Abstract
Prader-Willi syndrome (PWS) is a complex, genetic disorder characterized by multisystem involvement, including hyperphagia, maladaptive behaviors and endocrinological derangements. Recent developments in advanced neuroimaging have led to a growing understanding of PWS as a neural circuit disorder, as well as subsequent interests in the application of neuromodulatory therapies. Various non-invasive and invasive device-based neuromodulation methods, including vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS) have all been reported to be potentially promising treatments for addressing the major symptoms of PWS. In this systematic literature review, we summarize the recent literature that investigated these therapies, discuss the underlying circuits which may underpin symptom manifestations, and cover future directions of the field. Through our comprehensive search, there were a total of 47 patients who had undergone device-based neuromodulation therapy for PWS. Two articles described VNS, 4 tDCS, 1 rTMS and 2 DBS, targeting different symptoms of PWS, including aberrant behavior, hyperphagia and weight. Multi-center and multi-country efforts will be required to advance the field given the low prevalence of PWS. Finally, given the potentially vulnerable population, neuroethical considerations and dialogue should guide the field.
Keywords: Prader-Willi syndrome, Neural circuits, Deep brain stimulation, Transcranial magnetic stimulation, Transcranial direct current stimulation, Vagus nerve stimulation
Introduction
Prader-Willi syndrome (PWS) is a rare, complex genetic neurodevelopmental disorder occurring in 1:10,000 to 30,000 live births. PWS affects approximately 350,000 people globally [1]. First described by Andrea Prader in 1956 as syndromic obesity, the genetic underpinning of PWS is the loss of expression of a paternally expressed gene locus on chromosome 15q11-13 [2]. There are several subtypes of this imprinting disorder, most commonly due to paternal deletion (DEL, 60–70%), maternal uniparental disomy (UPD, 25–35%), or an imprinting defect (1–3%) [3]. There are five common breakpoints (BP1-BP5) along chromosome 15q11-q13, and the DEL subtype can be further classified (Type I or II) based on the length of the deletion [4]. Maternal UPD manifests because of meiosis errors in female gametogenesis [4,5]. Importantly, SNORD116, MAGEL2 and IPW genes are found in the chromosomal 15 gene locus and are critical in hypothalamic functions, and are thought to possibly account for many of the PWS clinical manifestations [6,7].
Multisystem clinical manifestations in three characteristic aspects are considered pathognomonic of PWS: (a) hyperphagia, which may lead to severe obesity and its related metabolic diseases; (b) maladaptive behavioral issues such as temper outbursts and cognitive disabilities, and (c) multiple endocrinological dysfunctions, including hypogonadism and growth-hormone axis dysfunction [8, 9, 10]. PWS individuals classically experience various nutritional phases over time [11]. Briefly, in the neonatal period, PWS newborns often present with severe hypotonia and poor feeding which may contribute to failure to thrive [12,13]. From later childhood, they manifest hyperphagia and related food-seeking behaviors, contributing to severe obesity (40% in children; 85% in adults) and related disorders such as diabetes mellitus, high blood pressure, metabolic syndrome, cardiorespiratory diseases, and sleep apnea [14]. Hyperphagia is the most extreme form of overeating. It occurs without the feeling of satiety and is severely debilitating for PWS individuals and their caregivers. Increased mortality and morbidity rates (1–4% annually) result from cardiorespiratory complications associated with morbid obesity, as well as hyperphagia-related incidents such as choking, gastrointestinal perforation, and necrosis [15]. Aberrant behaviors in PWS include temper outbursts, repetitive and ritualistic behaviors, mood swings, and skin picking. Individuals with PWS also tend to enjoy solitary pursuits, have poor interpersonal relationships, impaired language skills, and are prone to developing psychiatric conditions such as depression. Strikingly, certain behavioral manifestations such as autism spectrum features are more strongly associated with the UPD subtype of PWS [6].
