González MD (Hospital 12 de Octubre de Madrid) (6). María Fernández-Villán MD PhD (Hospital de Jove de Gijón (Asturias)) (7). Unai García De Cortázar MD, Mirentxu Arrieta MD, Daniel Escobar MD, Estíbaliz Castrillo MD (Hospital Universitario de Basurto (Bizkaia)) (8). Patricia Balvis Balvis MD, Mónica Rodríguez Arenas MD, Ángela García Pérez MD (Hospital Álvaro Cunqueiro de Vigo (Pontevedra)) (9). Jesús Moreta MD, Iñigo Bidea MD, Xabier Jiménez-Urrutia MD (Hospital Universitario de Galdakao-Usansolo (Bizkaia)) (10). Beatriz Olías-López MD, Juan Boluda-Mengod MD, David González-Martín MD PhD (Hospital Universitario de Canarias de Tenerife) (11). Leopoldo Bárcena Goitiandia MD, Daniel López Dorado MD (Hospital Infanta Elena de Valdemoro (Madrid)) (12). Juan Carlos Borrás-Cebrián MD (Hospital Universitario Dr. Peset de Valencia) (13). David García-Aguilera MD PhD, Patricio Andrés Freile Pazmiño MD (Hospital Royo Villanova de Zaragoza) (14). Miguel Ángel Suárez Suárez MD PhD, Lucía Lanuza Lagunilla MD, Antonio García Arias MD (Hospital Universitario de Cabueñes de Gijón) (15). Jaime Sánchez-Saz MD, Javier García-Coiradas MD, José Valle-Cruz MD, Jesús Mora-Fernández MD (Hospital Clínico San Carlos de Madrid) (16). María Ángeles Cano Leira MD, Guillermo Rieiro MD (Complejo Hospitalario Universitario de A Coruña) (17). Antonio Benjumea Carrasco MD, Rodrigo Jesús Priego Sánchez MD, Coral Sánchez Pérez MD (Hospital General Universitario Gregorio Marañón de Madrid) (18). Jorge Guadilla-Arsuaga MD Prof., Alexis Fernández-Juan MD (Hospital Universitario de Álava) (19). Plácido Sánchez MD (Hospital General Universitario Los Arcos del Mar Menor de Murcia) (20). F. Javier Ricón MD (Hospital Vega Baja de Orihuela (Alicante)) (21). Alfonso Fuentes-Díaz MD, Elena M. García García MD (Hospital General Universitario J.M. Morales Meseguer de Murcia) (22). Francisco Cuadrado Abajo MD, Gonzalo García Portal MD (Hospital Universitario Marqués de Valdecilla de Santander) (23). Pedro del Pozo Manrique MD, Virginia Castillo del Pozo MD, Francisco Manuel Garcia-Navas MD PhD (Hospital Universitario de Toledo) (24). Ester García-Paredero MD, Teresa Beteta Robles MD, Ainhoa Guijarro Valtueña MD, Gonzalo Gutiérrez Baiget MD (Hospital Puerta de Hierro de Majadahonda (Madrid)) (25). Noelia Alonso-García MD PhD, Inés Navas-Pernía MD (Complejo Asistencial de Segovia) (26). Diana Ariza Herrera MD, Joan Vilanova MD, Miquel Videla-Cés MD PhD (Consorci Sanitari Integral—Hospital Sant Joan Despí- Moisès Broggi de Barcelona) (27). Teresa Serra Porta MD PhD, César Vázquez García MD (Hospital Universitari Sagrat Cor—Quirónsalud de Barcelona) (28). Carmen Carrasco Becerra MD (Complejo Hospitalario de Llerena-Zafra (Badajoz)) (29). Silvia Pena Paz MD, Víctor Otero-Naveiro MD, Inés Fernández-Billón Castrillo MD (Hospital Universitario Lucus Augusti de Lugo) (30). Amaia Martínez-Menduiña MD, Carolina Hernández-Galera MD, Fátima Fernández-Dorado MD (Hospital Ramón y Cajal de Madrid) (31). María Madrigal López MD (Hospital Príncipe de Asturias de Alcalá de Henares) (32). Antonio Murcia-Asensio MD PhD, Elena Galián-Muñoz MD (Hospital General Universitario