Abstract
Although highly effective on average, exposure-based treatments do not work equally well for all patients with anxiety disorders. The identification of pre-treatment response-predicting patient characteristics may enable patient stratification. Preliminary research highlights the relevance of inhibitory fronto-limbic networks as such. We aimed to identify pre-treatment neural signatures differing between exposure treatment responders and non-responders in spider phobia and to validate results through rigorous replication. Data of a bi-centric intervention study comprised clinical phenotyping and pre-treatment resting-state functional connectivity (rsFC) data of n = 79 patients with spider phobia (discovery sample) and n = 69 patients (replication sample). RsFC data analyses were accomplished using the Matlab-based CONN-toolbox with harmonized analyses protocols at both sites. Treatment response was defined by a reduction of >30% symptom severity from pre- to post-treatment (Spider Phobia Questionnaire Score, primary outcome). Secondary outcome was defined by a reduction of >50% in a Behavioral Avoidance Test (BAT). Mean within-session fear reduction functioned as a process measure for exposure. Compared to non-responders and pre-treatment, results in the discovery sample seemed to indicate that responders exhibited stronger negative connectivity between frontal and limbic structures and were characterized by heightened connectivity between the amygdala and ventral visual pathway regions. Patients exhibiting high within-session fear reduction showed stronger excitatory connectivity within the prefrontal cortex than patients with low within-session fear reduction. Whereas these results could be replicated by another team using the same data (cross-team replication), cross-site replication of the discovery sample findings in the independent replication sample was unsuccessful. Results seem to support negative fronto-limbic connectivity as promising ingredient to enhance response rates in specific phobia but lack sufficient replication. Further research is needed to obtain a valid basis for clinical decision-making and the development of individually tailored treatment options. Notably, future studies should regularly include replication approaches in their protocols.
Subject terms: Predictive markers, Biomarkers
Introduction
Even though exposure-based cognitive behavioral therapy (CBT) provides a powerful approach for the treatment of anxiety disorders, a substantial proportion of patients does not respond in a clinically meaningful way [1, 2]. The combination of high prevalence of anxiety disorders [3] with high rates of non-responders and disorder-related high socio-economic costs [4] urges clinical research to identify pre-treatment patient characteristics associated with treatment response. Knowledge about these characteristics may enable patient stratification, the personalized and tailored application of modified or add-on treatments and thus the improvement of response rates.
Exposure-based CBT owes its efficacy to the extensive scientific efforts that have been made with respect to the underlying neurobiology of anxiety disorders [5] and the way how fear extinction alters those neural substrates [6, 7]. In line with calls for more translationally oriented clinical research [8–10], the present investigation aims to identify pre-treatment neural signatures of treatment response.
To investigate neural markers for treatment response, we selected spider phobia as a prototypical model of fear circuitry dysfunctions in anxiety disorders. Its neurocircuitry substantially overlaps with structures commonly referred to the defensive system network within the whole spectrum of anxiety disorders [5]. Furthermore, this neurocircuitry highly corresponds with the one involved in fear extinction, which is discussed as a core mechanism of action underlying exposure treatment [7]. A network for defensive mobilization comprising the amygdala, medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), insula, and thalamus has been shown to be hyperresponsive in specific phobia patients compared to healthy controls [11–19]. This hyperactivation is accompanied by decreased activation in medial and ventral portions of the prefrontal cortex [11, 14, 20–22] thus underlying emotion regulation deficits, including deficient top-down fear inhibition [11]. Regarding the animal subtype of specific phobia, there is especially high consistency within the results [11, 18].
Neurofunctional studies on pre-treatment neural signatures in anxiety disorders are accumulating within the last years [23–29] and hint at potential moderators of treatment outcome in anxiety disorders [24, 30, 31]. Recent reviews and meta-analyses identified activation differences in regions of salience and interoceptive processing, namely the right inferior gyrus and the anterior insular cortex, as well as the dorsomedial prefrontal cortex and the dorsal anterior cingulate cortex as relevant predictors for treatment outcome in anxiety disorders [24, 26, 31]. Additionally, one meta-analysis found evidence for an activation decrease from pre- to post-treatment in symptom provocation paradigms in the left anterior cingulate, the bilateral middle frontal gyrus and the right insula [32]. However, there is little knowledge on functional connections between the mentioned neural structures.
The few existing studies on (functional) connectivity in specific phobia point to a decoupling of prefrontal structures and the defensive system during stimulus presentation [33, 34]. This was also demonstrated for other anxiety disorders [35–42]. Stefanescu et al. [34] interpreted the decoupling in terms of deficient emotion regulation. Corroborating these results, Böhnlein et al. [43] found a hypoconnectivity of the amygdala within regions involved in the salience network in patients with spider phobia compared to healthy controls during disorder-unspecific emotion processing. Furthermore, Scharmüller et al. [44] have demonstrated that fronto-striatal connectivity is decreased in specific phobia compared to healthy controls suggesting an altered information flow in those patients. However, all studies used task-based approaches, leaving open the question as to which extent these alterations may represent overarching signatures. Intrinsic brain connectivity investigated via fMRI in a resting condition is an easy-to-acquire and paradigm-independent measure. The moderating role of resting-state functional connectivity (rsFC) with respect to treatment response in specific phobia patients remains unknown. Analyzing rsFC may therefore constitute an innovative approach to better understand large-scale intrinsic brain networks conferring treatment response towards exposure treatment in spider phobia.
The present investigation aims at identifying pre-treatment rsFC signatures that differ between exposure treatment responders and non-responders in spider phobia. To contribute to the conquest of the so-called ‘replication crisis’ [45, 46] we applied a rigorous replication design by a) trying to replicate results by another team using the same data (cross-team replication) and b) trying to replicate discovery sample results in the independent replication sample (cross-site replication).
We hypothesized (1) that treatment responders exhibit enhanced negative connectivity between prefrontal areas and defensive-system networks compared to non-responders. This should be pronounced in the amygdala and the ACC. (2) We assumed that comprehensive cross-team replication is possible in the case of harmonized analysis protocols. Cross-site replication would strengthen the validity of the results.
Methods
Study design and sample description
Analyses are part of the “SpiderVR” study, which was conducted as a bicentric study in Würzburg and Münster within the German Collaborative Research Center 58 “Fear, Anxiety, Anxiety Disorders”.
Spider phobia patients were recruited at both sites. Fulfillment of DSM-IV-TR diagnostic criteria for specific phobia, animal subtype was assessed via the structured clinical interview for DSM-IV-TR (SCID Axis I; German version [47]). Patients had to be right-handed, of Caucasian descent, without an acute or lifetime diagnosis of comorbid mental disorders except for mild to moderate depression (unless currently treated psychotherapeutically or pharmacologically) and further specific phobias of the animal subtype. A current pregnancy or fulfillment of MRI-contraindications led to exclusion. All patients needed to reach a minimum Spider Phobia Questionnaire (SPQ, [48]) score of 20 to be included (see Supplementary 1 for detailed description of clinical assessments). We included n = 79 patients in the analysis in Würzburg and n = 69 in Münster (for detailed sample description see Supplementary 2).
The study has been conducted in accordance with the Declaration of Helsinki and has been approved by the ethics committees of both participating medical faculties. All participants provided written informed consent and received financial compensation for participation. Furthermore, the study has been pre-registered at ClinicalTrials.gov (registration ID: NCT03208400).
Study protocol and response assessment
The study protocol is given in Schwarzmeier et al. [49]. Briefly, it comprised five visits (T1: clinical diagnostics and baseline measures, T2: Magnetic Resonance Imaging (MRI), T3: exposure treatment via virtual reality (VRET), T4: clinical post-assessment; T5: 6-month follow-up). VRET comprised psychoeducative information regarding the rationale of exposure treatment and a one-session exposure intervention in virtual reality. Treatment efficacy can be found in Leehr et al. [50]. The primary outcome was defined by a reduction of the sum score of >30% in the German version of the SPQ [48] from pre- to post-treatment. Further, we included a Behavior Avoidance Test (BAT) as a behavioral measure of avoidance as secondary outcome (a reduction of >50% in the initial minimal distance of the patient towards a living bird spider was defined as an indicator for response). The mean within-session fear reduction, resulting from the difference values between maximal and minimal fear in each of the 5 VRET scenarios (0–100) was used as process measure based on prominent learning theories on exposure [51]. Treatment details can be found in Supplementary 3.
