Abstract
The white-rot fungus Pleurotus eryngii secretes various laccases involved in the degradation of a wide range of chemical compounds. Since the laccase production is relatively low in fungi, many efforts have been focused on finding ways to increase it, so in this study, we investigated the effect of copper on the transcription of the pel3 laccase gene and extracellular laccase activity. The results indicate that adding 0.5 to 2 mM copper to liquid cultures of P. eryngii KS004 increased both pel3 gene transcription and extracellular laccase activity in a concentration-dependent manner. The most significant increase in enzyme activity occurred at 1 mM Cu2+, where the peak activity was 4.6 times higher than in control flasks. Copper also induced the transcription of the laccase gene pel3. The addition of 1.5 and 2 mM Cu2+ to fungal culture media elevated pel3 transcript levels to more than 13-fold, although the rate of induction slowed down at Cu2+ concentrations higher than 1.5 mM. Our findings suggest that copper acts as an inducer in the regulation of laccase gene expression in P. eryngii KS004. Despite its inhibitory effect on fungal growth, supplementing cultures with copper can lead to an increased extracellular laccase production in P. eryngii.
Keywords: Induction, Laccase, Pleurotus eryngii, Transcriptional regulation, White-rot fungi
Introduction
Fungi serve as cell factories for producing various proteins, including enzymes. Pleurotus eryngii, the king oyster mushroom, is both edible and medicinal, producing ligninolytic enzymes like peroxidase, aryl-alcohol oxidase, and laccase [1]. Laccases are extracellular enzymes that catalyze the oxidation of diverse substrates such as phenolic compounds, aromatic, and aliphatic amines by safe and eco-friendly reactions dealing with reduction of oxygen to water. High-redox-potential laccases which are more desirable for biotechnological purposes are mostly of fungal origin, while low-redox-potential laccases are found in bacteria, higher plants, and animals. In fungi, the reactions catalyzed by laccase are involved in decomposition of lignocellulose, defense responses, pathogenesis, pigmentation, and sporulation [2, 3]. These copper-containing enzymes find applications in industries like pulp, paper and textiles, detoxification of recalcitrant pollutants, bioremediation, and more [4, 5]. However, realizing laccase’s biotechnological potential faces challenges. A primary obstacle is the high production cost due to low enzyme yields in fungi. Consequently, efforts concentrate on determining molecular characteristics of fungal laccases, unraveling laccase gene regulation mechanisms, and enhancing expression or expanding enzyme properties for better production. One of the strategies practiced to produce larger quantities of laccase is using inducers such as aromatic compounds [6], amino acids [7], plant extracts [8], and metal ions [9–11]. In silico analysis of laccase gene promoters has revealed various responsive elements scattered across the promoter region. Correlation between specific cis-acting elements and laccase gene transcription exists, with potential mechanisms proposed. Differences in element copy number, location, or orientation contribute to the intricate landscape of laccase gene regulation [11, 12].
The fact that laccases are mostly coded by several genes within a given species is a usual phenomenon in eukaryotes [2]. In P. eryngii, four laccase genes, including pel3, have been isolated and characterized so far [13, 14]. Analysis of the pel3 promoter region has highlighted diverse regulatory elements, including a copper-sensing sequence, a yeast copper-response element, and two putative metal response elements (MREs) [14]. In certain fungi, MREs can bind to metal ions like Cu2+, Fe2+, and Cd2+ in culture media, stimulating extracellular laccase production [4, 15].
Although P. eryngii has a great potential to be used as a valuable source of enzymes due to its short growth period and easy and cheap cultivation compared to other basidiomycetes, there is relatively little knowledge about laccase induction and transcriptional regulation in this fungus. Since the influence of copper on laccase synthesis and secretion is species and isolate dependent [12, 16], this study explores the effect of copper as an inducer on laccase activity and pel3 gene transcription in P. eryngii KS004.