Reina Sofía de Murcia (33). Ángel Castro Sauras MD, Teresa Espallargas Doñate MD, María Royo Agustín MD (Hospital General Obispo Polanco de Teruel) (34). Nuria Plaza-Salazar MD, Carla Gámez-Asunción MD, Adrián Muñoz-Vicente MD, Teresa Pareja-Sierra MD PhD (Hospital Universitario de Guadalajara) (35). Jennifer Benito-Santamaría MD (Hospital Universitari Doctor Josep Trueta de Girona) (36). Alejandro Cuenca Copete MD, Ana Verdejo González MD, Blas González Montero MD PhD (Complejo Hospitalario Universitario de Albacete) (37). Luis Alejandro Giraldo Vegas MD, Laura Alonso Viana MD, Eduardo José Díez Pérez PhD (Hospital Sierrallana de Torrelavega (Cantabria)) (38). Ricardo Briso-Montiano MD, Ana Isabel Andrés MD, Juan Mingo-Robinet MD (Complejo Asistencial Universitario de Palencia) (39). María Naharro Tobío MD PhD, Emma Escudero Martínez PhD (Complexo Hospitalario Universitario de Pontevedra) (40). Jorge Serrano-Sanz MD, J. M. Peñalver-Matamoros MD, Núria Fernàndez-Poch MD, Laia Martínez-Carreres PhD (Hospital Universitari Parc Taulí de Sabadell (Barcelona)) (41). María Macho-Mier MD, Carlos Martín-Hernández MD PhD, Antonio Francisco Laclériga-Giménez MD PhD (Hospital Universitario Miguel Servet de Zaragoza) (42). José Carlos Saló Cuenca MD, César Salamanca Ontiveros MD, Jordi Espona Roselló MD, Victoria Altemir Martínez MD (Hospital Universitario Arnau de Vilanova de Lleida) (43). Guillermo Criado-Albillos MD, Jorge Cunchillos-Pascual MD, Mercedes Millán-Cid MD, Hugo Gabriel Cabello-Benavides MD (Complejo Asistencial Universitario de Burgos (44). Jorge Martínez-Íñiguez Blasco MD, Paloma Sevilla Ortega MD (Hospital Universitario San Pedro de Logroño) (45). Juan Ramón Cano MD PhD, Alicia Ramírez MD (Hospital Universitario Costa del Sol de Marbella (Málaga)) (46). Fernando Marqués-López MD PhD, Santos Martínez-Díaz MD PhD (Hospital Parc De Salut Mar de Barcelona) (47). Guido S. Carabelli MD, Pablo A. Slullitel MD, Ignacio Astore MD, Bruno R. Boietti MD (Hospital Italiano de Buenos Aires) (48). Carlos Hernández-Pascual MD, Javier Marín-Sánchez MD, Julio César Córdova-Peralta MD (Hospital Universitario de Salamanca) (49). Iván Dot Pascuet MD (Hospital Universitari Sant Joan de Reus (Tarragona)) (50). Eduardo Pereira Mosquera MD, Javier Martín Antúnez, MD, José María Pérez MD (Hospital Virgen del Rocío de Sevilla) (51). Alfonso Mandía Martínez MD, Julio De Caso MD, Jordi Martín Marcuello MD (Hospital de la Santa Creu i Sant Pau de Barcelona) (52). Miguel Benito-Mateo MD, A. David Murillo-Vizuete MD (Hospital Universitario Infanta Leonor de Madrid) (53). Luis Gracia Delgado MD (Hospital Alto Guadalquivir de Andújar (Jaén)) (54). Gaspar de la Herrán MD, Nahikari Nunes MD (Hospital Universitario de Donostia) (55). Ivan Pérez-Coto MD PhD (Hospital Carmen y Severo Ochoa de Cangas del Narcea (Asturias)) (56). María Rosa González-Panisello MD PhD (Hospital Reina Sofía de Tudela (Navarra)) (57). Susana Iglesias Fernández MD PhD, Gorka Luis Ruete Gil MD PhD, Sergio Ramos García MD PhD (Hospital San Agustín de Avilés (Asturias)) (58). Juan Pablo Villarreal MD (Hospital Universitario Virgen Macarena de Sevilla) (59).
