Abstract
Background and Aims
Nivolumab was the first immune checkpoint inhibitor approved for hepatocellular carcinoma (HCC). External beam radiation therapy (EBRT) is locally effective and may enhance the effectiveness of immunotherapy. This study investigated the efficacy and safety of concurrent nivolumab and EBRT in HCC with macrovascular invasion.
Methods
In this phase II multicenter trial, patients with HCC and macrovascular invasion were concurrently treated with intravenous nivolumab (3 mg/kg every 2 weeks) and EBRT, followed by maintenance nivolumab until progression or unacceptable toxicity. Primary endpoints were progression-free survival (PFS) and safety, and secondary endpoints were overall survival, time-to-progression, objective response rate, and disease control rate.
Results
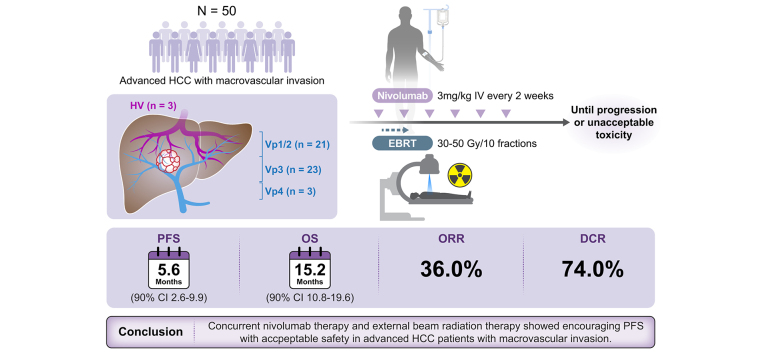
Between January 2020 and June 2021, 50 patients (male 84%, median age 62.5) were enrolled; 47 (94.0%) and 13 (26.0%) with portal (Vp1/2, n = 21; Vp3, n = 23; Vp4, n = 3) and hepatic vein invasion, respectively. Patients received EBRT (median dose: 50 [IQR 43–50] Gy) after the first nivolumab dose. The median number of nivolumab doses was 8.5. Median PFS was 5.6 (90% CI 3.6–9.9) months. Median overall survival and time-to-progression were 15.2 (90% CI 10.8–19.6) and 5.6 (90% CI 3.6–9.9) months, respectively. The objective response rate and disease control rate were 36.0% and 74.0%, respectively. The median duration of response was 9.9 months. Of 35 patients with follow-up data, 23 received subsequent systemic treatment, including atezolizumab-bevacizumab, sorafenib, lenvatinib, and regorafenib. Treatment-related any grade adverse events (AEs) and grade 3/4 AEs occurred in 40 (80.0%) and 6 (12.0%) patients, respectively. Common treatment-related AEs included pruritus (38.0%) and rash (16.0%), with no treatment-related deaths.
Conclusion
Concurrent nivolumab therapy and EBRT showed encouraging PFS with acceptable safety in patients with advanced HCC and macrovascular invasion.
Impact and implications
Immune checkpoint inhibitors, the standard care for advanced hepatocellular carcinoma (HCC), show relatively poor therapeutic effects in patients with advanced HCC and macrovascular invasion. In this investigator-initiated phase II study, we, for the first time, show that concurrent external beam radiation therapy with nivolumab, an immune checkpoint inhibitor, led to encouraging progression-free survival in patients with HCC and macrovascular invasion. The concurrent treatment was tolerable without significant safety concerns. Further randomized studies investigating the combination of immunotherapy and external beam radiation therapy are required.
ClinicalTrials.gov identifier
Keywords: hepatocellular carcinoma, nivolumab, radiotherapy, combination therapy
Graphical abstract
Highlights
-
•
EBRT plus nivolumab treatment led to encouraging PFS in patients with HCC and macrovascular invasion.
-
•
The combination therapy yielded higher ORR and DCR than in historical controls.
-
•
Concurrent nivolumab and EBRT has an acceptable safety profile.
Introduction
Hepatocellular carcinoma (HCC) is a common cause of cancer-related mortality.1 Despite efforts to adopt and improve surveillance for HCC, in actual clinical practice, a significant number of patients are diagnosed at advanced stages.2,3 The prognosis of advanced HCC is still dismal, with a median overall survival of less than 8 months; however, it has improved with advances in novel systemic therapies.[4], [5], [6], [7] Recently, the introduction of immune checkpoint inhibitors (ICIs), such as programmed cell death protein-1 or programmed cell death ligand-1 inhibitors, has further improved the oncologic outcomes for patients with HCC.[8], [9], [10], [11] While ICI monotherapy has shown relatively modest efficacy in terms of response rates and survival outcomes, ICI-based combination therapy confers further efficacy,[10], [11], [12] and recent HCC guidelines recommend these combination therapies as a first-line systemic treatment.13,14 However, these ICI-based first-line treatments showed relatively poor therapeutic effects in patients with HCC and macrovascular invasion (MVI)15 or excluded main portal invasion cases in clinical trials.11 In practice, MVI is still found in 10–29% of patients with HCC at the initial diagnosis and is a troublesome issue in HCC progression after other treatments.3 While systemic therapy is recommended for patients with MVI by many academic guidelines, various liver-directed therapies – such as surgical resection, transarterial chemoembolization (with or without radiotherapy), and radioembolization – have demonstrated significant efficacy.[16], [17], [18] There may be an unmet need for improved treatment strategies integrating systemic and liver-directed therapy in patients with HCC and MVI.