Human growth hormone (HGH) therapy remains the only FDA-approved treatment for PWS, and numerous pharmacological targets such as anorexigenic agents, endocannabinoid antagonists, and bariatric surgeries have proven ineffective. While HGH addresses some of the hormonal deficiencies in PWS, it has not been shown to have any significant impact on reducing hyperphagia or aberrant behaviors, especially in adulthood [16, 17, 18]. Bariatric surgery was less effective in PWS individuals and was also associated with higher post-operative complications and revision rates (12%) [19]. PWS individuals in general rely on forced, lifelong food security and restriction to prevent overeating, and some stay in group homes to prevent free access to food. Based on first-person and caregiver surveys, hyperphagia was repeatedly ranked as the most challenging and pervasive struggle on a day-to-day basis. Thus, this is a priority for effective therapy development [20, 21, 22]. Although some PWS individuals eventually progress into phase 4 disease, where they no longer have an insatiable appetite, this is actually a small minority of the population [11].
While the exact pathophysiological mechanism of PWS remains undeciphered, through advanced neuroimaging studies, there is growing evidence of neural circuits dysfunction in PWS that may account for its characteristic symptoms. Brain regions that have been implicated, consistent with the affected gene locus in PWS, include the hypothalamus, pre-frontal cortices responsible for inhibitory control and cognition (dorsolateral/medial prefrontal cortex, anterior cingulate cortex and insula), and the limbic reward system (orbitofrontal cortex, ventral striatum including nucleus accumbens, hippocampus, amygdala and anterior insula) [23]. The increasing appreciation of aberrant neural circuits such as hyperactive subcortical reward circuitry in PWS have led to recent application of various non-invasive and invasive neuromodulation techniques to treat aspects of PWS. These therapies include vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS) and deep brain stimulation (DBS). In this systematic review, we will consolidate the current literature on the use of neural stimulation therapies for the treatment of various PWS symptoms and we will suggest the possibility that shifting from a symptom-specific approach to a network-modulating strategy may have the potential to better address the clinical manifestations of PWS.
Methods
Search strategy
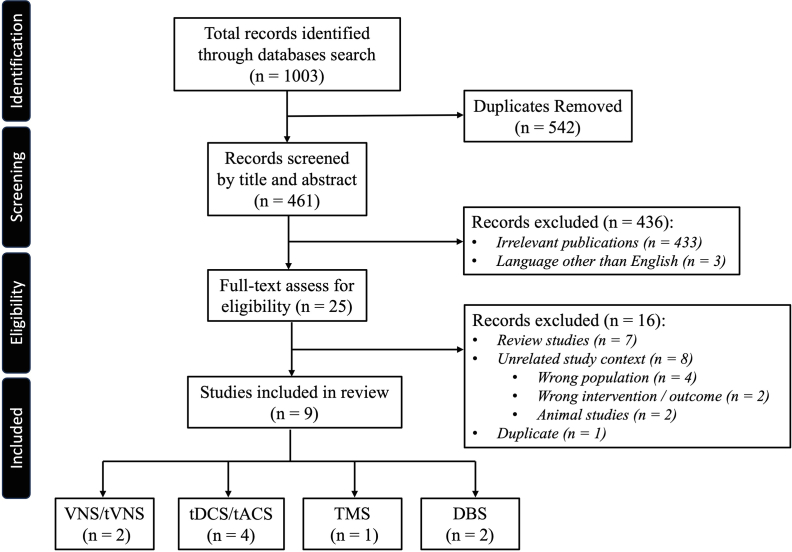
Following the Preferred Reporting Items for Systematic Review and Meta-Analysis 2020 guidelines (PRISMA, http://www.prisma-statement.org), a comprehensive and systematic literature search of four databases (PubMed, Scopus, Embase, and Web of Science) was performed in January 2023 and updated in June 2023 (Fig. 1) [24]. Relevant articles were identified using medical subject headings (MeSH) including the search terms “Prader-Willi” and “stimulation”. The search results were uploaded to Covidence Extraction 2.0 systematic review software (Veritas Health Innovation, Melbourne, Australia), and abstract screening and full text review were performed by three authors (AC, LQ and RM) independently. Any conflicts at any stage of screening or review were resolved by LQ. The following inclusion and exclusion criteria were used to select articles - Inclusion: 1) articles published in the English language, 2) original articles, including case reports, describing the use of neural stimulation therapy (invasive or non-invasive) in patients diagnosed with PWS. Exclusion criteria: 1) non-human preclinical studies, 2) studies not including a PWS population, 3) review papers, commentaries, and protocols. If duplicate data was encountered, the study with highest number of cases, or the longest follow-up was used and the other discarded.