Abbreviations
- PPF
Periprosthetic fractures
- UCS
Unified Classification System
- PIPPAS
Peri-Implant PeriProsthetic Survival Analysis
- THA
Primary total hip arthroplasty
- TKA
Total knee arthroplasty
- IQR
Interquartile range
- SD
Standard deviation
- ASA
American Society of Anesthesiologists physical status classification system
- CCI
Charlson comorbidity index
- CFS
Clinical frailty scale
- LL
Lower limb
Author contributions
The PIPPAS study was organized by seven trauma and orthopaedic surgery departments in Spain: the PIPPAS coordinating team. HJA was the principal investigator and Hospital Clínico Universitario de Valladolid was the lead centre. HJA, APM, EMA, SPO, and VGV were responsible for the database, data validation, analyses, and study-centre coordination. The coordinating team (HJA, PCB, JTS, YGS, JMMV, PCC, MJG, COT, PGP, and APM) designed the study and vouched for the accuracy of the data and analyses. JMM, RCG, and HJA performed the statistical analysis. HJA wrote the first draft of the manuscript, and HJA, PCB, JTS, YGS, JMMV, PCC, MJG, COT, PGP, and APM made critical revisions. All authors made comments, read and approved the final manuscript, and decided to submit the paper.
Funding
The study was funded by grants from the AO Trauma Foundation (Spain) (grant number: 20–2041) and SACYL (Sanidad de Castilla y León) (grant number: GRS: 2371/A/21). No funding entity had a role in the design or conduct of the study, the collection or analyses of data, or manuscript preparation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All patients or their relatives gave their written consent to be included in the study. The study coordinating centre and each participating hospital obtained institutional review board approval. The study was approved by the coordinating centre ethics committee board, Comité Ético de Investigación Médica Área de Salud Valladolid Este (code: PI 20-2041). The Comité Ético de Investigación Médica Área de Salud Valladolid Este is the reference ethics committee board for the PIPPAS study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
The PIPPAS Study Group:
Héctor J. Aguado, Pablo Castillón-Bernal, Jordi Teixidor-Serra, Yaiza García-Sánchez, Josep M. Muñoz-Vives, Pilar Camacho-Carrasco, Montsant Jornet-Gibert, Cristina Ojeda-Thies, Pablo García-Portabella, Adela Pereda-Manso, Elvira Mateos-Álvarez, Javier Manzano-Mozo, Raquel Carrillo-Gómez, Sergio País-Ortega, Virginia García-Virto, David Noriega-González, Begoña Aránzazu Álvarez-Ramos, Abel Ganso-Pérez, Carmen Cervera-Díaz, María Plata-García, Alina Ortega-Briones, Juan Berrocal-Cuadrado, Diego Criado del Rey-Machimbarrena, Jordi Salvador, Laura Rey, Jordi Tomás-Hernández, Jordi Selga-Marsà, José Vicente Andrés-Peiró, Jordi Querolt-Coll, Guillermo Triana, Marian Vives-Barquiel, Marina Renau-Cerrillo, Borja Campuzano-Bitterling, José M Hernández, Ricardo Ostilla, Anna Carreras-Castañer, Pere Torner, Rebeca Díaz-Suárez, Eliam Ajuria Fernández, Carlos Olaya-González, María Fernández-Villán, Unai García de Cortázar, Mirentxu Arrieta, Daniel Escobar, Estíbaliz Castrillo, Patricia Balvis, Mónica Rodríguez-Arenas, Ángela García-Pérez, Jesús Moreta, Iñigo Bidea, Xabier Jiménez-Urrutia, Beatriz Olías-López, Juan Boluda-Mengod, David González-Martín, Leopoldo Bárcena-Goitiandia, Daniel López-Dorado, Juan Carlos Borrás-Cebrián, David García-Aguilera, Patricio Andrés Freile-Pazmiño, Miguel Ángel Suárez-Suárez, Lucía Lanuza-Lagunilla, Antonio García-Arias, Jaime Sánchez-Saz, Javier García-Coiradas, José Valle-Cruz, Jesús Mora-Fernández, María Ángeles Cano-Leira, Guillermo Rieiro, Antonio Benjumea-Carrasco, Rodrigo Jesús Priego-Sánchez, Coral Sánchez-Pérez, Jorge Guadilla-Arsuaga, Alexis Fernández-Juan, Plácido Sánchez, Javier Ricón, Alfonso Fuentes-Díaz, Elena M. García-García, Francisco Cuadrado-Abajo, Gonzalo García-Portal, Pedro