External beam radiation therapy (EBRT) can be applied to patients with HCC in various situations,13,19,20 including those with symptomatic primary liver or metastatic lesions.19 Recent advances in EBRT techniques, including stereotactic body radiotherapy (SBRT), proton beam therapy (PBT), and carbon ion radiotherapy, have enabled the delivery of higher radiation doses to achieve excellent local control.[21], [22], [23] As a curative option for small residual or recurrent intrahepatic HCC, PBT (a type of EBRT) demonstrated non-inferiority to radiofrequency ablation in terms of progression-free survival (PFS).24 The combination of EBRT and transarterial chemoembolization has been shown to have acceptable tolerability and superior efficacy to sorafenib for HCC with MVI.17 Although little is known about the clinical outcomes of the concurrent use of radiotherapy and immunotherapy, it can be expected to exert a synergistic effect in cancer treatment.25,26 Radiation therapy can have immunostimulatory effects. Substantial preclinical studies have shown that radiotherapy may synergize with immunotherapy.27 Preliminary clinical studies have recently been reported. A phase I trial of SBRT combined with immunotherapy (nivolumab with or without ipilimumab) exhibited favorable outcomes, and the combination therapy of EBRT and atezolizumab/bevacizumab demonstrated acceptable safety.28,29
Based on the CheckMate-040 trial,8 which showed promising clinical activity and a favorable safety profile, nivolumab, a programmed cell death protein-1 inhibitor, obtained accelerated approval from regulatory agencies worldwide, including in South Korea, as a second-line treatment, and a global first-line nivolumab trial could be initiated. Nivolumab monotherapy demonstrated a durable response in some patients; however, the response rate remained at 20%. EBRT has shown good local control in HCC and may potentiate immunotherapy through immunomodulatory effects; therefore, we conducted a phase II study evaluating the efficacy and safety of concurrent therapy with nivolumab and EBRT in patients with advanced HCC and MVI.
Patients and methods
Study design and participants
This NEXTRAH study was an investigator-initiated, single-arm, multicenter, phase II trial designed to investigate the efficacy and safety of combination therapy with nivolumab and EBRT in patients with advanced HCC with MVI.
Eligible patients were 20 years or older and had unresectable HCC confirmed histologically or clinically according to criteria of the Korea Liver Cancer Association (KLCA)-National Cancer Center (NCC) Korea HCC guidelines.13,30 They also had vascular invasion in the portal vein, hepatic vein, or inferior vena cava with or without extrahepatic spread and measurable disease, as defined by RECIST version 1.1. Other inclusion criteria were Child-Pugh class A, Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1, and adequate hematological and hepatic function. Patients who received ≥2 prior systemic therapies were excluded from the study. Prior immunotherapy was allowed. Patients who had received any systemic anti-cancer therapy within 2 weeks prior to the enrollment were excluded. Prior EBRT was allowed; however, reirradiation to targeted lesion (i.e., vascular invasion area) was not allowed due to limits of dose-volumetric constraints for organs at risk (OAR) if targeted lesion was previously irradiated. The complete eligibility criteria are provided in the trial protocol (Appendix).
All the participants provided written informed consent before participating in the study. The study protocol and amendments were approved by the Institutional Review Board Committee of each center, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Procedures
Patients received nivolumab (3 mg/kg intravenously every 2 weeks) until disease progression (RECIST version 1.1), unacceptable toxicity, withdrawal of consent, or study closure (Fig. S1). Patients were allowed to continue nivolumab therapy beyond the initial RECIST version 1.1-defined disease progression as per the investigator's judgment if they were still experiencing benefits without any definite progression in the target lesion. All patients underwent EBRT, beginning 2-7 days after receiving the first dose of nivolumab. EBRT with intensity-modulated radiation therapy (IMRT) or PBT was performed mainly in and around areas of vascular invasion. The choice of IMRT or PBT was based on the available time slots to maintain the protocol schedule. PBT was the preferred choice when both modalities were available. The radiotherapy procedure and the dose-volume constraints to the OARs have been described in detail.23,24 In brief, gross tumor volume (GTV) was defined as vascular invasion and/or the contiguous HCC at average computed tomography images during the gated (exhalation) phase. Internal target volume (ITV) was delineated by summing the individual GTVs within the gated phases. Planning target volume (PTV) 1 was expanded to include a 3-5 mm margin from the ITV, excluding the 10 mm expanded volume of gastrointestinal organs, and PTV2 was expanded to include a 5-7 mm margin from the ITV, excluding the volume of gastrointestinal organs. The dose-volume constraints to OARs were as follows: the relative volumes of the total liver and remaining residual liver (total liver – GTV) receiving more than 26.5 Gy were less than 50% and 60%, respectively; the maximum dose to the spinal cord, small and large bowel, stomach, and esophagus was less than 37 Gy, 35 Gy, 37 Gy, and 40 Gy, respectively; the mean dose to each kidney was less than 15 Gy; and the relative volume of the lung was less than 35%. The radiotherapy plan was performed using 6 or 15 MV X-rays or 230 MeV proton beams and the radiation doses of 30–35 Gy and 30 Gy in 10 fractions were prescribed to PTV1 and PTV2, respectively, per protocol considering the dose-volume constraints to OARs. Patients were maintained on nivolumab therapy during and after the completion of EBRT.
Outcomes and assessments
The primary endpoints were PFS (time from the date of treatment initiation to progressive disease or death from any cause) and safety. Tumors were assessed using dynamic computed tomography or magnetic resonance imaging at baseline and every 8 weeks after the initiation of nivolumab therapy until disease progression or discontinuation of the study treatment. Tumor progression and response were assessed according to RECIST version 1.1. A maximum of 5 measurable lesions, with a maximum of 2 lesions per organ, were identified as target lesions at baseline. Any progression in target or non-target lesions or the appearance of any new lesion is considered progression. Follow-up survival data were collected every 12 weeks for at least 18 months after enrollment of the last participant. The investigator evaluated safety in patients who received at least one dose of nivolumab according to the CTCAE version 4.03, at baseline, on day one of every cycle, and for up to 100 days after the last dose. Adverse events were assessed by the investigator as related or unrelated to the study treatment.