MRI measurements and preprocessing
MR images were acquired using 3-T Siemens Skyra (WZ) and 3-T Siemens Prisma (MS). A T1 structural image was collected via magnetization prepared rapid gradient echo (MPRAGE; matrix = 256 × 256, slices = 176, FOV = 256, voxel size = 1 × 1 × 1 mm, WZ: TE = 2.26 ms, TR = 1.9 s, flip angle = 9°, MS: TE = 2.28 ms, TR 0 2.13 s, flip angle = 8°). During the resting state measurement, lasting 8 min (eyes closed, light switched off), functional images were acquired in ascending order using a T2* weighted echo planar imaging sequence (EPI BOLD; matrix = 64 × 64, slices = 33, FOV = 210, voxel size = 3.3 × 3.3 × 3.8 mm, slice thickness = 3.8 mm, slice gap = 10%, WZ: TE = 30 ms, TR = 2.0 s, flip angle = 90°, MS: TE = 29 ms, TR = 2.0 s, flip angle = 90°). Each slice covered the whole brain and was positioned transaxially parallel to the anterior-posterior commissural line with a tilted angle of 20°.
At each site a harmonized analysis pathway was performed, with different Matlab versions between sites. Structural and functional images were preprocessed in CONN 18a (www.nitrc. org/projects/conn, RRID: SCR_009550) implemented in MATLAB (WZ: R2012b; MS: R2019ba; MathWorks, Natick, Mass) and SPM12 (www.fil.ion.ucl.ac.uk). Due to potential inhomogeneities in initial magnetization the first five functional volumes were discarded. Besides functional realignment and unwarping, preprocessing (CONN default MNI pipeline) included slice-timing correction, structural segmentation (gray matter, white matter, cerebrospinal fluid) and normalization to MNI space, functional normalization to MNI space, and smoothing (5 mm FWHM Gaussian filter). As spurious correlations and thus functional connectivity may be easily introduced by head motions, we used the Artifact Detection Tools (ART, www.nitrc.org/projects/artifact_detect) implemented in CONN to identify outlier images (see Supplementary 2 for the quality control procedure). Patients with less than 10% invalid scans were included and invalid scans were entered as individual 1st-level covariate (scrubbing). For each region of interest (ROI; Automated anatomical labeling atlas, aal, https://www.gin.cnrs.fr/en/tools/aal/, [52]) an average timeseries was extracted from the unsmoothed dataset. Realignment parameters together with their first-order temporal derivatives, patient-specific artefactual covariates (ART-based scrubbing), the effect of rest, and white matter and CSF BOLD time series of each participant were removed from the BOLD signal by applying linear regression to reduce noise. Subsequently, we applied a bandpass-filter to the resulting BOLD time series (bounding box: 0.01Hz- 0.1 Hz) and checked the functional correlations for normal distribution.
Statistical analyses
On the 1st-level, we performed ROI-to-ROI as well as Seed-to-Voxel analyses of functional connectivity by computing bivariate correlation maps weighted by the hemodynamic response function. Baseline SPQ score, age and duration of exposure were included as 2nd-level covariates of no interest, as there were site and/or group differences. Effects of response group and site were compared using an ANOVA.
For seed-based 2nd-level analyses, we computed the main effect of the respective bilateral seed regions. The cluster-threshold was set to p < 0.05 (FDR). The height-threshold was set to p < 0.001.
Due to the hypothesis of deficient regulation of the defensive system network via prefrontal structures in non-responders, predefined AAL ROIs were grouped into “defensive system related ROIs” (amygdala, insula, ACC, hippocampus, and thalamus) and “executive control related ROIs” (superior (SFG) and middle frontal gyrus (MFG; including their orbital and medial parts), the IFG pars opercularis, triangularis and orbitalis and the gyrus rectus). For seed-based analyses, we focused on key regions of the defensive system being of utmost relevance in anxiety disorders (the amygdala, the ACC and the hippocampus).
Analyses were performed according to the following three-step protocol: (1) analysis of the presented ROI-to ROI and seed-to-voxel approach on the WZ data (discovery sample) by the WZ team based on three different response criteria, namely SPQ-response (SPQ pre to post reduction> 30%), BAT-response (BAT pre to post reduction> 50%) and low within-session fear reduction (low WS-ext) versus high within-session fear reduction (high WS-ext) using median split); (2) cross-team replication, as it has been shown, that it is by no means trivial to replicate results using the same methods on the same data, but by another team (i.e., analytical reproducibility, [53]): replication of findings from (1) on the WZ data by the MS team; Thus, the aim was to rigorously test our own reporting practices and the effect of different computing environments. (3) cross-site replication: replication from (1) on the MS dataset, to test replicability of results. Additional exploratory analyses using the combined sample (MS and WZ) can be found in Supplementary 7.
Results
Sample characteristics
For detailed information regarding the sample characteristics refer to Table 1. The replication sample did not differ from the discovery sample regarding any relevant variable, except for age, age of onset (being correlated with age) and duration of VRET. Besides these differences, clinical characteristics reflected a high homogeneity of the samples. The one session exposure treatment has proved effective in reducing self-reported symptoms and avoidance behavior [50].
Table 1.
Demographic and clinical characteristics of the included sample at pre-treatment, Means (SD), except where noted. The categorization as responders (vs. non-responders) is based on the primary outcome measure, i.e., SPQ reductions of 30% from pre- to post-treatment assessment.
| Variables | Sample Münster | Sample Würzburg | Differences between sites | Difference between response groups at baseline | ||||
|---|---|---|---|---|---|---|---|---|
| All (n = 69) | Responder (n = 33) | Non-responder (n = 36)a | All (n = 79) | Responder (n = 49)b | Non-responder (n = 30)c | Test statistic | Test statistic | |
| Demographic characteristics | ||||||||
| Female gender [n (%)] | 59 (85.5) | 29 (87.9) | 30 (83.3) | 68 (86.10) | 41 (83.7) | 27 (90.0) | 0.01 | 0.030 |
| Age (years) | 25.83 (6.56) | 24.33 (4.85) | 27.19 (7.62) | 29.16 (9.55) | 27.16 (7.47) | 32.43 (11.63) | 9.561* | 9.440* |
| Years of education | 15.22 (2.48) | 15.09 (2.35) | 15.34 (2.63) | 14.35 (3.32) | 14.22 (3.37) | 14.57 (3.28) | 2.545 | 0.206 |
| Pre-treatment Clinical characteristics | ||||||||
| Age of onset | 6.09 (5.08) | 5.64 (4.54) | 6.50 (5.55) | 8.43 (4.06) | 9.21 (4.30) | 7.14 (3.30) | 7.189* | |
| SPQ | 22.59 (1.90) | 22.82 (1.81) | 22.39 (1.99) | 23.17 (2.37) | 23.73 (2.32) | 22.26 (2.12) | 0.757 | 7.808* |
| BAT final distance | 173.07 (70.64) | 166.55 (70.24) | 179.06 (71.46) | 172.51 (61.55) | 181.99 (60.50) | 157.02 (61.09) | 0.055 | 0.334 |
| Comorbid major depression [n (%)] | 1 (1.40) | 0 (0.00) | 1 (2.80) | 1 (1.30) | 0 (0.00) | 1 (3.30) | 2.028 | 2.550 |
| STAI-Trait | 35.10 (7.80) | 34.64 (7.87) | 35.54 (7.82) | 36.85 (9.08) | 37.24 (9.07) | 36.20 (9.20) | 1.613 | 0.011 |
| BDI-II total | 3.18 (3.46) | 2.67 (2.62) | 3.66 (4.12) | 3.68 (4.39) | 3.67 (4.38) | 3.70 (4.47) | 0.855 | 0.620 |
| Scenarios completed | 4.33 (1.20) | 4.30 (1.26) | 4.36 (1.15) | 4.67 (1.01) | 4.73 (0.91) | 4.57 (1.17) | 2.837 | 0.133 |
| Duration of VRET | 76.54 (22.79) | 74.94 (25.52) | 78.00 (20.24) | 87.25 (25.21) | 84.98 (24.41) | 90.97 (26.46) | 7.896* | 0.940 |
Test-statistic = χ2 for categorical and F for metric data; df = 141;
SPQ Spider Phobia Questionnaire, BAT Behavioral Avoidance Test (smaller values equal smaller distance of the spider to the patient), STAI-Trait Trait-version of the State-Trait Anxiety Inventory, BDI-II Beck Depression Inventory II.