Materials and methods
Strains, medium, and culture conditions
The Pleurotus eryngii strain KS004 was provided by the Edible Mushrooms Research Center of Ferdowsi University of Mashhad, Iran, and maintained on potato dextrose agar (PDA) plates containing 200 g/L potato extract, 20 g/L dextrose, and 15 g/L agar at 26 °C. The potato dextrose broth (PDB) medium used for shaken cultures had the same ingredients, excluding agar, and included 5 g/L yeast extract as the best nitrogen source for laccase production [17]. The pH was adjusted to 6 before sterilization. Seven mycelial plugs (4 mm diameter) were taken from the periphery of PDA plates and inoculated into 100 mL of PDB medium in Erlenmeyer flasks. They were then grown for 8 days at 26 °C with agitation at 150 rpm. Cultures were supplemented with CuSO4.5H2O to final concentrations of 0, 0.5, 1, 1.5, and 2 mM. Previous studies on other Pleurotus species showed that the best laccase production in Pleurotus ostreatus was induced by adding copper during the mid-logarithmic phase of cultivation (the 5th day) [17]. In our study, filter-sterilized copper sulfate was added to actively growing 5-day-old P. eryngii cultures, followed by 3 more days of growth. Samples were taken from 8-day-old cultures, with a reference culture without any inducer serving as the control. All experiments were conducted in triplicate.
Determination of the mycelial dry weight
To assess the impact of copper on fungal growth, culture media were augmented with varying concentrations of copper sulfate at the time of inoculation [15]. After 8 days of growth, the dry weights of the mycelium were measured. The quantification was performed using the method proposed by Manubens et al. [18]. Fungal mycelia were harvested from triplicate flasks of 8-day-old cultures and filtered through Whatman No.1 papers that were previously dried at 100 °C and weighed. The retained mycelium was then dried at 100 °C to a constant weight, and the mycelial dry weight was determined by calculating the difference in measured values.
To better illustrate the effect of copper on fungal growth, the Pleurotus eryngii KS004 mycelia were also cultured on PDA plates containing different Cu2+ concentrations.
Enzyme activity assay
Laccase activity was assessed at room temperature by monitoring the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfononic acid) diammonium salt (ABTS) as the substrate (SIGMA, Saint Louis, Missouri, USA). The reaction mixture contained 5 mM ABTS, 0.1 M sodium acetate buffer (pH = 5), and 100 μL of culture supernatant [14]. It was then incubated at room temperature for 3 min, after which the laccase activity was determined by the increase in absorbance at 420 nm (ε = 36,000 M−1 cm−1). One unit of enzyme activity was defined as the amount of enzyme needed to oxidize 1 μmol of ABTS per minute. Measurements were conducted in 10-mm quartz cuvettes using a UV-Vis spectrophotometer (Unico S-2100-UV, USA).
Extraction of RNA and cDNA synthesis
Upon harvest, 8-day-old cultures of P. eryngii mycelia were collected by filtration, washed twice with distilled water, and rapidly frozen in liquid nitrogen. The frozen mycelia were subsequently pulverized using a mortar and pestle. Total RNA extraction was performed using the DENAzist total RNA extraction kit (DENAzist Asia, Mashhad, Iran), following the manufacturer’s instructions. All RNA samples were prepared in triplicate. RNA quality and quantity were assessed through gel electrophoresis and a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, USA). Next, 625 ng of total RNA was reverse transcribed into cDNA in a 20 μL reaction volume using the Parstous cDNA synthesis kit (Parstous, Mashhad, Iran), as per manufacturer’s protocol.
Primer design
The specific primer pairs used for the pel3 gene in real-time PCR (qPCR) were designed by the Primer3 program (version 4.1.0). These primers were based on the genomic DNA sequence of pel3 (accession no. AY686700) and amplified a 107 bp fragment in each reaction. The primer sequences were as follows: 5′-TCTTGCTATGGGCTTCGACT-3′ and 5′-GGCGTAGCACCTGACAGAAT-3′. The β-actin gene of P. eryngii was employed as an internal control. The primer pairs for β-actin were obtained from the literature [19]. The forward and reverse primers were 5′-CCCCTGAGCGAAAGTACTCC-3′ and 5′-AGGGCCTGACTCGTCGTATT-3′, respectively.