Secondary endpoints included overall survival (OS; the time from the date of treatment initiation to death from any cause), time-to-progression (TTP; the time from the date of treatment initiation to disease progression), objective response rate (ORR; the proportion of enrolled patients whose best overall response was complete response [CR] or partial response [PR]), and disease control rate (DCR; the proportion of enrolled patients whose best overall response was CR, PR, or stable disease).
Statistical analysis
The sample size was determined based on PFS, the study's primary endpoint. In a previous CheckMate-040 study,8 the median PFS in patients treated with nivolumab alone was 4.0 months (95% CI 2.9–5.4). With concurrent nivolumab treatment with EBRT, PFS was expected to increase by 50% compared to nivolumab alone. We calculated that 44 patients would provide a power of 80% at a one-sided 5% significance level to detect a median survival time of 6 months in patients treated with nivolumab and EBRT, allowing for a 12-month accrual period and 18-month follow-up period after the completion of enrollment. Considering a follow-up loss of 12%, 50 patients were finally enrolled in the study.
All statistical analyses were performed using an intention-to-treat population, defined as patients receiving at least one nivolumab dose. The confidence interval of the tumor response rates was calculated using the Clopper–Pearson exact method. The PFS, OS, and TTP curves were estimated using the Kaplan–Meier method, and median survival times and 90% CIs are presented. In post hoc subgroup analysis, the survival curves were compared using the log-rank test, and hazard ratios (HRs) and 90% CIs were calculated using the Cox proportional hazard model. All statistical analyses were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA), and R software, version 4.0.5 (R Project for Statistical Computing).
Results
Patients
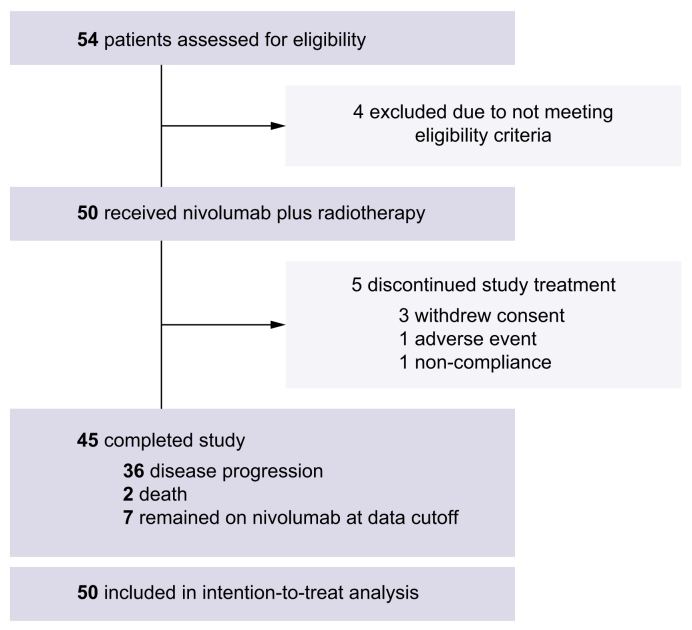
Between January 29, 2020, and June 29, 2021, 50 patients with advanced HCC who received nivolumab and EBRT were included in the intention-to-treat population (Fig. 1). The baseline characteristics of the patients are shown in Table 1. While 47 (94.0%) and 13 (26.0%) patients had portal vein and hepatic invasion, respectively, 46.0% and 6.0% had tumor invasion in the first-order branches (Vp3) or main portal vein (Vp4), respectively. Twenty-eight (56.0%) patients received treatment for HCC before participating in this study; 16 and 5 patients received transarterial chemoembolization and prior systemic therapy (1 sorafenib, 3 lenvatinib, and 1 atezolizumab plus bevacizumab), respectively.
Fig. 1.
Trial profile.
Table 1.
Baseline characteristics.
| Characteristics | N = 50 |
|---|---|
| Age, median [IQR], years | 62.5 [56–67] |
| Male sex, n (%) | 42 (84.0) |
| ECOG performance status, n (%) | |
| 0 | 47 (94.0) |
| 1 | 3 (6.0) |
| Etiology | |
| HBsAg-positive | 31 (62.0) |
| Anti-HCV-positive | 2 (4.0) |
| HBsAg-negative & HBcIgG-positive | 11 (22.0) |
| All-negative | 7 (14.0) |
| Diabetes | 16 (32.0) |
| Child-Pugh score, n (%) | |
| 5 | 38 (76.0) |
| 6 | 12 (24.0) |
| ALBI grade, n (%) | |
| 1 | 31 (62.0) |
| 2 | 19 (38.0) |
| Intrahepatic tumor burden | 13 (26.0) |
| Median size [IQR], cm | 7.0 [2.9–9.0] |
| Single lesion, n (%) | 30 (60.0) |
| Portal vein invasion, n (%) | 47 (94.0) |
| Vp1 | 2 (4.0) |
| Vp2 | 19 (38.0) |
| Vp3 | 23 (46.0) |
| Vp4 | 3 (6.0) |
| Hepatic vein invasion, n (%) | 13 (26.0) |
| IVC invasion, n (%) | 2 (4.0) |
| Bile duct invasion, n (%) | 2 (4.0) |
| Extrahepatic spread | 6 (12.0) |
| Alpha-fetoprotein† | |
| Median [IQR], ng/ml | 229.8 [7.8–2265.0] |
| ≥400 ng/ml, n (%) | 19 (38.0) |
| PIVKA-II† | |
| Median [IQR], mAU/ml | 421.0 [79.0–3008.0] |
| Prior therapy for HCC, n (%)‡ | |
| Any prior therapy | 28 (56.0) |
| Surgery | 8 (16.0) |
| Local ablation | 16 (32.0) |
| TACE | 16 (32.0) |
| Radiotherapy | 6 (12.0) |
| Systemic therapy | 5 (10.0) |
| Sorafenib | 1 (2.0) |
| Lenvatinib | 3 (6.0) |
| Atezolizumab + bevacizumab | 1 (2.0) |
Vp1, portal vein invasion in portal branches distal to the second branch; Vp2, portal vein invasion in the second portal branch; Vp3, portal vein invasion in the first portal branch; Vp4, portal vein invasion in the main portal trunk.