Fig. 1.
PRISMA flowchart of the conduct of systematic literature review. DBS = deep brain stimulation; tACS = transcranial alternating current stimulation; tDCS = transcranial direct current stimulation; TMS = transcranial magnetic stimulation; tVNS = transcutaneous vagus nerve stimulation; VNS = vagus nerve stimulation.
Data extraction
Data extracted from full texts included study design, patient demographics, PWS symptoms treated, neural stimulation modality, brain target, stimulation parameters, treatment outcome, follow-up period and any adverse events. Each study was assigned a risk of bias (low, moderate, serious or critical) according to the ROBINS-I tool for non-randomized studies [25] and RoB 2.0 (low, some concerns or high) for randomized clinical trials [26]. Case reports were classified as critical, while small case series (less than 10 patients) were considered moderate if patient management and outcomes were homogeneous. Risk of bias was considered serious if management and/or outcomes varied substantially across individual patients.
Results
A total of 1003 abstracts were identified from the collective databases. After removal of duplicate studies, 461 articles remained. There were 436 articles excluded after abstract screening for the following reasons: 1) irrelevant publications (n = 433); 2) language other than English (n = 3). Twenty-five full text articles were reviewed, and another 16 articles were excluded due to duplicates (n = 1), identification as review articles (n = 7) and due to unrelated study context (n = 8). A total of 9 articles, ranging from case reports, case series and to randomized clinical trials were included in the final analysis, with a sum total of 47 patients across all modalities (Table 1). Two studies reported the use of VNS, 4 on tDCS, 1 on TMS, and 2 on DBS (Fig. 2). Outcome measures included physical measures such as body weight and body mass index (BMI), behavioral measures including number of temper outbursts, and standardized inventories, as well as eating and hyperphagia assessments. In the following sections, we detail the findings.
Table 1.
Summary of clinical studies examining the effects of neurostimulation in PWS patients.
| Author, year | Study design | Study size, n | Stimulation target | Stimulation settings | Target PWS symptom | Outcome | Follow up | Adverse events | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| (a) Vagusnerve stimulation (VNS)/Transcutaneous vagusnerve stimulation (tVNS) | |||||||||
| Manning, 2016 [27] | CS | 3 | VNS: Left vagus nerve | 30 Hz/500 μs/0.25–1.5 mA | Hyperphagiaa | Benefits in maladaptive behavior, temperament and social functioning (n = 2) | 12 months | Voice hoarseness; dyspnea | Serious |
| 30 s-on, 5 min-off cycle | |||||||||
| Manning, 2019 [29] | CS | 5 | tVNS: Left vagus nerve (cymba conchae) | 25 Hz/250 μs/0.1–5.0 mA | Maladaptive behaviors | Significant improvement in mean daily outbursts and CBI (n = 4) | 13 months | Nil | Moderate |
| 4 h stimulation/day | |||||||||
| (b) Transcranialdirect current stimulation (tDCS) | |||||||||
| Bravo, 2016 [33] | RCT | 10 | Right DLPFC | 2.0 mA × 30 min × 5 days | Food drive & craving | Improved food drive and cravings, hyperphagia | 30 days | Nil | Low |