*p < 0.01.
aYears of education, STAI-Trait and BDI-II total reported for n = 35;
bAge of onset reported for n = 48;
cAge of onset reported for n = 29.
Analyses on the Würzburg data set (discovery sample)
ROI-to-ROI approach
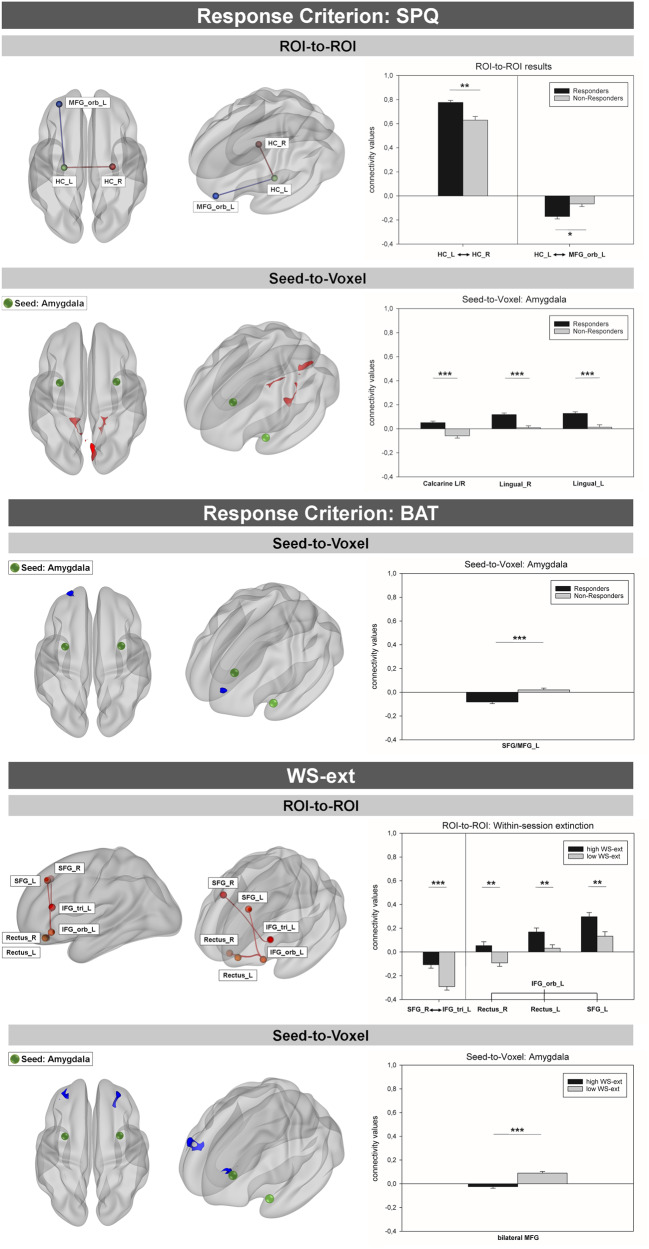
The comparison of SPQ-responders to SPQ-Non-responders revealed a differential connection of two brain regions to the left hippocampus: There was significantly increased negative functional connectivity between the orbital part of the left MFG and left hippocampus in responders, T(74) = −3.38, p < 0.05, d = −0.78. Furthermore, left and right hippocampus exhibited increased positive connectivity among responders, T(74) = 3.77, p < 0.01, d = 0.87 (see Fig. 1 and Table 2).
Fig. 1. Differential functional connectivity in SPQ- and BAT-responders vs. non-responders and high vs. low WS-ext groups as identified by ROI-to-ROI and Seed-to-Voxel analyses in the Würzburg sample.
Clusters/Edges in red indicate responders to exhibit stronger positive connectivity compared to non-responders. Clusters/Edges in blue indicate stronger negative connectivity in responders. The corresponding bar graphs show connectivity values (Pearson’s correlation coefficients extracted from the respective cluster(s)/edges. ROI-to-ROI: Spheres indicate ROIs. Edges indicate significant connectivity between ROIs. Seed-to-Voxel: Green spheres indicate seeds. SPQ Spider Phobia Questionnaire, BAT Behavior Avoidance Test, WS-ext within-session fear reduction, L left, R right, ROI Region of Interest, MFG_orb middle frontal gyrus pars orbitalis, HC Hippocampus, SFG superior frontal gyrus, MFG middle frontal gyrus, cluster threshold: p < 0.05 (FDR); height-threshold: p < 0.001 (uncorr.); *p < 0.05; **p < 0.01; ***p < 0.001.
Table 2.
Differential functional resting-state connectivity within SPQ-responders and non-responders (ROI-to-ROI and Seed-to-Voxel).
| Analyses discovery sample | Cross-team replication | Cross-site replication | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI-to-ROI | T | pFDR | T | pFDR | T | pFDR | |||||||||||||||||||
| t-contrast: resp > non-resp | |||||||||||||||||||||||||
| Hippocampus L ⟷ | Hippocampus R | 3.77 | <0.01 | Hippocampus L ⟷ | Hippocampus R | 3.83 | <0.01 | Middle frontal gyrus, medial part R ⟷ | Middle frontal gyrus, orbital part R | 3.53 | <0.05 | ||||||||||||||
| Inferior frontal gyrus, pars opercularis L ⟷ | Inferior frontal gyrus, pars triangularis R | 3.32 | <0.05 | Insula L ⟷ | Thalamus R | − 3.72 | <0.05 | ||||||||||||||||||
| t-contrast: non-resp > resp | |||||||||||||||||||||||||
| Hippocampus L ⟷ | Inferior frontal gyrus, pars orbitalis | 3.38 | <0.05 | Hippocampus L ⟷ | Inferior frontal gyrus, pars orbitalis | 3.65 | <0.05 | ||||||||||||||||||
| Seed-to-Voxel | Side | k | x | y | z | T | pFDR | Side | k | x | y | z | T | pFDR | Side | x | y | z | T | pFDR | |||||
| Amygdala | |||||||||||||||||||||||||
| t-contrast: resp > non-resp | |||||||||||||||||||||||||
| Cluster 1: | 174 | 6 | −88 | 6 | 4.85 | <0.001 | Cluster 1: | 137 | 6 | −86 | 6 | 3.20 | <0.05 | No differential connectivity | |||||||||||
| Calcarine | R | 99 | Calcarine | R | 88 | ||||||||||||||||||||
| Lingual gyrus | R | 46 | Lingual gyrus | R | 35 | ||||||||||||||||||||
| L | 17 | Calcarine | L | 12 | |||||||||||||||||||||
| Calcarine | L | 11 | Lingual gyrus | L | 2 | ||||||||||||||||||||
| AAL not-labeled | -- | 1 | |||||||||||||||||||||||
| Cluster 2: | 151 | −10 | −42 | −12 | 5.27 | <0.001 | |||||||||||||||||||
| Cerebellum 4/5 | L | 105 | |||||||||||||||||||||||
| Lingual gyrus | L | 25 | |||||||||||||||||||||||
| Fusiform gyrus | L | 14 | |||||||||||||||||||||||
| Vermis 4/5 | -- | 5 | |||||||||||||||||||||||
| Cerebellum 3 | L | 2 | |||||||||||||||||||||||
| Cluster 3: | 108 | 16 | −52 | −10 | 4.93 | <0.001 | |||||||||||||||||||
| Cerebellum 4/5 | R | 58 | |||||||||||||||||||||||
| Fusiform gyrus | R | 24 | |||||||||||||||||||||||
| Lingual gyrus | R | 17 | |||||||||||||||||||||||
| Cerebellum 6 | R | 9 | |||||||||||||||||||||||
| Seed-to-Voxel | Side | k | x | y | z | T | pFDR | Side | k | x | y | z | T | pFDR | Side | x | y | z | T | pFDR | |||||
| t-contrast: non-resp > resp | |||||||||||||||||||||||||
| No differential connectivity | No differential connectivity | ||||||||||||||||||||||||
| Seed-to-Voxel | Side | k | x | y | z | T | pFDR | Side | k | x | y | z | T | pFDR | Side | x | y | z | T | pFDR | |||||
| Anterior cingulate cortex | |||||||||||||||||||||||||
| t-contrast: resp > non-resp | |||||||||||||||||||||||||
| Cluster 1: | 158 | −14 | 28 | 10 | 6.32 | <0.001 | Cluster 1: | 175 | −12 | 28 | 8 | 3.2 | <0.001 | No differential connectivity | |||||||||||
| Anterior cingulate cortex | L | Anterior cingulate cortex | L | 24 | |||||||||||||||||||||
| R | R | 6 | |||||||||||||||||||||||
| Mid cingulate cortex | L | Mid cingulate cortex | L | 3 | |||||||||||||||||||||
| R | R | 3 | |||||||||||||||||||||||
| AAL not-labeled | -- | AAL not-labeled | -- | 139 | |||||||||||||||||||||
| t-contrast: non-resp > resp | |||||||||||||||||||||||||
| No differential connectivity | No differential connectivity | No differential connectivity | |||||||||||||||||||||||
| Hippocampus | |||||||||||||||||||||||||
| t-contrast: resp > non-resp | |||||||||||||||||||||||||
| Cluster 1: | 102 | 26 | −78 | −14 | 4.00 | <0.001 | Cluster 1: | 118 | 26 | −78 | −14 | 3.2 | <0.05 | No differential connectivity | |||||||||||
| Lingual gyrus | R | 72 | Lingual gyrus | 95 | |||||||||||||||||||||
| Fusiform gyrus | R | 25 | Fusiform gyrus | 18 | |||||||||||||||||||||
| Cerebellum 6 | R | 5 | Cerebelum 6 | 5 | |||||||||||||||||||||
| t-contrast: non-resp > resp | |||||||||||||||||||||||||
| No differential connectivity | No differential connectivity | No differential connectivity | |||||||||||||||||||||||
FDR false discovery rate, k number of voxels per cluster/region, non-resp non-responder, resp responder, ROI region of interest, SPQ spider phobia questionnaire, x, y, z, MNI coordinates.