ALBI, albumin-bilirubin; ECOG, Eastern Cooperative Oncology Group; HBcIgG, Immunoglobulin G antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; IVC, inferior vena cava; TACE, transarterial chemoembolization.
Except missing values (2.0%).
Missing values (6.0%) were included in the percentage calculation.
As of the data cut-off date (December 30, 2022), 45 patients had completed the study treatment, and 7 out of these 45 patients with CR (n = 3) or PR (n = 4) maintained the study treatment and discontinued nivolumab treatment upon study termination (Fig. 1). The most common reason for discontinuing the study treatment was progressive disease (n = 36, 72.0%). The median number of nivolumab treatments received was 8.5 (IQR 4–26).
Overall, 23 (46.0%) and 27 (54.0%) patients received IMRT and PBT, respectively. The median interval from the first dose of nivolumab to EBRT was 5 days (IQR 3–6). The radiation field included vascular invasion and surrounding primary liver tumors in 47 patients, while 3 patients received radiation only for vascular tumor invasion. The median irradiation dose was 50 Gy (IQR 43–50 Gy) administered in 10 fractions. One patient did not complete EBRT (21 Gy in 6 fractions) and withdrew consent.
Twenty-six of the 35 patients with available follow-up data underwent anti-cancer therapy after the study treatment. Of them, 23 received subsequent systemic treatment (8 atezolizumab-bevacizumab, 13 sorafenib, 7 lenvatinib, and 7 regorafenib). Eight patients received ≥2 lines of systemic therapy after the study treatment.
Efficacy
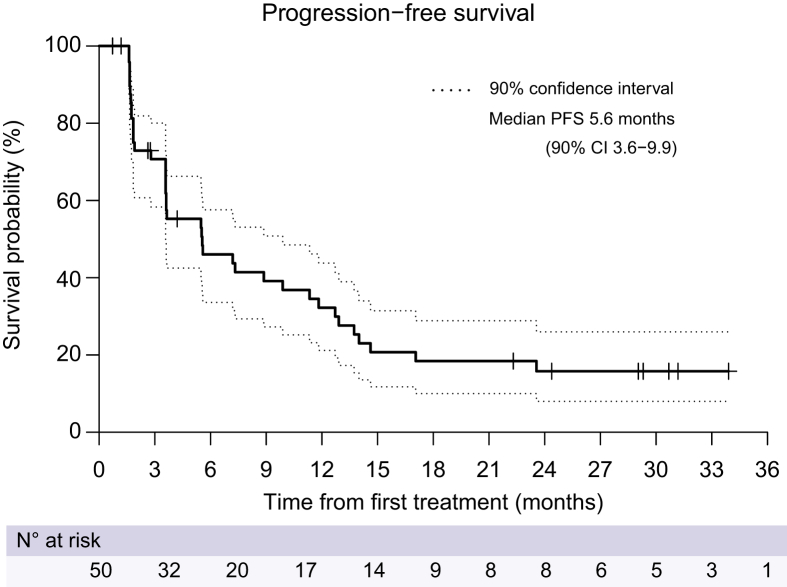
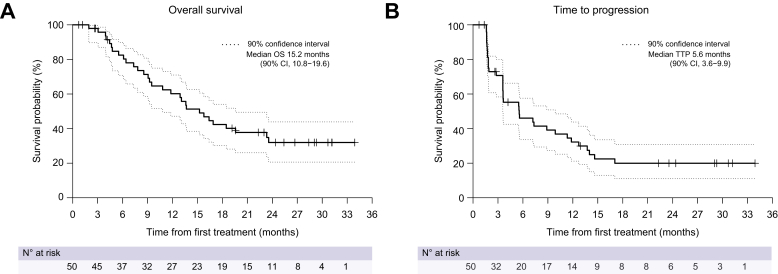
After the median follow-up of 29.3 months (IQR 24.4–31.2), 38 (76.0%) patients progressed or died, and the median PFS was 5.6 months (90% CI 3.6–9.9; Fig. 2). Six-month, 12-month, and 18-month PFS was 46.0% (90% CI 33.6–57.6), 32.2% (90% CI 21.2–43.8), and 18.4% (90% CI 10.0–28.9), respectively. The median OS and TTP were 15.2 (90% CI 10.8–19.6; Fig. 3A) and 5.6 (90% CI 3.6–9.9; Fig. 3B) months, respectively. Six-month, 12-month, and 18-month OS was 82.5% (90% CI 70.8–89.8), 60.2% (90% CI 47.2–71.0), and 42.4% (90% CI 30.2–54.0), respectively. Among 36 patients who exhibited radiologic progression, 7 patients experienced progression within the EBRT treatment field, while 29 patients demonstrated progression outside the treatment field.
