Abstract
Phosphate-starved Chlorella pyrenoidosa cells formed polyphosphate bodies (PB) upon transfer into nutrient solutions containing phosphate and potassium, or another monovalent cation, such as Na+, NH4+, Li+, or Rb+. The phenomenon was studied by chemical analyses, light microscopy, and electron microscopy.
When the P-starved cells were transferred into a complete nutrient solution containing 100 micromolar P, they accumulated large quantities of P and K within several hours. The accumulation was accompanied by a corresponding appearance of PB in the cells. The absence of K from the medium prevented appreciable P accumulation and PB formation, but omitting Ca or Mg did not.
The P-starved cells exposed to a simple solution of at least 20 micromolar H3PO4 and 100 micromolar KHCO3 responded in a similar manner as the cells exposed to the complete nutrient solution. However, the PB appeared structurally different.
It is proposed that monovalent cations are essential for PB formation in C. pyrenoidosa. K is suggested to be a major component of PB formed in K-sufficient media.
Full text
PDF




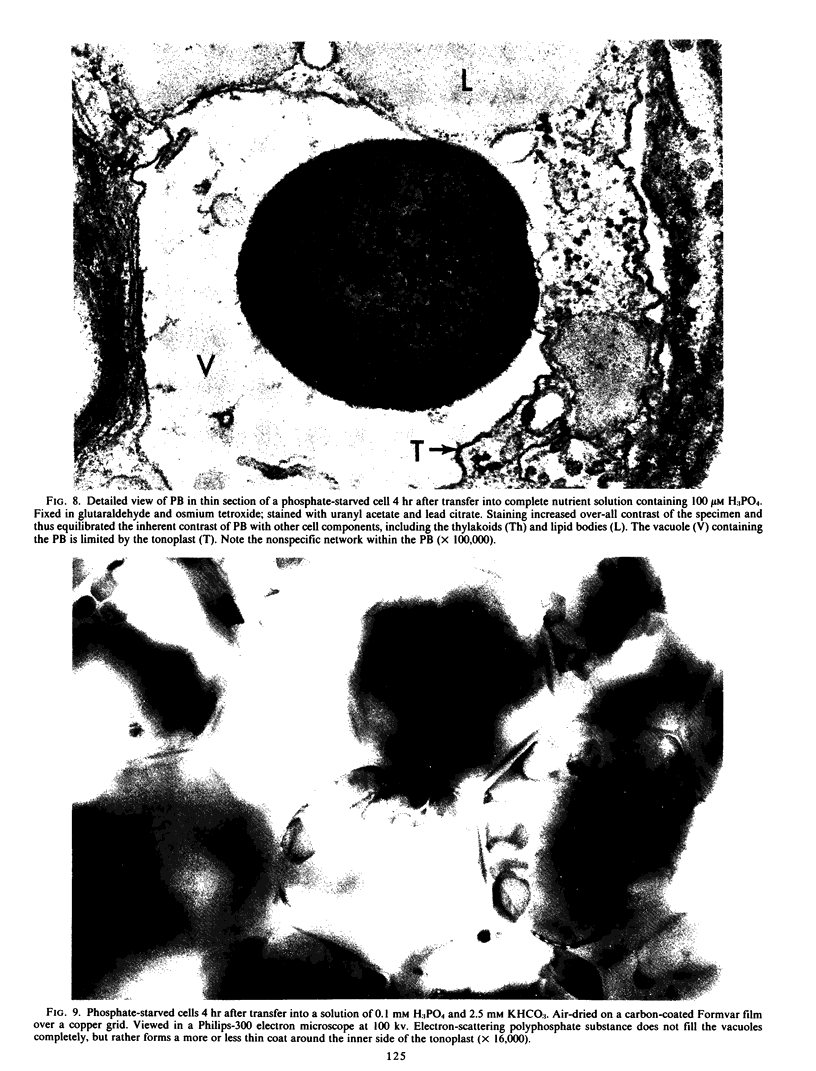

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson A. W., Jr, John P. C., Gunning B. E. The growth and division of the single mitochondrion and other organelles during the cell cycle of Chlorella, studied by quantitative stereology and three dimensional reconstruction. Protoplasma. 1974;81(1):77–109. doi: 10.1007/BF02055775. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Avigad G. Structures containing polyphosphate in Micrococcus lysodeikticus. J Bacteriol. 1968 Aug;96(2):544–553. doi: 10.1128/jb.96.2.544-553.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M. Accumulation of inorganic polyphosphate in mutants of Neurospora crassa. Biochim Biophys Acta. 1960 Dec 4;45:172–188. doi: 10.1016/0006-3002(60)91438-4. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYACHI S. Inorganic polyphosphate in spinach leaves. J Biochem. 1961 Oct;50:367–371. doi: 10.1093/oxfordjournals.jbchem.a127459. [DOI] [PubMed] [Google Scholar]
- Terry K. R., Hooper A. B. Polyphosphate and orthophosphate content of Nitrosomonas europaea as a function of growth. J Bacteriol. 1970 Jul;103(1):199–206. doi: 10.1128/jb.103.1.199-206.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDRA A. Metachromatic granules of microorganisms. J Bacteriol. 1959 Nov;78:664–670. doi: 10.1128/jb.78.5.664-670.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]