We observed no differential functional connectivity between BAT-responders and BAT-non-responders with respect to ROI-to-ROI analyses (see Supplementary 4 Table S1).
When comparing patients with high vs. low WS-ext values we found significantly decreased negative connectivity between the right SFG and left triangular IFG in patients exhibiting more fear reduction during exposure treatment, T(74) = 4.36, p < 0.001, d = 1.01. Additionally, positive connectivity between the left orbital IFG and the bilateral gyrus rectus, left: T(74) = 3.02, p < 0.01, d = 0.70, right: T(74) = 3.23, p < 0.01, d = 0.75, and the left SFG was significantly increased in patients showing more fear reduction during exposure, T(74) = 3.17, p < 0.01, d = 0.74 (see Supplementary 5 Table S2).
Seed-to-voxel approach
Amygdala
The bilateral amygdalae were significantly higher positively connected to three adjacent clusters within the occipital cortex and the occipito-temporal junction in SPQ-responders. The first cluster was located within the medial occipital cortex and comprised the bilateral calcarine and lingual gyri, T(74) = 4.85, p < 0.001, d = 1.12. The two further clusters were lateralized but mirrored each other congruently. They extended over the lingual gyrus, cerebellar lobules 4 and 5 and parts of the fusiform gyrus, right: T(74) = 5.27, p < 0.001, d = 1.22; left: T(74) = 4.93, p < 0.001, d = 1.14 (see Table 2 and Fig. 1).
When using the BAT as response criterion, we found activity in a cluster extending over the left superior and middle frontal gyrus to be stronger negatively associated with amygdala activity in responders compared to non-responders, T(74) = −4.91, p < 0.001, d = −1.14 (see Supplementary 4 Table S1 and Fig. 1).
Seed-to-voxel analyses of the amygdala revealed the bilateral amygdalae to be connected significantly less positive with the bilateral MFG and the left SFG in patients exhibiting more anxiety reduction during exposure, T(74) = −5.57, p < 0.001, d = −1.29 (see Supplementary 5 Table S2).
Anterior cingulate cortex
We observed significantly increased positive connectivity between bilateral ACC seeds among SPQ-responders. The cluster predominantly comprised the left anterior and mid cingulum but also portions of the corresponding regions within the right hemisphere, T(74) = 6.32, p < 0.001, d = 1.47 (see Table 2).
No results were found using the secondary outcome or within-session fear reduction as response criteria (see Supplementary 4 Table S1 and Supplementary 5 Table S2).
Hippocampus
Among responders, the bilateral hippocampi exhibited significantly increased positive connectivity with a cluster at the junction of occipital and temporal lobe. It comprised parts of the right lingual and fusiform gyrus but also of the sixth cerebellar lobule, T(74) = 4.00, p < 0.001, d = 0.93 (see Table 2).
No results were found using the secondary outcome or within-session fear reduction as response criterion (see Supplementary 4 Table S1 and Supplementary 5 Table S2).
Cross-team replication on the Würzburg data set
ROI-to-ROI approach
Cross-team replication of the ROI-to-ROI results supported the increased positive connectivity between the bilateral hippocampi in SPQ-responders, compared to non-responders, T(74) = 3.83, p < 0.01, d = 0.89, and decreased negative functional connectivity between the left hippocampus and the orbital part of the left MFG, T(74) = −3.32, p < 0.05, d = −0.77. In contrast to the analyses in Würzburg, cross-team analyses further showed an increased positive connectivity in responders between the left IFG pars opercularis and right IFG pars triangularis, T(74) = 3.65, p < 0.05, d = 0.85.
As in the discovery sample, no differential functional connectivity between BAT-responders and BAT-non-responders with respect to ROI-to-ROI analyses could be revealed (see Supplementary 4 Table S1).
Cross-team replication of the ROI-to-ROI results comparing patients with high vs. low WS-ext succeeded (see Supplementary 5 Table S2). Thus, we were able to replicate the findings of decreased negative connectivity between SFG and triangular IFG, and increased positive connectivity between the left orbital IFG and the bilateral rectus in patients with more within-session fear reduction during exposure treatment.
Seed-to-Voxel approach
Amygdala
Cross team replication only yielded one cluster comprising the intracalcarine cortex, the lingual gyrus, as well as the occipital pole, significantly higher positively connected to the bilateral amygdalae: T(74) = 3.20, p = 0.0113, d = 0.74 (see Table 2). The two other clusters could be replicated by slightly lowering the cluster threshold to p < 0.06 (compare Supplementary 6 Table S3).
We replicated the decreased negative association in BAT-responders compared to non-responder between the amygdala and a cluster extending over the left superior and middle frontal gyrus, T(74) = −3.2, p < 0.05, d = −0.74 (see Supplementary 4 Table S1).
Cross-team replication revealed only one cluster being less positively connected in patients exhibiting high WS-ext compared to the low WS-ext group, T(74) = −3.2, p < 0.01, d = −0.74 (see Supplementary 5 Table S2).
Anterior cingulate cortex
Corroborating results from the Würzburg team, responders exhibited significantly increased positive connectivity between bilateral ACC seeds resulting in a cluster predominantly comprising the left anterior and mid cingulum but also small parts of the right anterior and mid cingulum, T(74) = 3.20, p < 0.001, d = 0.74 (see Table 2).
As in the primary analyses, no results were found using the secondary outcome or within-session fear reduction as response criteria (see Supplementary 4 Table S1 and Supplementary 5 Table S2).
Hippocampus
The bilateral hippocampi showed significantly increased positive connectivity with one cluster localized at the border of occipital and temporal lobe. It extended over the right lingual and fusiform gyrus but also small parts of the sixth cerebellar lobule, T(74) = 4.00, p < 0.001, d = 0.93.
No results were found using the secondary outcome or within-session fear reduction as response criteria (see Supplementary 4 Table S1 and Supplementary 5 Table S2).