| Azevedo, 2017 [34] | CR | 1 | Left DLPFC | 2.0 mA × 20 min/day × 10 weekdays | Food craving, aberrant behaviors | Improved food cravings and behavioral disturbance, up to 3 months. | 3 months | Nil | Critical |
| Azevedo, 2021 [35] | Open-label CT | 12 | Left DLPFC | 2.0 mA × 20 min/day × 10 weekdays (1.0 mA for age 11–12) | Hyperphagic, food craving, and aberrant behaviors | Improved hyperphagia, food craving and behavioral symptoms | 1 month | Nil | Moderate |
| Poje, 2021 [36] | CS | 10 | Right DLPFC | 2.0 mA, 30 min | Event-related potentials (ERPs) | Decreased NoGo amplitude and response time after tDCS | 30 min | NR | Moderate |
| (c) Repetitive transcranial magnetic stimulation (rTMS) | |||||||||
| Ferruli, 2022 [42] | CR | 1 | Bilateral DLPFC and insula | 3 times/week × 5 weeks dTMS (H coil) - HF: 18 Hz LF: 1 Hz |
Obesity, cognitive status | 4.4 kg (3.5%) weight loss after 5 weeks; 5.9 kg (4.6%) after 3 months. Improved cognition | 3 months | No severe adverse effects reported | Critical |
| (d) Deep brain stimulation (DBS) | |||||||||
| Talakoub, 2017 [50] | CR | 1 | Lateral hypothalamus area | 90 μs, 130 Hz, 3.5 V 8 Hz, 90 μs, 3 V |
Food cravings | Alpha-band frequency stimulations caused early sensation of fullness, but it didn't stop the patient from craving. | 4 h | NR | Critical |
| Franco, 2018 [51] | CS | 4 | Bilateral lateral hypothalamus | LF: 40 Hz, 210 μs, 3 mA HF: 130 Hz, 91 μs, 2 mA |
Obesity | No significant weight reduction | 6 months | Manic symptoms, skin picking, infection. | Moderate |
Abbreviations: CR: case report; CS: case series; CT: clinical trial; DBS: deep brain stimulation; DLPFC: dorsolateral prefrontal cortex; HF: high frequency; LF: low frequency; NR: not reported; RCT: randomized controlled trial; rTMS: repetitive transcranial magnetic stimulation; tDCS: transcranial direct current stimulation; tVNS: transcutaneous vagus nerve stimulation; VNS: vagus nerve stimulation.
Initial primary outcome measure.
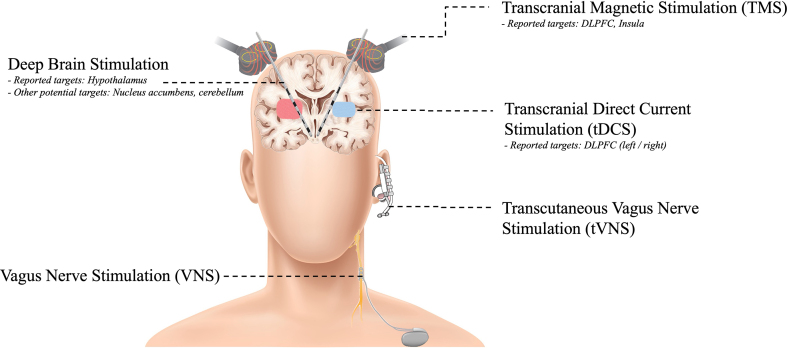
Fig. 2.
Reported neural stimulation therapies applied to Prader-Willi syndrome, including non-invasive (right) and invasive (left) modalities.
Vagus nerve stimulation (VNS)
Implanted VNS
The first clinical trial of VNS was conducted in three individuals with PWS in the United Kingdom, and the results were published in 2016 [27]. The impetus for this study was drawn from neuroimaging investigations suggesting insensitivity of satiety and reward pathways involved in eating behaviors in PWS, knowledge of the role of vagal nerve feedback between the gut and hypothalamus, and anecdotal reports of the weight reduction effects in patients implanted with VNS. In this study, the VNS system was implanted with electrical coils around the left vagal nerve. Stimulation parameters of 30 Hz, 500 us and 30 s of on-time, 5 min off-time cycle were used, with current amplitude ranging from 0.25 mA to 1.5 mA. In these 3 participants, VNS did not achieve the primary objective of hyperphagia reduction. However, significant improvement in pre-existing maladaptive behaviors in 2 of the 3 patients were documented, and there were reported improvements in emotional and cognitive flexibility, reactivity, social functioning, and control over food. Both patients in this study requested continuation of stimulation after conclusion of the trial.