del PozoManrique, Virginia Castillo del Pozo, Francisco Manuel Garcia-Navas, Ester García-Paredero, Teresa Beteta-Robles, Ainhoa Guijarro-Valtueña, Gonzalo Gutiérrez-Baiget, Noelia Alonso-García, Inés Navas-Pernía, Diana Ariza-Herrera, Joan Vilanova, Miquel Videla-Cés, Teresa Serra-Porta, César Vázquez-García, Carmen Carrasco-Becerra, Silvia Pena-Paz, Víctor Otero-Naveiro, Inés Fernández-Billón-Castrillo, Amaia Martínez-Menduiña, Carolina Hernández-Galera, Fátima Fernández-Dorado, María Madrigal-López, Antonio Murcia-Asensio, Elena Galián-Muñoz, Ángel Castro-Sauras, Teresa Espallargas-Doñate, María Royo-Agustín, Nuria Plaza-Salazar, Carla Gámez-Asunción, Adrián Muñoz-Vicente, Teresa Pareja-Sierra, Jennifer Benito-Santamaría, Alejandro Cuenca-Copete, Ana Verdejo-González, Blas González-Montero, Luis Alejandro Giraldo-Vegas, Laura Alonso-Viana, Eduardo José Díez-Pérez, Ricardo Briso-Montiano, Ana Isabel Andrés, Juan Mingo-Robinet, María Naharro-Tobío, Emma Escudero-Martínez, Jorge Serrano-Sanz, J. M. Peñalver-Matamoros, Núria Fernàndez-Poch, Laia Martínez-Carreres, María Macho-Mier, Carlos Martín-Hernández, Antonio Francisco Laclériga-Giménez, José Carlos Saló-Cuenca, César Salamanca-Ontiveros, Jordi Espona-Roselló, Victoria Altemir-Martínez, Guillermo Criado-Albillos, Jorge Cunchillos-Pascual, Mercedes Millán-Cid, Hugo Gabriel Cabello-Benavides, Jorge Martínez-Íñiguez-Blasco, Paloma Sevilla-Ortega, Juan Ramón Cano, Alicia Ramírez, Fernando Marqués-López, Santos Martínez-Díaz, Guido S. Carabelli, Pablo A Slullitel, Ignacio Astore, Bruno R. Boietti, Carlos Hernández-Pascual, Javier Marín-Sánchez, Julio César Córdova-Peralta, Iván Dot-Pascuet, Eduardo Pereira-Mosquera, Javier Martín-Antúnez, José María Pérez, Alfonso Mandía-Martínez, Julio De Caso, Jordi Martín-Marcuello, Miguel Benito-Mateo, A. David Murillo-Vizuete, Luis Gracia Delgado, Gaspar dela Herrán, Nahikari Nunes, Ivan Pérez-Coto, María Rosa González-Panisello, Susana Iglesias-Fernández, Gorka Luis Ruete-Gil, Sergio Ramos-García, and Juan Pablo Villarreal
References
- 1.Khan T, Middleton R, Alvand A, Manktelow ARJ, Scammell BE, Ollivere BJ. High mortality following revision hip arthroplasty for periprosthetic femoral fracture: a cohort study using national joint registry data. Bone Jt J. 2020;102(B12):1670–1674. doi: 10.1302/0301-620X.102B12.BJJ-2020-0367.R1. [DOI] [PubMed] [Google Scholar]
- 2.Slullitel PA, Garcia-Barreiro GG, Oñativia JI, Zanotti G, Comba F, Piccaluga F, et al. Selected Vancouver B2 periprosthetic femoral fractures around cemented polished femoral components can be safely treated with osteosynthesis. Bone Jt J. 2021;103(B7):1222–1230. doi: 10.1302/0301-620X.103B7.BJJ-2020-1809.R1. [DOI] [PubMed] [Google Scholar]
- 3.Biggi F, Di Fabio S, D’Antimo C, et al. Periprosthetic fractures of the femur: the stability of the implant dictates the type of treatment. J Orthopaed Traumatol. 2010;11:1–5. doi: 10.1007/s10195-010-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruchholtz S, El-Zayat B, Kreslo D, Bücking B, Lewan U, Krüger A et al (2013) Less invasive polyaxial locking plate fixation in periprosthetic and peri-implant fractures of the femur—a prospective study of 41 patients. Injury 44(2):239–248 [DOI] [PubMed]
- 5.Konan S, Sandiford N, Unno F, Masri BS, Garbuz DS, Duncan CP. Periprosthetic fractures associated with total knee arthroplasty: an update. Bone Jt J. 2016;98(B11):1489–1496. doi: 10.1302/0301-620X.98B11.BJJ-2016-0029.R1. [DOI] [PubMed] [Google Scholar]
- 6.Gitajn IL, Heng M, Weaver MJ, Casemyr N, May C, Vrahas MS, et al. Mortality following surgical management of Vancouver B periprosthetic fractures. J Orthop Trauma. 2017;31(1):9–14. doi: 10.1097/BOT.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 7.Wadhwa H, Salazar BP, Goodnough LH, Van Rysselberghe NL, DeBaun MR, Wong HN, et al. Distal femur replacement versus open reduction and internal fixation for treatment of periprosthetic distal femur fractures: a systematic review and meta-analysis. J Orthop Trauma. 2022;36(1):1–6. doi: 10.1097/BOT.0000000000002141. [DOI] [PubMed] [Google Scholar]