Cross-site replication on the Münster data set
ROI-to-ROI approach
Instead of increased negative functional connectivity between the orbital part of the left MFG and left hippocampus and the increased positive functional connectivity within the hippocampus in SPQ-responders from the discovery sample, we observed increased positive connectivity in SPQ-responders between the medial and the orbital part of the right MFG, T(64) = 3.53, p < 0.05, d = 0.85 and between the left insula and the right thalamus, T(64) = 3.72, p < 0.05, d = 0.90, compared to SPQ-non-responders (see Table 2).
Neither the BAT nor the within-session fear reduction response criterion revealed any results in the replication sample (see Supplementary 4 Table S1 and Supplementary 5 Table S2).
Seed-to-Voxel approach
Amygdala
Cross-site replication did not show any differential connectivity based on an amygdala seed approach. Neither the BAT nor the within-session fear reduction response criterion revealed any results in the replication sample.
Anterior cingulate cortex
Seed-based analyses with the ACC as seed region revealed no differential connectivity in the replication sample.
BAT-responders compared to non-responders showed increased positive connectivity between ACC and left thalamus, T(64) = 3.22, p < 0.001, d = 0.78. BAT-non-responders showed increased positive connectivity between the ACC and a cluster including a small part of the caudate nucleus, T(64) = 3.22, p < 0.05, d = 0.78.
No differential connectivity was found regarding the within-session fear reduction criterion.
Hippocampus
No connectivity differences between responders and non-responders could be found for the Hippocampus.
BAT-responders did not show differential connectivity compared to BAT-non-responders, except for a small cluster (k = 2) indicating that BAT-responders show increased positive connectivity between the hippocampus and the Cerebellum, T(64) = 3.22, p < 0.05, d = 0.78.
Patients with high WS-ext showed increased positive connectivity between the hippocampus seed and an occipital cluster comprising parts of the superior occipital cortex, the cuneus, T(64) = 3.22, p < 0.05, d = 0.78 and a second cluster comprising the right lingual and the calcarine gyrus, T(64) = 3.22, p < 0.05, d = 0.78.
Discussion
The present investigation aimed to identify pre-treatment resting-state connectivity signatures that moderate response towards exposure as a first-line treatment for specific phobia. Analyses were embedded within a strict replication approach. Main findings on moderators of treatment response in the discovery sample suggest: (a) increased inhibitory fronto-limbic rsFC, (b) increased positive amygdala-visual rsFC, and (c) more excitatory “cross-talk” between prefrontal regions in patients with a high within-session fear extinction. Regarding the replication, results were mixed: while the cross-team replication showed largely comparable results, replicating findings in the replication sample was not successful, highlighting the importance of further replications.
In line with our overarching hypothesis, SPQ-responders in the discovery sample exhibited increased inhibitory fronto-limbic connectivity between left hippocampus and left orbital MFG and increased positive connectivity between the bilateral hippocampi. Those results could be interpreted with respect to hippocampal replay, which has been related to rumination and worry in anxiety disorders and depression [54], memory-guided behavior [55] as well as the retrieval of (fear) memory contents in rodents and humans [56–59]. It is defined as “the rapid, coordinated reactivation of encoding-activated cellular ensembles during sleep and resting wakefulness” [54]. In rodents, an inhibition of ventral hippocampal projections to the medial PFC via an optogenetic approach seems to disrupt anxiety and avoidance behavior [60]. In contrast, increased synchrony between hippocampus and medial PFC has been observed during states of anxiety [61]. The present results might therefore indicate altered retrieval of fear-relevant memory contents among patients who will benefit well from exposure. This may support the acquisition of a new fear-inhibitory memory trace or facilitate competing with the old fear-related memory trace thus resulting in better treatment success.
Findings on stronger fronto-limbic connectivity as a prerequisite for treatment response were also reflected in our secondary, more behavioral outcome measure (BAT response), where frontal activity in the left superior and middle frontal gyrus was more negatively associated with amygdala activity in responders compared to non-responders.
Supplementing our primary hypothesis on fronto-limbic connectivity, treatment responders in the discovery sample were additionally characterized by increased positive limbic-occipital to temporal connectivity: the amygdala and hippocampus exhibited significantly increased positive connectivity with clusters in the occipital and posterior temporal lobe in responders following the bilateral ventral visual pathway [62, 63]. Previous findings on backward projections of the amygdala to visual cortices suggest this connection to account for saliency of emotional visual information [64–69]. Within this framework, responders seem to be characterized by pronounced amygdala-driven processing of fear-relevant stimuli within the visual cortex, which may facilitate information processing within fear-relevant structures and stronger inhibitory fronto-limbic connectivity. Moreover, this may lead to attenuated fear memory retrieval. This interpretation is supported by recent findings on effective connectivity between amygdala, hippocampus and VMPFC and their interplay with respect to the elaboration and retrieval of autobiographical memories [70]. Here, hippocampus-VMPFC connectivity was increased when reliving emotionally arousing events.
Additionally, we exploratively introduced within-session fear reduction as a process measure of inhibitory learning. We found dorsolateral and ventromedial prefrontal areas to be stronger connected to the orbital IFG in high WS-ext patients compared to low WS-ext patients. Additionally, the right SFG was less inhibitorily connected to the triangular IFG in high WS-ext patients. Repeatedly, dorsolateral, ventrolateral and ventromedial prefrontal areas have been named as neural substrates of fear extinction and anxiety disorders [71–74]. The structures further correspond to the three steps of (voluntary) emotion regulation as suggested by the extended process model of emotion regulation [75]: the evaluation of the need to regulate (VLPFC [75]); the selection of adequate regulation strategies plus their implementation (DLPFC [76, 77]); Heightened connectivity among those regions might therefore reflect enhanced reconciliation and balancing of those processes [78], which may facilitate extinction during subsequent exposure [79, 80].
When comparing high and low WS-ext groups with respect to seed-to-voxel connectivity of the amygdala significant differential connectivity with the bilateral MFG was identified as it was true for the same analysis among BAT responders and non-responders. Profound investigation of the absolute connectivity values suggests that increased connectivity between amygdala and MFG within the low WS-ext group may indicate a heightened need for regulatory control.
As expected, cross-team replication, produced largely overlapping results. Of note, some results could only be replicated by lowering the cluster threshold to p < 0.06, thus the performance of the same analyses still seems to add some variance to the results. This is a common finding in reproducibility research [81] and can be traced back to the lack of standardized reporting practices, sharing analysing codes, and differences in the computing environment. In our case, we did not use the same code, but only written reports to transfer analysing methods and used different computing environments.
In line with the so-called replication crisis, the results from the discovery sample could not be replicated in the independent replication sample. This warrants an explanation. Firstly, only few cross-site replications of rsCF results in anxiety disorders exist [82], thus the generalizability of the results from one sample to another remains unclear. Besides, replication literature might be subject to a strong publication bias, hence lacking publication of unsuccessful replication studies. Further, studies are needed that dive deeper into the sources of variance that might affect replication. In our case, different scanner types faced a highly harmonized protocol regarding assessment and pre-processing of resting-state data. A potential reason for our replication failure may be the rather small sample sizes [83], which are however quite large for clinical neuroimaging studies (for results using the combined sample see Supplementary 7). Marek et al.[83]. suggest reproducibility of brain-wide association studies to require samples with thousands of individuals, while further stating that reproducibility also depends on the effect sizes. Regarding our discovery sample, we had large effects ranging from Cohen’s d 0.07 to 1.47, which resulted in a theoretical minimal power of 89% in a sample of the size of the replication sample. As sample sizes as proposed by Marek et al. [83] may not be feasible within single clinical studies, there is a high need for research initiatives to develop harmonized analysis protocols [84, 85] and to pool data to achieve sufficient statistical power [83], e.g., as promoted by the ENIGMA consortium (https://enigma.ini.usc.edu/).
All this opens the field to a broader discussion regarding the means to increase transparency and reproducibility in research, which has gained upwind the last couple of years, e. g. registered reports [86] and open science [87].