Quantitative real-time PCR
To quantify the transcription level of the pel3 gene, quantitative real-time PCRs were conducted using a QIAGEN Rotor Gene 3000. SYBR green fluorescent dye was employed for product detection. Each reaction was prepared in a final volume of 20 μL, containing 10 μL of 2× master mix green (Ampliqon), 0.5 μM of both forward and reverse primers, and 1250 ng of cDNA, and the remaining volume was adjusted to 20 μL using nuclease-free water. The qRT-PCR was performed as follows: an initial step of 15 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 57 °C, and 30 s at 72 °C. Subsequently, a melting cycle ranging from 50 to 99 °C was performed to ensure amplification specificity. Each sample’s amplification experiment was conducted in triplicate, with three biological replicates, each run in three technical replicates. All data were normalized to the internal control, and relative expression was calculated using the 2−ΔΔCt method.
Statistical analysis
All measurements were conducted in three independent experiments and were analyzed using one-way analysis of variance (ANOVA). Post hoc Tukey HSD test was employed to compare the treatments. Statistical analysis was carried out using IBM SPSS Statistics 23, and the charts were generated using GraphPad Prism (version 9.5.1.733). The plotted results represent the mean values derived from the collected data. A p-value less than or equal to 0.05 has been interpreted statistically significant. Significant differences between treatments are denoted by letters on graph bars.
Results
The presence of multiple regulatory elements related to heavy metal response in the promoter region of pel3 gene implied that laccase gene transcription in P. eryngii may be regulated by metal ions, specifically copper. To validate this hypothesis and also efficiently improve enzyme yield in this strain, here we examined the impact of varying concentrations of copper in the culture media on laccase activity and pel3 laccase gene transcription in P. eryngii KS004.
In terms of enzyme activity, laccase activity remained relatively low in the absence of inducer. But when copper was added, the extracellular laccase activity clearly started to rise. With the addition of only 0.5 mM Cu2+, laccase activity increased by more than 2-fold. The most significant increase was observed at 1 mM Cu2+, reaching a peak activity of 381.1 U/L approximately 4.6 times higher than that in control flasks. As the amount of copper in the culture media was further elevated, the increasing trend was interrupted; as in 1.5 mM and 2 mM Cu2+, the extracellular laccase activity began to slightly decline, reaching 371.85 U/L and 290.43 U/L, respectively. The changes in laccase activity are illustrated in Fig. 1.
Fig. 1.
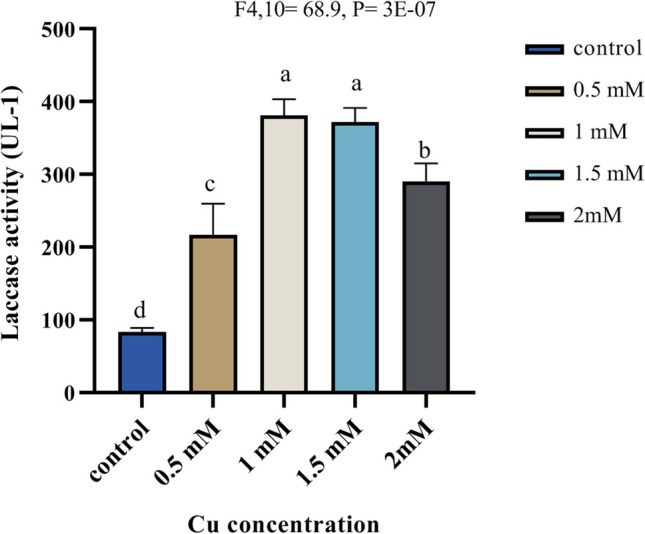
Influence of various Cu concentrations on extracellular laccase activity (enzyme activity units per liter of culture). Vertical bars represent the standard deviation of three independent replicates