Fig. 2.
Kaplan–Meier estimates of progression-free survival in the intention-to-treat population.
Fig. 3.
Kaplan–Meier estimates of overall survival and time-to-progression in the intention-to-treat population.
(A) Overall survival; (B) time-to-progression.
Post hoc subgroup analysis was performed according to the EBRT modality, site of portal vein invasion, baseline albumin-bilirubin (ALBI) grade, presence of extrahepatic spread, and prior history of systemic therapy (Table 2). The median PFS was significantly lower in patients with major portal vein invasion: 3.6 months (90% CI 1.9–9.9) in patients with Vp3-4 vs. 7.3 months (90% CI 5.6–17.1) in patients with Vp1-2 (HR 1.82, 90% CI 1.03–3.22; p = 0.079). The median OS significantly differed between the IMRT (9.1 [90% CI 6.1–19.6] months) and PBT (16.9 [90% CI 13.6–not estimable] months) groups (HR 0.54, 90% CI 0.29–0.98; p = 0.086), and between the ALBI grade 1 (19.6 [90% CI 13.7–not estimable] months) and ALBI grade 2 (7.9 [90% CI 4.8–13.1] months) groups (HR 3.09, 90% CI 1.68–5.67; p = 0.001). Patients who have received nivolumab as a first-line or second-line treatment showed no significant difference in median PFS (5.5 vs. 5.9 months; p = 0.679) or OS (15.8 vs. 12.4 months; p = 0.350).
Table 2.
Subgroup analysis of progression-free survival and overall survival.
| Subgroup | Patients(n) | Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| Median PFS,months (90% CI) | Hazard ratio (90% CI) | p value | Median OS,months (90% CI) | Hazard ratio(90% CI) | p value | ||
| EBRT modality | |||||||
| IMRT | 23 | 3.7 (1.9-7.3) | reference | 9.1 (6.1-19.6) | reference | ||
| PBT | 27 | 8.9 (3.6-12.7) | 0.86 (0.50-1.47) | 0.638 | 16.9 (13.6- NE) | 0.54 (0.29-0.98) | 0.086 |
| Site of PV invasion | |||||||
| Vp1/Vp2 | 21 | 7.3 (5.6-17.1) | reference | 18.5 (12.9-NE) | reference | ||
| Vp3/Vp4 | 26 | 3.6 (1.9-9.9) | 1.82 (1.03-3.22) | 0.079 | 10.8 (7.3-16.9) | 1.77 (0.94-3.32) | 0.132 |
| Baseline ALBI grade | |||||||
| Grade 1 | 31 | 8.9 (5.5-12.7) | reference | 19.6 (13.7- NE) | reference | ||
| Grade 2 | 19 | 3.2 (1.8-5.6) | 1.65 (0.96-2.85) | 0.122 | 7.9 (4.8-13.1) | 3.09 (1.68-5.67) | 0.001 |
| Extrahepatic spread | |||||||
| No | 44 | 5.6 (3.6-9.9) | reference | 15.2 (10.8-18.5) | reference | ||
| Yes | 6 | 1.9 (1.6- NE) | 0.92 (0.38-2.21) | 0.871 | 23.6 (5.5-NE) | 0.72 (0.27-1.97) | 0.593 |
| Prior systemic therapy | |||||||
| No | 42 | 5.5 (3.6-8.9) | reference | 15.8 (9.2-19.6) | reference | ||
| Yes | 5 | 5.9 (1.6-23.6) | 1.24 (0.52-2.98) | 0.679 | 12.4 (4.8-23.6) | 1.65 (0.68-4.01) | 0.350 |
In a 90% confidence interval, a p value of less than 0.10 is statistically significant.
ALBI, albumin-bilirubin; EBRT, external beam radiation therapy; IMRT, intensity-modulated radiation therapy; NE, not estimable; PBT, proton beam therapy; PV, portal vein.
Vp1, portal vein invasion in portal branches distal to the second branches; Vp2, portal vein invasion in the second portal branches; Vp3, portal vein invasion in the first portal branches; Vp4, portal vein invasion in the main portal trunk.
The hazard ratio and 90% CI were calculated using the Cox proportional hazard model.
Of the 50 patients, 4 (8.0%) and 14 (28.0%) achieved a CR and PR, respectively, according to RECIST version 1.1 (Table 3). Two patients were excluded because they withdrew consent before the first assessment. ORR and DCR were 36.0% (90% CI 24.7–48.6) and 74.0% (90% CI 61.9–83.9), respectively. The median duration of the objective response was 9.9 months (IQR 3.6–18.6).
Table 3.
Tumor response rates determined by RECIST version 1.1.
| Best response | |
|---|---|
| Objective response, n (%)∗ | 18 (36.0; 24.7–48.6) |
| Complete response | 4 (8.0) |
| Partial response | 14 (28.0) |
| Stable disease, n (%) | 19 (38.0) |
| Disease control rate, n (%)∗ | 37 (74.0; 61.9–83.9) |
| Progressive disease, n (%) | 11 (22.0) |
| Not evaluable, n (%) | 2 (4.0) |
| Patients with an ongoing response, n (%) | 7 (14.0) |
| Duration of response, median [IQR], months | 9.9 [3.6-18.6] |
90% confidence interval, shown in parentheses, was calculated using the Clopper-Pearson exact method. Seven patients maintained a response (CR or PR) at the time of study closure.