Taken the inconclusive interpretation of our cross-site replication approach, deviant results in the replication sample also need to be discussed. We found limbic and frontal regions to be stronger interconnected in responders. Especially the increased positive connectivity between the thalamus and the insula might be of interest. The insula is regarded as integration hub for emotional and interoceptive information [88], whereas the thalamus is the main input of bottom-up stimuli information before being transferred to the cortex and is involved in prefrontal inhibition processes relevant in anxiety [89]. Further, both regions belong to the extended defensive system [90]. Corroborating the finding of stronger interconnection of limbic structures in SPQ-responders, BAT-responders also showed increased limbic interconnection. BAT-non-response was associated with increased positive connectivity of the defensive system with the caudate nucleus (Supplementary Table S1). In line with the primary results of increased connectivity of limbic structures with the visual cortices in responders, high within-session fear reduction was associated with increased connectivity between limbic structures and the visual system (Supplementary Table S2).
Interpretation of the results requires the consideration of limitations. Due to strict inclusion/exclusion criteria, the present investigation was based on a rather homogeneous patient sample, resulting in high internal validity but limiting generalizability to clinical samples. We did control for variables showing differences between the two samples. Generalizability is further limited by the use of VRET, which allowed for high experimental control and standardization in the execution of exposure as well as the application of the very same exposure for each participant on the one hand, but involved characteristics (e.g., disconfirmation of certain beliefs is not possible in VR) that might have condensed within treatment response and the associated pre-treatment characteristics and restricted individualization of exposure on the other. However, as VRET relies on the same mechanism of action as in-vivo exposure and has been demonstrated to be equally effective [91], we believe the use of VRET does not substantially impair the transfer of our findings toward traditional exposure-based CBT.
The comparison of the observed results of the two response criteria as well as regarding WS-ext shows an overlap with respect to inhibitory fronto-limbic connectivity, which characterizes responders and high WS-ext patients. However, results also differ substantially between classification methods. As SPQ and BAT groups overlap for only about 61.22%, and overlap is even smaller with the WS-ext groups differences in group composition might have led to differing results. Future studies should try to achieve a more comprehensive understanding of the individual aspects covered by the different criteria for classifying treatment response [1].
Furthermore, replication is necessary, also with respect to treatments beyond a one-session exposure as well as within other anxiety disorders.
As cross-site replication of our findings within a second independent sample was not successful even though the prerequisite of a prior successful cross-team replication was met and study setup and data analysis pathways were fully harmonized. On the other hand, published replication data are rarely available, thus limiting the interpretation of findings in terms of replication results in general. Clearly, more studies are needed that explicitly address the replicability of their findings.
Clinically, findings in the discovery sample suggest that treatment adaptations supporting inhibitory fronto-limbic connectivity might enhance response rates. This might be achieved e.g., via the implementation of an emotion regulation training prior to treatment (see e.g., [92, 93]), frontal neurofeedback [94–96], or the application of gamma amino butyric acid (GABA) related drugs [97, 98]. Furthermore, brain stimulation techniques like repetitive transcranial magnetic stimulation (rTMS) as well as transcranial direct current stimulation (tDCS) may constitute options for directly manipulating brain activity and thus also connectivity (see e.g., [99–103]). First evidence suggests an effect of tDCS stimulation of the ventromedial prefrontal cortex on neural fear generalization patterns in a non-clinical sample [104]. Still, as we were not able to replicate results, interpretation remains inconclusive.
In summary, we identified three potential pre-treatment signatures of treatment response in large-scale intrinsic brain networks: i) inhibitory fronto-limbic connectivity which may confer emotion regulation via fear-inhibitory learning, ii) pronounced visuo-limbic connectivity which may be a clinical correlate of reduced avoidance behavior, and iii) enhanced crosstalk within the dorsolateral to ventromedial PFC supporting fear reduction during the exposure process, possibly via conscious and/or verbal cognitive processing of contingencies. Those three patient features may represent three distinct routes to facilitate fear reduction via exposure and hence inform treatment augmentation strategies for patients particularly vulnerable for treatment non-response. Findings are however limited by the lack of cross-site replication and warrant critical reflection as well as considerations how to foster replications in clinical neuroimaging studies.
Supplementary information
Acknowledgements
We would like to thank Tina Jocham, Jana Scharnagl, and Inge Gröbner (Dept. of Psychiatry, University Hospital of Würzburg); Dominik Grotegerd, Manuel Kraft, Merle Gebauer, Elena Wilkens, Jonathan Repple, Nina Muck, Stella Fingas, Janina Werner, Anna Kraus, and Kordula Vorspohl (Dept. of Psychiatry, University of Münster); Harald Kugel, Jochen Bauer, and Birgit Vahrenkamp (Dept. of Clinical Radiology, University of Münster), Lea Borgmann, Jaqueline Brieke, Aylin Fuchs, Carolin Heinemann, Annika Hense, Valeria Kleinitz, Kaja Loock, Johannes Lücke, and Kathrin Rüb (Institute of Medical Psychology and Systems Neuroscience); Tilman Coers, Julia Wandschura, Marielle Clerc, Hannah Casper, Sarah Hein, Karin Wilken, Andreas Wollbrink, Ute Trompeter, and Hildegard Deitermann (Institute for Biomagnetism and Biosignalanalysis) for their help and support.
Author contributions
EJL, FRS, JB, BG, TS, KR, MJ, HS, MJH, UD, and UL contributed to the design of the study. EJL, FRS, JB, BG, TS, KR, MJ, HS, NS, MJH, UD, and UL contributed to the acquisition of the data. EJL, FRS, JB, and UL provided the analysis and interpretation of the data for the work and did the primary drafting of the manuscript. EJL, FRS, JB, BG, TS, KR, MJ, HS, MJH, UD, UL, TL, JG, DG, SM, and NRW interpretation of the study and revised the work critically for important intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG)—Projektnummer 44541416-TRR 58 (CRC-TRR58, Projects C09 and Z02 to UD and UL, Project C08 to MJ and TS, Project C07 to TS and MJH) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (Grant Dan3/012/17 to UD), and the “Innovative Medizinische Forschung” (IMF) of the medical faculty of Münster (EJL, grant number LE121703 and LE121904). Open Access funding enabled and organized by Projekt DEAL.
Data availability
The study protocol of the study is published (Schwarzmeier et al., [49]). The data are not publicly available due to privacy or ethical restrictions.
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was reviewed by the Ethics Committees of the Medical Faculties at Würzburg University (proposal number 330/15) and Münster University (proposal number 216–212-b-S). After explaining the study protocol, written informed consent was obtained. The study protocol was preregistered at ClinicalTrials.gov (ID: NCT03208400).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Elisabeth J. Leehr, Fabian R. Seeger.
Supplementary information
The online version contains Supplementary material available at 10.1038/s41398-024-02799-x.
