Abstract
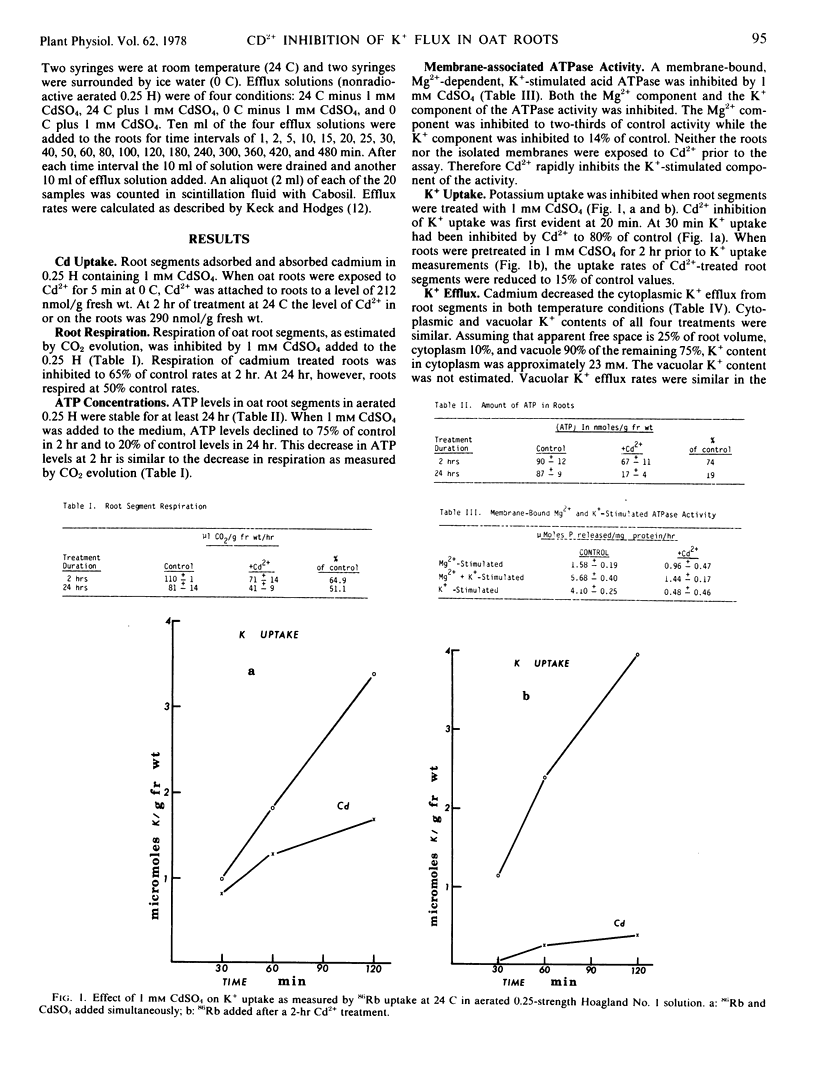
Segments of oat (Avena sativa L.) roots which had been exposed to 1 millimolar CdSO4 in quarter-strength Hoagland No. 1 solution exhibited decreased respiratory rates, ATP levels, membrane-bound ATPase activity, and reduced K+ fluxes. Respiration and ATP levels were decreased after a 2-hour treatment with 1 millimolar CdSO4 to 65 and 75%, respectively, of control rates. A membrane-bound, Mg2+-dependent, K+-stimulated acid ATPase was rapidly inhibited to 12% of control activity in the presence of 1 millimolar CdSO4. Potassium uptake into root segments was inhibited to 80% of control values after 30 minutes in the presence of CdSO4. A 2-hour pretreatment of root segments with CdSO4 inhibited K+ uptake to 15% of control values. Cytoplasmic K+ efflux was inhibited with 1 millimolar CdSO4.
The rates and the degree of Cd2+ inhibition of the parameters listed above suggest that one of the first sites of Cd2+ action is the plasmalemma K+ carrier (ATPase) in oat roots.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Cram W. J. Respiration and energy-dependent movements of chloride at plasmalemma and tonoplast of carrot root cells. Biochim Biophys Acta. 1969 Mar 11;173(2):213–222. doi: 10.1016/0005-2736(69)90105-9. [DOI] [PubMed] [Google Scholar]
- Cutler J. M., Rains D. W. Characterization of cadmium uptake by plant tissue. Plant Physiol. 1974 Jul;54(1):67–71. doi: 10.1104/pp.54.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Steady State Sodium and Rubidium Effluxes in Pisum sativum Roots. Plant Physiol. 1967 May;42(5):685–690. doi: 10.1104/pp.42.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A., Epstein E. Transport of potassium and rubidium in plant roots: the significance of calcium. Plant Physiol. 1970 May;45(5):639–641. doi: 10.1104/pp.45.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M. G., Cross C. E. Pulmonary alveolar macrophage. Oxidative metabolism of isolated cells and mitochondria and effect of cadmium ion on electron- and energy-transfer reactions. Biochemistry. 1971 Nov;10(23):4176–4185. doi: 10.1021/bi00799a004. [DOI] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARLS R. L., PETERS J. M., SANADI D. R. alpha-Ketoglutaric dehydrogenase. X. On the mechanism of dihydrolipoyl dehydrogenase reaction. J Biol Chem. 1961 Aug;236:2317–2322. [PubMed] [Google Scholar]