Safety
Overall, 48 (96.0%) and 9 (18.0%) patients experienced adverse events (AEs) and serious AEs (SAEs), respectively, regardless of attribution. Grade 3/4 events occurred in 21 (42.0%) and 5 (10.0%) patients, respectively, regardless of attribution. Treatment-related AEs and SAEs occurred in 40 (80.0%) and 4 (8.0%) patients, respectively. Grade 3/4 treatment-related AEs and SAEs occurred in 6 (12.0%) and 2 (4.0%) patients, respectively. Treatment-related AEs are listed in Table 4. The most common treatment-related any grade AEs were pruritus (38.0%), rash (16.0%), pneumonitis (10.0%), nausea (10.0%), fatigue (10.0%), and dyspepsia (10.0%). Two (4.0%) patients experienced grade 3 treatment-related SAEs: one had grade 3 bilirubin elevation, and the other had pneumonitis related to nivolumab. Five (10.0%) patients experienced any grade treatment-related pneumonitis; related to nivolumab in 4/5 patients (grade 2 in 3 patients and grade 3 in 1 patient) and to both nivolumab and EBRT in the fifth patient (grade 1). Other treatment-related SAEs included grade 2 rash and grade 2 colitis related to nivolumab. Two patients discontinued the treatment because of treatment-related toxicities (rash and hyperbilirubinemia).
Table 4.
Treatment-related adverse events.
| Any grade | Grade 3/4 | |
|---|---|---|
| Treatment-related AE | 40 (80.0%) | 6 (12.0%) |
| Pruritus | 19 (38.0%) | 0 |
| Rash | 8 (16.0%) | 0 |
| Pneumonitis | 5 (10.0%) | 1 (2.0%) |
| Nausea | 5 (10.0%) | 0 |
| Fatigue | 5 (10.0%) | 0 |
| Dyspepsia | 5 (10.0%) | 0 |
| ALT increase | 4 (8.0%) | 2 (4.0%) |
| Anorexia | 4 (8.0%) | 0 |
| AST increase | 3 (6.0%) | 3 (6.0%) |
| Hypothyroidism | 3 (6.0%) | 0 |
| Treatment-related SAE | 4 (8.0%) | 2 (4.0%) |
Data are presented as n (%). Adverse events were graded using the NCI-CTCAE version 4.03.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event.
The mean ALBI score did not change significantly (-2.57 at baseline to -2.58 at 6 months). Twenty-two patients (44.0%) had ≥1 nivolumab dose delay due to AEs (n = 16) or for other reasons (n = 6), whereas 5 patients (10.0%) had ≥2 dose delays due to AEs. No treatment-related death occurred during the study period.
Discussion
To the best of our knowledge, this NEXTRAH study is the first phase II study to evaluate concurrent immunotherapy with EBRT in patients with HCC and MVI. When this study was designed, nivolumab was the only available ICI for HCC under accelerated approval. Obtaining approval from the regulatory agency was crucial for this investigator-initiated trial. Nivolumab showed promising results in HCC at the initiation of this study and demonstrated manageable toxicity. Still, nivolumab monotherapy is associated with response rates of 20%, which clearly need to be improved. EBRT has the potential to enhance the efficacy of nivolumab via its immunomodulatory effects. Therefore, nivolumab is considered suitable for concurrent treatment with radiotherapy.8 The safety profile is essential because the primary hurdle of combination treatment is increasing toxicity.31
In the present study, the median PFS and OS were 5.6 (90% CI 3.6–9.9) and 15.2 months (90% CI 10.8–19.6), respectively. Compared to the PFS of 4.0 months in the historical control group (CheckMate-040) treated with nivolumab monotherapy, nivolumab in combination with EBRT improved PFS, given that the proportion of patients receiving nivolumab as second-line therapy was different (10% in this study vs. 68% in CheckMate-040).8 The median PFS of 5.6 months reported here was also higher than the 3.7 months reported in systemic treatment-naïve patients receiving nivolumab in CheckMate-459.12 Considering that only 33% of the patients included in CheckMate-459 had vascular invasion, the clinical outcomes (PFS and OS) in this study, where patients had vascular invasion (Vp3 or Vp4 invasion in 52.0% and hepatic vein invasion in 26.0%), are encouraging for patients with advanced HCC and MVI. Moreover, the ORR (36.0%) and DCR (74.0%) in this study were both higher than those in CheckMate-040 (ORR 20% and DCR 64%) and CheckMate-459 (ORR 15% and DCR 55%).8,12
In the post hoc subgroup analysis based on the EBRT modality, PBT led to longer median PFS (8.9 months) than IMRT (3.7 months); however, the difference was not significant. The median OS with PBT (16.9 months) was significantly higher than with IMRT (9.1 months) (Table 2). The incidental selection of IMRT or PBT, as mentioned in the Methods section, could have resulted in unintentional randomization; had we included more patients in the study, we might also have observed a significant difference in PFS between these modalities. Therefore, PBT may be a better option as an EBRT modality in combination with immunotherapy. However, some favorable clinical features (e.g., younger age, lower ALBI grade, or etiology) might have contributed to the differences. Patients receiving PBT tended to be younger (median age 59 vs. 65, p = 0.0343). The proportion of ALBI grade 2 was lower in patients receiving PBT (14.8% vs. 65.2%, p = 0.0003). Furthermore, the proportion of HBsAg-positive patients was higher in patients receiving PBT (81.5% vs. 39.1%, p = 0.0021), whereas that of all-negative patients was lower in those receiving PBT (3.7% vs. 26.1%, p = 0.0386). Additional comprehensive studies are required to confirm this.