References
- 1.Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin Psychol Rev. 2015;42:72–82. doi: 10.1016/j.cpr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Taylor S, Abramowitz JS, McKay D. Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord. 2012;26:583–9. doi: 10.1016/j.janxdis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–79. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015;11:115–26. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messina I, Sambin M, Palmieri A, Viviani R. Neural correlates of psychotherapy in anxiety and depression: a meta-analysis. Mechelli A, editor. PloS One. 2013;8:e74657–e74657. [DOI] [PMC free article] [PubMed]
- 7.Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol. 2013;9:215–48. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- 8.Holmes EA, Craske MG, Graybiel AM. Psychological treatments: a call for mental-health science. Nature. 2014;511:287–9. doi: 10.1038/511287a. [DOI] [PubMed] [Google Scholar]
- 9.Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5:237–86. doi: 10.1016/S2215-0366(17)30513-8. [DOI] [PubMed] [Google Scholar]
- 10.Holmes EA, O’Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–60. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Casale A, Ferracuti S, Rapinesi C, Serata D, Piccirilli M, Savoja V, et al. Functional neuroimaging in specific phobia. Psychiatry Res. 2012;202:181–97. doi: 10.1016/j.pscychresns.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Garcia R. Neurobiology of fear and specific phobias. Learn Mem. 2017;24:462–71. doi: 10.1101/lm.044115.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinze J, Röder A, Menzie N, Müller U, Domschke K, Riemenschneider M, et al. Spider phobia: neural networks informing diagnosis and (virtual/augmented reality-based) cognitive behavioral psychotherapy—a narrative review. Front Psychiatry. 2021;0:1435. doi: 10.3389/fpsyt.2021.704174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci. 2013;67:311–22. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- 15.Lueken U, Kruschwitz JD, Muehlhan M, Siegert J, Hoyer J, Wittchen HU. How specific is specific phobia? Different neural response patterns in two subtypes of specific phobia. NeuroImage. 2011;56:363–72. doi: 10.1016/j.neuroimage.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Lueken U, Hilbert K, Stolyar V, Maslowski NI, Beesdo-Baum K, Wittchen HU. Neural substrates of defensive reactivity in two subtypes of specific phobia. Soc Cogn Affect Neurosci. 2014;9:1668–75. doi: 10.1093/scan/nst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Münsterkotter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, et al. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress Anxiety. 2015;32:656–63. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- 18.Peñate W, Fumero A, Viña C, Herrero M, Marrero RJ, Rivero F. A meta-analytic review of neuroimaging studies of specific phobia to small animals. Eur J Psychiatry. 2017;31:23–36. doi: 10.1016/j.ejpsy.2016.12.003. [DOI] [Google Scholar]
- 19.Zilverstand A, Sorger B, Kaemingk A, Goebel R. Quantitative representations of an exaggerated anxiety response in the brain of female spider phobics-a parametric fMRI study: quantitative brain representations of phobia. Hum Brain Mapp. 2017;38:3025–38. doi: 10.1002/hbm.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biol Psychol. 2007;75:124–30. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schienle A, Schäfer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia: an fMRI study on exposure therapy. Eur Arch Psychiatry Clin Neurosci. 2007;257:486–93. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- 23.Hilbert K, Kunas SL, Lueken U, Kathmann N, Fydrich T, Fehm L. Predicting cognitive behavioral therapy outcome in the outpatient sector based on clinical routine data: a machine learning approach. Behav Res Ther. 2020;124:103530. doi: 10.1016/j.brat.2019.103530. [DOI] [PubMed] [Google Scholar]
- 24.Lueken U, Zierhut KC, Hahn T, Straube B, Kircher T, Reif A, et al. Neurobiological markers predicting treatment response in anxiety disorders: A systematic review and implications for clinical application. Neurosci Biobehav Rev. 2016;66:143–62. doi: 10.1016/j.neubiorev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Maron E, Nutt D. Biological predictors of pharmacological therapy in anxiety disorders. Dialogues Clin Neurosci. 2015;17:305–17. doi: 10.31887/DCNS.2015.17.3/emaron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picó-Pérez M, Fullana MA, Albajes-Eizagirre A, Vega D, Marco-Pallarés J, Vilar A, et al. Neural predictors of cognitive-behavior therapy outcome in anxiety-related disorders: a meta-analysis of task-based fMRI studies. Psychol Med. 2023;53:3387–95. doi: 10.1017/S0033291721005444. [DOI] [PubMed] [Google Scholar]
- 27.Roesmann K, Leehr EJ, Böhnlein J, Steinberg C, Seeger F, Schwarzmeier H, et al. Behavioral and magnetoencephalographic correlates of fear generalization are associated with responses to later virtual reality exposure therapy in spider phobia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:221–30. doi: 10.1016/j.bpsc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Roesmann K, Toelle J, Leehr EJ, Wessing I, Böhnlein J, Seeger F, et al. Neural correlates of fear conditioning are associated with treatment-outcomes to behavioral exposure in spider phobia—evidence from magnetoencephalography. NeuroImage Clin. 2022;35:103046. doi: 10.1016/j.nicl.2022.103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin LM, Davis F, VanElzakker MB, Dahlgren MK, Dubois SJ. Neuroimaging predictors of treatment response in anxiety disorders. Biol Mood Anxiety Disord. 2013;3:15. doi: 10.1186/2045-5380-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwood L, Wise T, Perkins AM, Cleare AJ. Meta-analyses of the neural mechanisms and predictors of response to psychotherapy in depression and anxiety. Neurosci Biobehav Rev. 2018;95:61–72. doi: 10.1016/j.neubiorev.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos VA, Carvalho DD, Van Ameringen M, Nardi AE, Freire RC. Neuroimaging findings as predictors of treatment outcome of psychotherapy in anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:60–71. doi: 10.1016/j.pnpbp.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Schrammen E, Roesmann K, Rosenbaum D, Redlich R, Harenbrock J, Dannlowski U, et al. Functional neural changes associated with psychotherapy in anxiety disorders—a meta-analysis of longitudinal fMRI studies. Neurosci Biobehav Rev. 2022;142:104895. doi: 10.1016/j.neubiorev.2022.104895. [DOI] [PubMed] [Google Scholar]
- 33.Åhs F, Pissiota A, Michelgård Å, Frans Ö, Furmark T, Appel L, et al. Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Res Neuroimaging. 2009;172:103–8. doi: 10.1016/j.pscychresns.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Stefanescu MR, Endres RJ, Hilbert K, Wittchen HU, Lueken U. Networks of phobic fear: functional connectivity shifts in two subtypes of specific phobia. Neurosci Lett. 2018;662:167–72. doi: 10.1016/j.neulet.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Geiger MJ, Domschke K, Ipser J, Hattingh C, Baldwin DS, Lochner C, et al. Altered executive control network resting-state connectivity in social anxiety disorder. World J Biol Psychiatry. 2016;17:47–57. doi: 10.3109/15622975.2015.1083613. [DOI] [PubMed] [Google Scholar]
- 36.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52:1549–58. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Makovac E, Meeten F, Watson DR, Herman A, Garfinkel SN, D. Critchley H, et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol Psychiatry. 2016;80:786–95. doi: 10.1016/j.biopsych.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJA, Demenescu LR, Aleman A, et al. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013;23:186–95. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Van Dam NT, Feng C, Luo Y, Ai H, Gu R, et al. Anxious brain networks: a coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019;96:21–30. doi: 10.1016/j.neubiorev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Böhnlein J, Leehr EJ, Roesmann K, Sappelt T, Platte O, Grotegerd D, et al. Neural processing of emotional facial stimuli in specific phobia: an fMRI study. Depress Anxiety. 2021;38:846–59. doi: 10.1002/da.23191. [DOI] [PubMed] [Google Scholar]
- 44.Scharmüller W, Leutgeb V, Schöngaßner F, Hermann A, Stark R, Schienle A. Altered functional connectivity of basal ganglia circuitry in dental phobia. Soc Cogn Affect Neurosci. 2014;9:1584–8. doi: 10.1093/scan/nst150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxwell SE, Lau MY, Howard GS. Is psychology suffering from a replication crisis? What does “failure to replicate” really mean? Am Psychol. 2015;70:487–98. doi: 10.1037/a0039400. [DOI] [PubMed] [Google Scholar]
- 46.Shrout PE, Rodgers JL. Psychology, science, and knowledge construction: broadening perspectives from the replication crisis. Annu Rev Psychol. 2018;69:487–510. doi: 10.1146/annurev-psych-122216-011845. [DOI] [PubMed] [Google Scholar]
- 47.Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. Göttingen: Hogrefe; 1997.