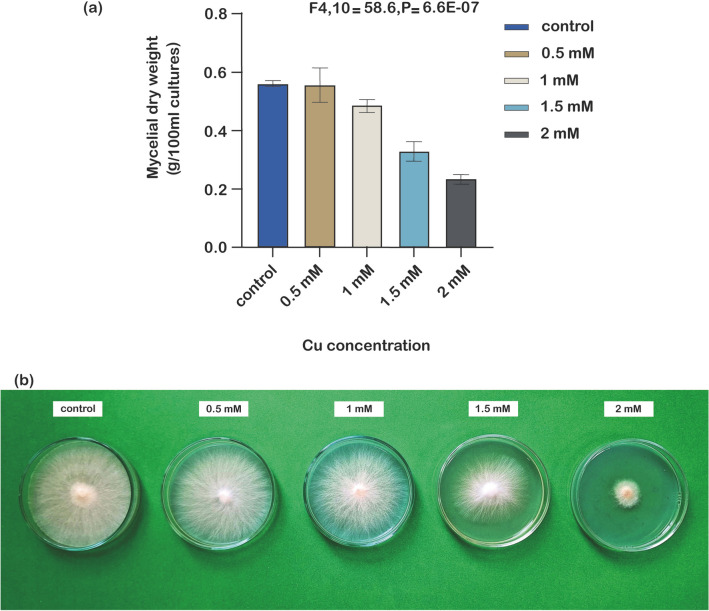
To investigate the potential impact of copper as a heavy metal on the growth of P. eryngii KS004, the dry weight of fungal cultures exposed to escalating Cu2+ amounts was measured. As depicted in Fig. 2, the addition of copper to P. eryngii KS004 cultures resulted in slower mycelial growth. Eight days after the addition of metal, the average dry weight of the control group increased by 0.56 g per 100 mL of culture. While 0.5 mM Cu2+ did not significantly affect fungal biomass, mycelial growth started to decelerate with increasing copper concentration, reaching only 0.33 g and 0.23 g per 100 mL at 1.5 mM and 2 mM Cu2+, respectively.
Fig. 2.
Impact of copper sulfate on mycelial growth of P. eryngii KS004. a Changes in dry weight of mycelia cultured on PDB medium. Vertical bars represent the standard deviation of three independent replicates. b Mycelial growth of P. eryngii KS004 on PDA culture medium with different concentrations of Cu2+
To explore the impact of copper on laccase gene transcription, 5-day-old P. eryngii cultures were exposed to various Cu2+ concentrations. After a 3-day incubation period, the transcription levels of the laccase gene pel3 were assessed through qRT-PCR. As depicted in Fig. 3, copper significantly stimulated the transcription of the pel3 gene in P. eryngii KS004. While the transcription level of this laccase gene was notably low in cultures without any inducer, the transcription levels of the pel3 gene subjected to 0.5 mM Cu2+ began to gradually increase. The pel3 gene transcription continued to rise consistently with escalating Cu2+ amounts, reaching 6.6-fold at 1 mM and over 13-fold in 1.5 and 2 mM CuSO4 treatments. It is worth noting that, despite the continuous increase in pel3 gene transcription, the rate of induction slowed down at Cu2+ concentrations higher than 1.5 mM.
Fig. 3.
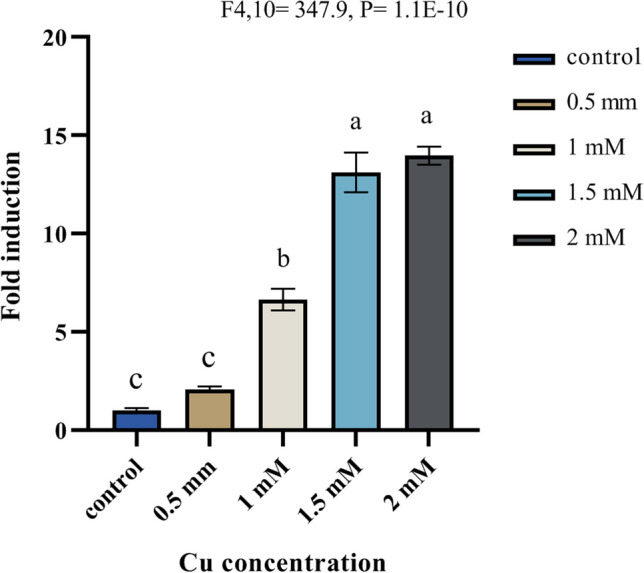
Relative induction effect of copper on pel3 laccase gene transcription
This study examined the impact of Cu2+ as a potential inducer on total production of extracellular laccase and how this heavy metal affects pel3 gene expression on transcriptional level in liquid cultures of P. eryngii KS004. The results indicated that the addition of copper to actively growing P. eryngii cultures could lead to a significant increase in transcription of pel3 gene, with the effect of copper being concentration-dependent. Moreover, it was demonstrated that the cultures supplemented with copper experienced an enhancement in extracellular laccase activity; however, higher Cu2+ concentrations curtailed mycelial growth. Consequently, while pel3 laccase gene transcription displayed a notable increase at higher Cu2+ concentrations, the increase in extracellular laccase activity followed a different pattern, reaching its peak at 1 mM Cu2+ and declining at higher copper concentrations.