The safety of concurrent therapy with nivolumab and EBRT was acceptable, with no new safety concerns. This is consistent with a previous report that administering an ICI within 90 days following EBRT did not increase the risk of AEs.32 In this study, the most common treatment-related AEs were dermatologic AEs, such as pruritus (38.0%) and rash (16.0%). The pruritis was nivolumab-related in 17/19 patients, EBRT-related in one, and nivolumab plus EBRT-related in one patient. The rash was related to both nivolumab and EBRT in 1/8 of patients. The incidence of dermatologic AEs in this study was higher than in those receiving nivolumab monotherapy in the CheckMate-459 study (pruritus in 12% and rash in 11%).12 However, nivolumab combined with radioembolization resulted in a higher incidence of pruritus (50%) and rash (39%).33 Although most cases of pneumonitis were likely related to nivolumab, the incidence was slightly higher than that observed with ICI monotherapy.9,12 Nevertheless, the incidence of pneumonitis was not notably concerning when considering previous studies on EBRT in HCC, which reported incidences of grade 1 asymptomatic pneumonitis ranging from 12% to 32%.24,34 A recent retrospective study on the combination of EBRT and atezolizumab/bevacizumab reported that the most common toxicities were grade 1 fatigue (57.1%) and nausea (47.6%), higher incidences than in the present study, while 3 (14.3%) patients experienced gastrointestinal bleeding.28 A higher proportion of patients in this retrospective study had Child-Pugh class B or C cirrhosis (33.3%), which may have contributed to a higher incidence of AEs compared with the present study. A phase I trial of the combination of SBRT and nivolumab, with or without ipilimumab, demonstrated that grade 3 hepatotoxicity occurred in 4/13 patients (30.8%).29 A higher incidence may be attributable to dual ICI therapy.
Since this study commenced, two ICI-based combination therapy trials for advanced HCC have been successfully completed; atezolizumab plus bevacizumab or tremelimumab plus durvalumab are now the standard of care for the first-line systemic treatment of advanced HCC.10,11,35 Although first-line nivolumab monotherapy did not significantly improve OS compared with sorafenib,12 concurrent therapy with nivolumab and EBRT showed meaningful efficacy without any major safety concerns. These data suggest the potential clinical benefit of combination therapy with ICI and EBRT in HCC. A recent prospective study of SBRT with camrelizumab and apatinib also reported better tumor response and survival than camrelizumab and apatinib alone.36 Further studies investigating ICI-based combination therapies with EBRT are anticipated.
Recently, promising results have been reported for pembrolizumab and SBRT in non-small cell lung cancer.37,38 However, the results are conflicting in other solid tumors.39 To successfully combine immunotherapy and radiotherapy, several issues must be addressed. Notably, radiotherapy outcomes can be influenced by the experience and skill of the radiation oncologist, similar to a surgeon. Therefore, a phase III study involving more institutions is required to determine the universal outcomes that can be achieved with ICI and EBRT combination therapies.
Our study has a few limitations. First, this was a single-arm study that compared the findings with the historical data of another study. Second, this study started with the expectation that IMRT and PBT would show similar clinical outcomes, but the results showed some differences. Therefore, additional research is needed on this issue. Third, given the small sample size, stratification based on the site of portal vein invasion and baseline ALBI grade was not possible in advance, and these factors influenced clinical outcomes. Fourth, the outcomes in patients who responded well to nivolumab and EBRT until study closure were affected by the forced termination of nivolumab treatment at the end of the study. The primary and secondary endpoints were also affected accordingly, which is considered a limitation of investigator-initiated trials.
In conclusion, concurrent EBRT and nivolumab treatment was associated with encouraging PFS in patients with HCC and MVI. Radiotherapy may offer a synergistic benefit to patients receiving nivolumab with acceptable tolerability. A phase III study to thoroughly investigate the clinical implications of these findings is warranted.
Financial support
We thank the Ono Pharmaceutical Company for providing nivolumab and for financial support. This study was partially supported by the National Cancer Center Korea (grants #2110351 and #2010162). The funding source had no role in the study design, data curation, or data analysis and interpretation.
Author’s contributions
Concept and study design: J-WP, BP, BHK, THK, and HCP. Funding acquisition: J-WP; Supervision: J-WP. Investigation and data curation: J-WP, BHK, HCP, THK, HCP, Y-HK, JYH, YC, DHS. Data analysis and interpretation: BP, Y-HK, BHK, HCP, THK, and J-WP. Original draft: BHK. Writing, review, and editing: J-WP, BHK, HCP, THK, and BP. All authors reviewed and approved the final draft.
Data availability statement
The data shown in this article are available from the corresponding authors upon a reasonable request.