- 8.Powell-Bowns MFR, Oag E, Ng N, Pandit H, Moran M, Patton JT et al (2021) Vancouver B periprosthetic fractures involving the Exeter cemented stem. Bone Jt J 103(B2):309–320 [DOI] [PubMed]
- 9.Gibbs VN, McCulloch RA, Dhiman P, McGill A, Taylor AH, Palmer AJR, et al. Modifiable risk factors for mortality in revision total hip arthroplasty for periprosthetic fracture. Bone Jt J. 2020;102(B5):580–585. doi: 10.1302/0301-620X.102B5.BJJ-2019-1673.R1. [DOI] [PubMed] [Google Scholar]
- 10.Märdian S, Perka C, Schaser KD, Gruner J, Scheel F, Schwabe P. Cardiac disease and advanced age increase the mortality risk following surgery for periprosthetic femoral fractures. Bone Jt J. 2017;99(B7):921–926. doi: 10.1302/0301-620X.99B7.BJJ-2016-0974.R1. [DOI] [PubMed] [Google Scholar]
- 11.The COMPOSE Study Team (2022) Management and outcomes of femoral periprosthetic fractures at the hip: data from the characteristics, outcomes and management of periprosthetic fracture service evaluation (COMPOSE) cohort study. Bone Jt J 104-B(8):997–1008 [DOI] [PubMed]
- 12.Anon (2018) Unified Classification System for Periprosthetic Fractures (UCPF). J Orthop Trauma 32:S141–S144 [DOI] [PubMed]
- 13.Pflüger P, Bolierakis E, Wurm M, Horst K, Hildebrand F, Biberthaler P (2022) Revision rate is higher in patients with periprosthetic femur fractures following revision arthroplasty in comparison with ORIF following our algorithm: a two-center 1 analysis of 129 patients. Eur J Trauma Emerg Surg 48(3):1913–1918 [DOI] [PMC free article] [PubMed]
- 14.Ragland K, Reif R, Karim S, Sexton KW, Cherney SM, Stambough JB, et al. Demographics, treatment, and cost of periprosthetic femur fractures: fixation versus revision. Geriatr Orthop Surg Rehabil. 2020;11:2151459320939550. doi: 10.1177/2151459320939550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carli AV, Negus JJ, Haddad FS. Periprosthetic femoral fractures and trying to avoid them: what is the contribution of femoral component design to the increased risk of periprosthetic femoral fracture? Bone Joint J. 2017;99:50–59. doi: 10.1302/0301-620X.99B1.BJJ-2016-0220.R1. [DOI] [PubMed] [Google Scholar]
- 16.Corten K, Vanrykel F, Bellemans J, Frederix PR, Simon JP, Broos PL. An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well-fixed femoral component. J Bone Joint Surg Br. 2009;91(11):1424–1430. doi: 10.1302/0301-620X.91B11.22292. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim KI, Park KC, Shon OJ, Sim JA, Kim GB. New classification for periprosthetic distal femoral fractures based on locked-plate fixation following total knee arthroplasty: a multicenter study. J Arthroplasty. 2022;37(5):966–973. doi: 10.1016/j.arth.2022.01.078. [DOI] [PubMed] [Google Scholar]
- 18.Reeves RA, Schairer WW, Jevsevar DS. The national burden of periprosthetic hip fractures in the US: costs and risk factors for hospital readmission. Hip Int J Clin Exp Res Hip Pathol Ther. 2019;29(5):550–557. doi: 10.1177/1120700018803933. [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Won SH, Kim HS, Won SJ, Lee YK, Koo KH (2022) Current incidence and future projection of periprosthetic fractures in South Korea: a study based on national claim database. Orthop Surg 14(3):530–535 [DOI] [PMC free article] [PubMed]
- 20.Spina M, Scalvi A. Vancouver B2 periprosthetic femoral fractures: a comparative study of stem revision versus internal fixation with plate. Eur J Orthop Surg Traumatol Orthop Traumatol. 2018;28(6):1133–1142. doi: 10.1007/s00590-018-2181-3. [DOI] [PubMed] [Google Scholar]