The authors hypothesized that the beneficial effects of VNS were modulated via modification of afferent and efferent vagal projections on specific neural networks. As the vagal nerve influences widespread activity in the brain through the autonomic nervous system, it was postulated that the favorable effects on emotional outbursts and improved global functioning might have resulted from a rebalance of aberrant neural communications between motivational or emotional circuitries that are involved in higher cognitive control [28].
Transcutaneous VNS (tVNS)
The same investigators who first applied invasive VNS then went on to study the effects of non-invasive, transcutaneous VNS (t-VNS) on temper outbursts in a small cohort of 5 individuals with PWS (all DEL subtypes). The results were published in 2019 [29]. t-VNS was delivered non-invasively by an earpiece connected to the cymba conchae of the left ear. The programming parameters for stimulation ranged from 0.1 mA to 5 mA with a 0.1 mA step until the patient reported a tingling sensation. A pulse width of 250 μs and frequency of 25 Hz was used. The study was conducted in a non-blinded single case repeat measures modified ABA methodology. Participants served as their own control since it was not possible to deliver sham stimulation. Sham stimulation could not be used as it was important for the participants of this early study to experience the tingling sensation. Each participant received 4-h of daily t-VNS stimulation for 12 months, followed by a month of daily 2-h t-VNS stimulation and clinical effects were documented. Unlike the duty-cycle stimulation of implanted VNS, stimulation is continuously delivered by the t-VNS during the hours when worn (2-h or 4-h per day). The primary outcome was the frequency of daily behavioral outbursts as reported by caregivers, and the secondary outcomes included questionaries and qualitative interviews.
Of 5 participants, 4 displayed statistically significant decreases in the severity and number of temper outbursts following 4 h of daily t-VNS treatment (p < 0.05). The effect was observed at approximately the 9-month point of active stimulation. Objective questionnaires and interview results also corroborated with the behavioral improvements. The Challenging Behavior Interview significantly improved in 4 patients (p < 0.05). These improvements translated into reduced care demands and improved quality of life for study participants and their caregivers. Subsequent reduction of daily t-VNS to 2 h resulted in an increase in temper outburst episodes for all the participants, with 2 reflecting a statistically significant increase (p < 0.05) [29]. All participants consequently requested an increase of stimulation to 4 h/day after the 1-month follow-up. The collective data from this experience suggested a dose-related effect of t-VNS in reducing the temper outbursts and challenging behaviors in PWS.
Transcranial direct current stimulation (tDCS)
Transcranial direct current stimulation (tDCS) is a non-invasive technique that applies a low and constant direct current to the scalp. The current courses through the brain parenchyma from the anodic to cathodic electrode in order to modulate neuronal and cognitive functioning in cortical brain regions [30,31]. The treatment may enhance cortical excitability under the anode or hyperpolarize and inhibit excitability under the cathode. Previous applications of anodal tDCS have revealed success in modulating decision-making and food/substance cravings in healthy controls as well as patient populations [32,33]. tDCS was first proposed as a therapeutic approach to control hyperphagia in PWS based on the finding that PWS was associated with altered prefrontal activity; a brain activation pattern which is also known to be associated with food consumption [32]. Four studies have since reported the effect of tDCS on PWS. Available data include randomized controlled study, open-label study and case reports (Table 1).