Discussion
Extracellular laccases are typically produced in limited quantities during secondary metabolism in fungi. Amplifying laccase production becomes imperative for the enzyme’s industrial applications. Laccase transcription has been observed to be regulated by metal ions [20], aromatic compounds [6], and nitrogen and carbon sources [21] in culture media. The induction of laccase by copper has been documented in various fungi [12, 15, 17]. Copper’s influence on transcript levels has been reported in Trametes versicolor [22], Ceriporiopsis subvermispora [23], Pleurotus ostreatus [24], Pleurotus sajor-caju [21], Trametes pubescens [20], and Aspergillus flavus [25], although the degree of induction in transcription varies among different species .This study marks the first report of copper-induced laccase gene transcription for the pel3 gene of P. eryngii. Our findings suggest that copper regulates pel3 gene expression at the transcriptional level, with the impact of Cu2+ being dose-dependent. Two putative metal-responsive elements (MREs), along with the copper-sensing sequence and yeast copper-response element found in the promoter region of the pel3 gene [14], might contribute to the induction of pel3 gene transcription by Cu2+. Our findings align with similar findings in other fungi, particularly those within the Pleurotus genus.
This study also delved into the effect of copper on laccase activity. P. eryngii KS004 exhibited laccase activity in both the absence and presence of Cu2+, but the addition of copper to the culture media significantly augmented laccase activity, reaching its peak at 1 mM Cu2+ concentration. Baldrian and Gabriel (2002) reported analogous results for copper induction in Pleurotus ostreatus within a nitrogen-limited liquid medium. Their findings proposed that the increased laccase activity upon CuSO4 addition stems from both heightened laccase production through improved expression of laccase genes and the stabilization of the enzyme in the extracellular environment [26]. Results from Palmieri et al. also indicated that the inclusion of 1 mM Cu2+ led to a decrease in extracellular proteolytic activity, thereby hindering laccase degradation [24].
Copper concentration is also variable in studies, as exemplified by Zhu et al. [9] and Baldrian and Gabriel [26], who explored CuSO4 effects ranging from 1 to 5 mM. Gomaa and Momtaz [25] employed 1, 5, and 10 mM for Aspergillus flavus, and Yang et al. [15] assessed 0.5 and 1 mM for copper induction in Trametes velutina. To have an accurate picture of the effect of copper on laccase induction in P. eryngii KS004, we examined four distinct copper concentrations: 0.5, 1, 1.5, and 2 mM. Previous investigations have shown that Cu can hinder mycelial growth in select Pleurotus species [17, 27], aligning with our findings. It has been suggested that concentrations beyond the fungal tolerance inhibit fungal growth as a result of oxidative stress that is extremely toxic to fungal cells [16, 28, 29]. Consequently, the inhibitory impact of copper on growth should be factored in when interpreting extracellular laccase activity results.
It has been suggested that fungi produce laccases to scavenge reactive oxygen species (ROS) in order to protect themselves from oxidative stress caused by copper [16, 30] and as a defense mechanism against this heavy metal [5]. Considering the results obtained in the present study, the elevated gene expression and enzyme activity despite the lowered growth, facing stress conditions caused by copper seems to be the case for P. eryngii KS004.