- 48.Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specific-fear questionnaires. Behav Ther. 1974;5:401–9. doi: 10.1016/S0005-7894(74)80008-0. [DOI] [Google Scholar]
- 49.Schwarzmeier H, Leehr EJ, Böhnlein J, Seeger FR, Roesmann K, Gathmann B, et al. Theranostic markers for personalized therapy of spider phobia: Methods of a bicentric external cross‐validation machine learning approach. Int J Methods Psychiatr Res. 2019;e1812–e1812. [DOI] [PMC free article] [PubMed]
- 50.Leehr EJ, Roesmann K, Böhnlein J, Dannlowski U, Gathmann B, Herrmann MJ, et al. Clinical predictors of treatment response towards exposure therapy in virtuo in spider phobia: a machine learning and external cross-validation approach. J Anxiety Disord [Internet]. 2021;83. Available from: https://pubmed.ncbi.nlm.nih.gov/34298236/. [DOI] [PubMed]
- 51.Rupp C, Doebler P, Ehring T, Vossbeck-Elsebusch AN. Emotional processing theory put to test: a meta-analysis on the association between process and outcome measures in exposure therapy. Clin Psychol Psychother. 2017;24:697–711. doi: 10.1002/cpp.2039. [DOI] [PubMed] [Google Scholar]
- 52.Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. NeuroImage. 2020;206:116189. doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- 53.Botvinik-Nezer R, Wager TD. Reproducibility in neuroimaging analysis: challenges and solutions. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:780–8. doi: 10.1016/j.bpsc.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Heller AS, Bagot RC. Is hippocampal replay a mechanism for anxiety and depression? JAMA Psychiatry. 2020;77:431. doi: 10.1001/jamapsychiatry.2019.4788. [DOI] [PubMed] [Google Scholar]
- 55.Zielinski MC, Tang W, Jadhav SP. The role of replay and theta sequences in mediating hippocampal‐prefrontal interactions for memory and cognition. Hippocampus. 2020;30:60–72. doi: 10.1002/hipo.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schapiro AC, McDevitt EA, Rogers TT, Mednick SC, Norman KA. Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nat Commun. 2018;9:3920. doi: 10.1038/s41467-018-06213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuck NW, Niv Y. Sequential replay of nonspatial task states in the human hippocampus. Science. 2019;364:eaaw5181. doi: 10.1126/science.aaw5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staresina BP, Alink A, Kriegeskorte N, Henson RN. Awake reactivation predicts memory in humans. Proc Natl Acad Sci. 2013;110:21159–64. doi: 10.1073/pnas.1311989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu CT, Haggerty D, Kemere C, Ji D. Hippocampal awake replay in fear memory retrieval. Nat Neurosci. 2017;20:571–80. doi: 10.1038/nn.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. Reif A, editor. PLoS One. 2011;6:e21714. [DOI] [PMC free article] [PubMed]
- 62.DeYoe EA, Felleman DJ, Van Essen DC, McClendon E. Multiple processing streams in occipitotemporal visual cortex. Nature. 1994;371:151–4. doi: 10.1038/371151a0. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert CD. The constructive nature of visual processing. In: Principles of Neural Science, 5th Edition [Internet]. New York, NY: McGraw-Hill Education; 2014 [cited 2023 Feb 8]. Available from: neurology.mhmedical.com/content.aspx?aid=1125179264.
- 64.Catani M. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 65.Furl N, Henson RN, Friston KJ, Calder AJ. Top-down control of visual responses to fear by the amygdala. J Neurosci. 2013;33:17435–43. doi: 10.1523/JNEUROSCI.2992-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci. 2009;106:16841–6. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris J. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 68.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–8. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 69.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Nawa NE, Ando H. Effective connectivity within the ventromedial prefrontal cortex-hippocampus-amygdala network during the elaboration of emotional autobiographical memories. NeuroImage. 2019;189:316–28. doi: 10.1016/j.neuroimage.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 71.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–8. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- 72.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. Gendelman HE, editor. PLoS One. 2009;4:e5865. [DOI] [PMC free article] [PubMed]
- 74.Shin LM, Liberzon I. The neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Ann Rev Clin Psychol. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- 76.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation–an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–55. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PloS One. 2012;7:e48107–e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phillips ML, Ladouceur CD, Drevets WC. Neural systems underlying voluntary and automatic emotion regulation: toward a neural model of bipolar disorder. Mol Psychiatry. 2008;13:829–829. doi: 10.1038/mp.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. 2017;151:105–16. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botvinik-Nezer R, Wager T. Analysis reproducibility in mental health research: challenges and solutions [Internet]. OSF Preprints; 2022 [cited 2023 Feb 13]. Available from: https://osf.io/ujnmp/.
- 82.Ashar YK, Clark J, Gunning FM, Goldin P, Gross JJ, Wager TD. Brain markers predicting response to cognitive-behavioral therapy for social anxiety disorder: an independent replication of Whitfield-Gabrieli et al. 2015. Transl Psychiatry. 2021;11:260. doi: 10.1038/s41398-021-01366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–84. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Botvinik-Nezer R, Iwanir R, Holzmeister F, Huber J, Johannesson M, Kirchler M, et al. fMRI data of mixed gambles from the Neuroimaging Analysis Replication and Prediction Study. Sci Data. 2019;6:106. doi: 10.1038/s41597-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chambers CD, Tzavella L. The past, present and future of Registered Reports. Nat Hum Behav. 2021;6:29–42. doi: 10.1038/s41562-021-01193-7. [DOI] [PubMed] [Google Scholar]
- 87.Munafò MR, Nosek BA, Bishop DVM, Button KS, Chambers CD, Percie du Sert N, et al. A manifesto for reproducible science. Nat Hum Behav. 2017;1:1–9. doi: 10.1038/s41562-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kenwood MM, Kalin NH. Nonhuman primate models to explore mechanisms underlying early-life temperamental anxiety. Biol Psychiatry. 2021;89:659–71. doi: 10.1016/j.biopsych.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ledoux J, Daw ND. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat Rev Neurosci. 2018;19:269–82. doi: 10.1038/nrn.2018.22. [DOI] [PubMed] [Google Scholar]
- 91.Wechsler TF, Kümpers F, Mühlberger A. Inferiority or even superiority of virtual reality exposure therapy in phobias?—A systematic review and quantitative meta-analysis on randomized controlled trials specifically comparing the efficacy of virtual reality exposure to gold standard in vivo exposure in agoraphobia, specific phobia, and social phobia. Front Psychol. 2019;10:1758. doi: 10.3389/fpsyg.2019.01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emot Wash DC. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neacsiu AD, Eberle JW, Kramer R, Wiesmann T, Linehan MM. Dialectical behavior therapy skills for transdiagnostic emotion dysregulation: a pilot randomized controlled trial. Behav Res Ther. 2014;59:40–51. doi: 10.1016/j.brat.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–80. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Lorenzetti V, Melo B, Basílio R, Suo C, Yücel M, Tierra-Criollo CJ, et al. Emotion regulation using virtual environments and real-time fMRI neurofeedback. Front Neurol. 2018;9:390. doi: 10.3389/fneur.2018.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zotev V, Phillips R, Young KD, Drevets WC, Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. Soriano-Mas C, editor. PLoS One. 2013;8:e79184. [DOI] [PMC free article] [PubMed]
- 97.Bach DR, Korn CW, Vunder J, Bantel A. Effect of valproate and pregabalin on human anxiety-like behaviour in a randomised controlled trial. Transl Psychiatry. 2018;8:157. doi: 10.1038/s41398-018-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haag L, Quetscher C, Dharmadhikari S, Dydak U, Schmidt-Wilcke T, Beste C. Interrelation of resting state functional connectivity, striatal GABA levels, and cognitive control processes: Striatal GABA modulates cognitive control. Hum Brain Mapp. 2015;36:4383–93. doi: 10.1002/hbm.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dittert N, Hüttner S, Polak T, Herrmann MJ. Augmentation of fear extinction by transcranial direct current stimulation (tDCS) Front Behav Neurosci. 2018;12:76. doi: 10.3389/fnbeh.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D’Urso G, Mantovani A, Patti S, Toscano E, de Bartolomeis A. Transcranial direct current stimulation in obsessive-compulsive disorder, posttraumatic stress disorder, and anxiety disorders. J ECT. 2018;34:172–81. doi: 10.1097/YCT.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 101.Herrmann MJ. Non-invasive brain stimulation and fear extinction. A systematic review [Internet]. PsyArXiv. 2019. [cited 2023 Feb 6]. Available from: https://osf.io/u65va.
- 102.Kekic M, Boysen E, Campbell IC, Schmidt U. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J Psychiatr Res. 2016;74:70–86. doi: 10.1016/j.jpsychires.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 103.Raij T, Nummenmaa A, Marin MF, Porter D, Furtak S, Setsompop K, et al. Prefrontal cortex stimulation enhances fear extinction memory in humans. Biol Psychiatry. 2018;84:129–37. doi: 10.1016/j.biopsych.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roesmann K, Kroker T, Hein S, Rehbein M, Winker C, Leehr EJ, et al. Transcranial direct current stimulation of the ventromedial prefrontal cortex modulates perceptual and neural patterns of fear generalization. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:210–20. doi: 10.1016/j.bpsc.2021.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol of the study is published (Schwarzmeier et al., [49]). The data are not publicly available due to privacy or ethical restrictions.