Conflict of interest
J-WP has served in a consulting or advisory role for Roche, AstraZeneca, and Bigene; received honoraria from Roche, AstraZeneca, Bayer, and Eisai; and participated in the research sponsored by Ono, BMS; AstraZeneca; Roche; Eisai; Exelixis; Merk-MSD. BHK has served in an advisory role for Eisai and Roche, received honoraria from Eisai, Roche, and Hanmi, received a research grant from Hanmi, and participated in research sponsored by Ono-BMS. JYH has served as an advisory role for Eisai and AstraZeneca; received honoraria from Eisai, Dong-A, and Kyowa Kirin; and has participated in research sponsored by Roche, AstraZeneca, MSD, and Ono-BMS. HCP, THK, Y-HK, YC, DHS, and BP have no conflicts of interest to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We are grateful to all NEXTRAH trial patients, their families, and collaborators, including the research nurses involved in this trial.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100991.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Akinyemiju T., Abera S., Ahmed M., et al. Global Burden of Disease Liver Cancer C The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon J.S., Lee H.A., Kim H.Y., et al. Hepatocellular carcinoma in Korea: an analysis of the 2015 Korean nationwide cancer registry. J Liver Cancer. 2021;21:58–68. doi: 10.17998/jlc.21.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J.W., Chen M., Colombo M., et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase III non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Meyer T., Cheng A.L., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S., Chen Z., Fang W., et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40:383. 383. [Google Scholar]
- 10.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa Ghassan K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 12.Yau T., Park J.W., Finn R.S., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase III trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 13.Korean Liver Cancer Association, National Cancer Center Korea 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:583–705. doi: 10.3350/cmh.2022.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reig M., Forner A., Rimola J., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng A.L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Kokudo T., Hasegawa K., Matsuyama Y., et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Yoon S.M., Ryoo B.Y., Lee S.J., et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur M.H., Cho Y., Kim D.Y., et al. Transarterial radioembolization versus tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol. 2023;29:763–778. doi: 10.3350/cmh.2023.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apisarnthanarax S., Barry A., Cao M., et al. External beam radiation therapy for primary liver cancers: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12:28–51. doi: 10.1016/j.prro.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Yoon S.M. External beam radiotherapy for hepatocellular carcinoma: a review of the current guidelines in the east and the west. J Liver Cancer. 2021;21:25–33. doi: 10.17998/jlc.21.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis S., Dawson L., Barry A., et al. Stereotactic body radiation therapy for hepatocellular carcinoma: from infancy to ongoing maturity. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J.I., Park H.C. Considerations for radiation therapy in hepatocellular carcinoma: the radiation oncologists' perspective. Dig Dis. 2014;32:755–763. doi: 10.1159/000368018. [DOI] [PubMed] [Google Scholar]
- 23.Kim T.H., Park J.W., Kim B.H., et al. Optimal time of tumour response evaluation and effectiveness of hypofractionated proton beam therapy for inoperable or recurrent hepatocellular carcinoma. Oncotarget. 2018;9:4034–4043. doi: 10.18632/oncotarget.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim T.H., Koh Y.H., Kim B.H., et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2021;74:603–612. doi: 10.1016/j.jhep.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Chajon E., Castelli J., Marsiglia H., et al. The synergistic effect of radiotherapy and immunotherapy: a promising but not simple partnership. Crit Rev oncology/hematology. 2017;111:124–132. doi: 10.1016/j.critrevonc.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Bent E.H., Wehrenberg-Klee E., Koay E.J., et al. Integration of systemic and liver-directed therapies for locally advanced hepatocellular cancer: harnessing potential synergy for new therapeutic horizons. J Natl Compr Canc Netw. 2021;19:567–576. doi: 10.6004/jnccn.2021.7037. [DOI] [PubMed] [Google Scholar]
- 27.Galluzzi L., Aryankalayil M.J., Coleman C.N., et al. Emerging evidence for adapting radiotherapy to immunotherapy. Nat Rev Clin Oncol. 2023;20:543–557. doi: 10.1038/s41571-023-00782-x. [DOI] [PubMed] [Google Scholar]
- 28.Manzar G.S., De B.S., Abana C.O., et al. Outcomes and toxicities of modern combined modality therapy with atezolizumab plus bevacizumab and radiation therapy for hepatocellular carcinoma. Cancers (Basel) 2022;14 doi: 10.3390/cancers14081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juloori A., Katipally R.R., Lemons J.M., et al. Phase 1 randomized trial of stereotactic body radiation therapy followed by nivolumab plus ipilimumab or nivolumab alone in advanced/unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2023;115:202–213. doi: 10.1016/j.ijrobp.2022.09.052. [DOI] [PubMed] [Google Scholar]
- 30.Korean Liver Cancer Association, National Cancer Center Korea 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut and liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boshuizen J., Peeper D.S. Rational cancer treatment combinations: an urgent clinical need. Mol Cel. 2020;78:1002–1018. doi: 10.1016/j.molcel.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Anscher M.S., Arora S., Weinstock C., et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: a pooled analysis of trials in the US food and drug administration database. JAMA Oncol. 2022;8:232–240. doi: 10.1001/jamaoncol.2021.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai D., Loke K., Gogna A., et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase II trial. Lancet Gastroenterol Hepatol. 2021;6:1025–1035. doi: 10.1016/S2468-1253(21)00305-8. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S.M., Kim S.Y., Lim Y.S., et al. Stereotactic body radiation therapy for small (</=5 cm) hepatocellular carcinoma not amenable to curative treatment: results of a single-arm, phase II clinical trial. Clin Mol Hepatol. 2020;26:506–515. doi: 10.3350/cmh.2020.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korean Liver Cancer Association, National Cancer Center Korea 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023;23:1–120. doi: 10.17998/jlc.2022.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y., Zhou M., Tang J., et al. Efficacy and safety of stereotactic body radiotherapy combined with camrelizumab and apatinib in patients with hepatocellular carcinoma with portal vein tumor thrombus. Clin Cancer Res : official J Am Assoc Cancer Res. 2023;29:4088–4097. doi: 10.1158/1078-0432.CCR-22-2592. [DOI] [PubMed] [Google Scholar]
- 37.Theelen W., Peulen H.M.U., Lalezari F., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theelen W., Chen D., Verma V., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9:467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 39.Spaas M., Sundahl N., Kruse V., et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: the CHEERS phase 2 randomized clinical trial. JAMA Oncol. 2023 doi: 10.1001/jamaoncol.2023.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data shown in this article are available from the corresponding authors upon a reasonable request.