- 21.Johnson-Lynn S, Ngu A, Holland J, Carluke I, Fearon P. The effect of delay to surgery on morbidity, mortality and length of stay following periprosthetic fracture around the hip. Injury. 2016;47(3):725–727. doi: 10.1016/j.injury.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Jt J. 2016;98(B4):461–467. doi: 10.1302/0301-620X.98B4.37201. [DOI] [PubMed] [Google Scholar]
- 23.Song JSA, Dillman D, Wilson D, Dunbar M, Richardson G. Higher periprosthetic fracture rate associated with use of modern uncemented stems compared to cemented stems in femoral neck fractures. Hip Int. 2019;29(2):177–183. doi: 10.1177/1120700018772291. [DOI] [PubMed] [Google Scholar]
- 24.Karam J, Campbell P, Desai S, Hunter M. Periprosthetic proximal femoral fractures in cemented and uncemented stems according to Vancouver classification: observation of a new fracture pattern. J Orthop Surg Res. 2020;15(1):100. doi: 10.1186/s13018-020-01619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nugent M, Wyatt MC, Frampton CM, Hooper GJ. Despite improved survivorship of uncemented fixation in total knee arthroplasty for osteoarthritis, cemented fixation remains the gold standard: an analysis of a national joint registry. J Arthroplasty. 2019;34(8):1626–1633. doi: 10.1016/j.arth.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):275–282. doi: 10.2106/00004623-200609001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Lamb JN, Nix O, Al-Wizni A, West R, Pandit H. Mortality after postoperative periprosthetic fracture of the femur after hip arthroplasty in the last decade: meta-analysis of 35 cohort studies including 4841 patients. J Arthroplasty. 2022;37(2):398–405.e1. doi: 10.1016/j.arth.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Marsland D, Mears SC. A review of periprosthetic femoral fractures associated with total hip arthroplasty. Geriatr Orthop Surg Rehabil. 2012;3(3):107–120. doi: 10.1177/2151458512462870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondanelli N, Troiano E, Facchini A, Ghezzi R, Di Meglio M, Nuvoli N, Peri G, Aiuto P, Colasanti GB, Giannotti S. Treatment algorithm of periprosthetic femoral fracturens. Geriatr Orthop Surg Rehabil. 2022;10(13):21. doi: 10.1177/21514593221097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudiganty S, Hughes L, Choudry Q, Bokhari A. Managing periprosthetic fractures—a review of the hub and spoke model. SICOT-J. 2022;8:2. doi: 10.1051/sicotj/2022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennison T, Yarlagadda R. A case series of mortality and morbidity in distal femoral periprosthetic fractures. J Orthop. 2020;18:244–247. doi: 10.1016/j.jor.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haughom BD, Basques BA, Hellman MD, Brown NM, Della Valle CJ, Levine BR. Do mortality and complication rates differ between periprosthetic and native hip fractures? J Arthroplasty. 2018;33(6):1914–1918. doi: 10.1016/j.arth.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 33.Welford P, Jones CS, Davies G, Kunutsor SK, Costa ML, Sayers A, et al. The association between surgical fixation of hip fractures within 24 hours and mortality: a systematic review and meta-analysis. Bone Jt J. 2021;103(B7):1176–1186. doi: 10.1302/0301-620X.103B7.BJJ-2020-2582.R1. [DOI] [PubMed] [Google Scholar]
- 34.Francony F, Montbarbon E, Pailhé R, Rubens Duval B, Saragaglia D (2022) Assessment of morbidity and mortality after periprosthetic hip fracture. Influence of Vancouver stage in a retrospective single-centre study of 88 patients. Orthop Traumatol Surg Res OTSR 108(1):102985 [DOI] [PubMed]
- 35.Keenan OJF, Ross LA, Magill M, Moran M, Scott CEH. Immediate weight-bearing is safe following lateral locked plate fixation of periprosthetic distal femoral fractures. Knee Surg Relat Res. 2021;33(1):19. doi: 10.1186/s43019-021-00097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Data collected from patients presenting with a Periprosthetic fracture.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.