Conclusion
The outcomes presented in this study show that Pleurotus eryngii KS004 laccase is not only constitutive but also inducible and establish a link between laccase enzyme production in this fungus and the presence of Cu2+ ions in culture media. This underscores that introducing this metal at low concentrations can significantly induce laccase gene transcription. Considering the toxic and inhibitory effect of copper on fungal growth and increased pel3 gene transcription, it is suggested that pel3 is a stress-regulated gene in P. eryngii KS004, although further investigations into this are required. Our result seen as a whole aids in comprehending laccase gene regulation within Pleurotus eryngii KS004 through a variety of cis-acting elements and offers a promising approach to enhance native laccase enzyme production for biotechnological applications.
Funding
This work was partly supported by the research council of Ferdowsi University of Mashhad (Project number 3/47998).
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villani A, Galli E, Paciolla C, Stea G, Logrieco AF, Siniscalco C, Mulè G, Susca A. Molecular characterization of Pleurotus eryngii varieties occurring in Italy. Sydowia. 2015;67:37. doi: 10.12905/0380.sydowia67-2015-0033. [DOI] [Google Scholar]
- 2.Janusz G, Pawlik A, Świderska-Burek U, Polak J, Sulej J, Jarosz-Wilkołazka A, Paszczyński A. Laccase properties, physiological functions, and evolution. Int J Mol Sci. 2020;21(3):966. doi: 10.3390/ijms21030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mate DM, Alcalde M. Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol. 2017;10(6):1457–1467. doi: 10.1111/1751-7915.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugnari T, Braga DM, dos Santos CS, Torres BH, Modkovski TA, Haminiuk CW, Maciel GM. Laccases as green and versatile biocatalysts: from lab to enzyme market—an overview. Bioresour Bioprocess. 2021;8(1):1–29. doi: 10.1186/s40643-021-00484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Research 2014. 10.1155/2014/163242 [DOI] [PMC free article] [PubMed]
- 6.Terrón MC, González T, Carbajo JM, Yagüe S, Arana-Cuenca A, Téllez A, Dobson AD, González AE. Structural close-related aromatic compounds have different effects on laccase activity and on lcc gene expression in the ligninolytic fungus Trametes sp. I-62. Fungal Genet Biol. 2004;41(10):954–962. doi: 10.1016/j.fgb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Dhawan S, Kuhad RC. Effect of amino acids and vitamins on laccase production by the bird’s nest fungus Cyathus bulleri. Bioresour Technol. 2002;84(1):35–38. doi: 10.1016/S0960-8524(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 8.Ardon O, Kerem Z, Hadar Y. Enhancement of laccase activity in liquid cultures of the ligninolytic fungus Pleurotus ostreatus by cotton stalk extract. J Biotechnol. 1996;51(3):201–207. doi: 10.1016/S0168-1656(96)01597-0. [DOI] [Google Scholar]
- 9.Lettera V, Del Vecchio C, Piscitelli A, Sannia G. Low impact strategies to improve ligninolytic enzyme production in filamentous fungi: the case of laccase in Pleurotus ostreatus. Comptes Rendus Biologies. 2011;334(11):781–788. doi: 10.1016/j.crvi.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Monjaraz WS, Caudillo-Pérez C, Salazar-Sánchez PU, Macías-Sánchez KL. Influence of iron and copper on the activity of laccases in Fusarium oxysporum f. sp. lycopersici. Braz J Microbiol. 2018;49:269–275. doi: 10.1016/j.bjm.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durán-Sequeda D, Suspes D, Maestre E, Alfaro M, Perez G, Ramírez L, Pisabarro AG, Sierra R. Effect of nutritional factors and copper on the regulation of laccase enzyme production in Pleurotus ostreatus. J Fungi. 2021;8(1):7. doi: 10.3390/jof8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V. Induction and transcriptional regulation of laccases in fungi. Curr Genom. 2011;12(2):104–112. doi: 10.2174/138920211795564331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz C, Guillen F, Martinez AT, Martinez MJ. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol. 1997;34:1–5. doi: 10.1007/s002849900134. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez E, Ruiz-Dueñas FJ, Kooistra R, Ram A, Martínez ÁT, Martínez MJ. Isolation of two laccase genes from the white-rot fungus Pleurotus eryngii and heterologous expression of the pel3 encoded protein. J Biotechnol. 2008;134(1-2):9–19. doi: 10.1016/j.jbiotec.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Wei F, Zhuo R, Fan F, Liu H, Zhang C, Ma L, Jiang M, Zhang X. Enhancing the laccase production and laccase gene expression in the white-rot fungus Trametes velutina 5930 with great potential for biotechnological applications by different metal ions and aromatic compounds. PLoS One. 2013;8(11):e79307. doi: 10.1371/journal.pone.0079307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buddhika UV, Savocchia S, Steel CC. Copper induces transcription of BcLCC2 laccase gene in phytopathogenic fungus, Botrytis cinerea. Mycology. 2021;12(1):48–57. doi: 10.1080/21501203.2020.1725677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu C, Bao G, Huang S. Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol Biotechnol Equip. 2016;30(2):270–276. doi: 10.1080/13102818.2015.1135081. [DOI] [Google Scholar]
- 18.Manubens A, Canessa P, Folch C, Avila M, Salas L, et al. Manganese affects the production of laccase in the basidiomycete Ceriporiopsis subvermispora. FEMS Microbiol Lett. 2007;275:139–145. doi: 10.1111/j.1574-6968.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Lee HS, Kwon HJ, Patnaik BB, Nam KW, Han YS, Bang IS, Han MD. Effects of different selenium levels on growth and regulation of laccase and versatile peroxidase in white-rot fungus, Pleurotus eryngii. World J Microbiol Biotechnol. 2014;30:2101–2109. doi: 10.1007/s11274-014-1636-x. [DOI] [PubMed] [Google Scholar]
- 20.Galhaup C, Wagner H, Hinterstoisser B, Haltrich D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzym Microb Technol. 2002;30(4):529–536. doi: 10.1016/S0141-0229(01)00522-1. [DOI] [Google Scholar]
- 21.Soden DM, Dobson AD. Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology. 2001;147(7):1755–1763. doi: 10.1099/00221287-147-7-1755. [DOI] [PubMed] [Google Scholar]
- 22.Collins PJ, Dobson A. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol. 1997;63(9):3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Álvarez JM, Canessa P, Mancilla RA, Polanco R, Santibáñez PA, Vicuña R. Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copper-fist transcription factor. Fungal Genet Biol. 2009;46(1):104–111. doi: 10.1016/j.fgb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol. 2000;66(3):920–924. doi: 10.1128/AEM.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomaa OM, Momtaz OA. Copper induction and differential expression of laccase in Aspergillus flavus. Braz J Microbiol. 2015;46:285–292. doi: 10.1590/S1517-838246120120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldrian P, Gabriel J. Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol Lett. 2002;206(1):69–74. doi: 10.1111/j.1574-6968.2002.tb10988.x. [DOI] [PubMed] [Google Scholar]
- 27.Mohamadhasani F, Rahimi M. Growth response and mycoremediation of heavy metals by fungus Pleurotus sp. Sci Rep. 2022;12(1):19947. doi: 10.1038/s41598-022-24349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzl C, Enrich J, Ebner H, Dallinger R, Krumschnabel G. Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology. 2004;196(1-2):57–64. doi: 10.1016/j.tox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Kannaiyan R, Mahinpey N, Mani T, Martinuzzi RJ, Kostenko V. Enhancement of Dichomitus squalens tolerance to copper and copper-associated laccase activity by carbon and nitrogen sources. Biochem Eng J. 2012;67:140–147. doi: 10.1016/j.bej.2012.06.007. [DOI] [Google Scholar]
- 30.Schouten A, Wagemakers L, Stefanato FL, Kaaij RM, Kan JA. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol Microbiol. 2002;43(4):883–894. doi: 10.1046/j.1365-2958.2002.02801.x. [DOI] [PubMed] [Google Scholar]