In a pilot double-blind, sham-controlled, multi-center study, anodal tDCS (2.0 mA, 30 min for 5 days) was applied to the right dorsolateral prefrontal cortex (DLPFC) in 10 PWS patients, 11 obese adults and 11 healthy-weight adult controls [34]. Active tDCS stimulation in PWS was associated with a significant reduction in the Three-Factor Eating Questionnaire (TFEQ) and the Dykens Hyperphagia Questionnaire (DHQ) at the 30-day timepoint. Significant reductions in self-reported measures of disinhibition, severity and food cravings were also noted. Using the TFEQ scale, PWS individuals who received active stimulation revealed an improved disinhibition (factor II) score as well as total score (p < 0.02) when compared to the sham group. Additionally, hyperphagia severity, as graded by participants and caregivers on the DHQ, showed improvements in the active stimulation group at 15 days (p < 0.05), although this difference narrowed at the 30-day timepoint. These results support the hypothesis that tDCS neuromodulation of prefrontal activity reduced food drive and the behavior contributing to hyperphagia, although this did not result in any significant weight changes during the short study period. These findings were specific to the PWS population and were not present in the obese and healthy weight control participants.
Following this study, a group in Brazil reported the use of ambulatory home tDCS in a single adult PWS individual [35]. This participant displayed severe intellectual disability and aggressive food-related behaviors prior to treatment. His food intake was up to three times that of the recommended amount for his age and energy expenditure and he had failed multiple medical treatments. Using a new tDCS montage with the anode over the left DLPFC and cathode over the right DLPFC, 2.0 mA of direct current was applied for 20 min per day over 10 consecutive weekdays (total of 2 weeks). The aim was to treat both food cravings and behavioral symptoms. The patient showed significant improvement in caregiver-rated hyperphagia and aberrant behaviors following treatment as reflected in the DHQ and Aberrant Behavior Checklist (ABC). This effect was sustained at the 90-day measurement.
A subsequent open-label study involving 12 patients was conducted using the same methodology and stimulation protocol as above in order to increase left PFC excitability and to inhibit right PFC [36]. It was hypothesized that increasing left PFC excitability with anodal stimulation would increase inhibitory control in these individuals and impact food cravings and aberrant, aggressive, and social avoidant behaviors. Results of this study reported significant improvements in hyperphagia and food-related behaviors, as assessed by the caregiver reported DHQ and Food Craving Questionnaire (FCQ). In addition, improvements in the irritability, hyperactivity, lethargy and inappropriate behavior subscales of the ABC were also demonstrated after one month. There was also substantial reduction in the frequency of food-related behaviors such as food grabbing, blackmailing, and denying food. There was no significant change in weight noted in the 2-week study period.
To investigate the neural effects of tDCS in PWS, Poje et al. conducted a sham-controlled study using Go (non-food) and NoGo (food) behavioral tasks in 10 PWS individuals with concurrent continuous EEG recordings [37]. Baseline EEG readings were obtained while participants viewed food and non-food pictures. Participants were instructed to click on a mouse button only in response to non-food pictures (Go trials). Thirty minutes of tDCS (2.0 mA) to the right DLPFC or sham stimulation was then delivered. After the stimulation, the Go/NoGo EEG task was repeated, and event-related potentials (ERPs) were recorded. The authors found significant reduction of N2 amplitude in the active group as compared to the sham group after viewing food images (NoGo trials). This study supported the hypothesis that tDCS of the right DLPFC modulated dysfunctional attention, decision-making and response inhibition circuits. The authors hypothesized that these circuits may underlie altered food-related information-processing and hyperphagia behavior in PWS.
In all 4 tDCS studies, no significant adverse events were reported. Common side effects included tingling at the site of stimulation (32.1%), skin redness (29.5%), sleepiness (12.5%) and headache (8.9%). There were no differences in adverse effects comparing active and sham groups.
Repetitive transcranial magnetic stimulation (rTMS)
Transcranial magnetic stimulation (TMS) is a non-invasive stimulation of brain tissue through the production of high or low-intensity magnetic fields designed to potentially modulate cortical excitability. Repetitive TMS (rTMS) refers to applying recurring TMS pulses to a specific brain region, and this method is commonly used to treat psychiatric and neurological disorders such as treatment-refractory depression, obsessive-compulsive disorder and posttraumatic stress disorder [38, 39, 40]. Treatment targets include the DLPFC, the dorsomedial prefrontal cortex (DMPFC), the orbital frontal cortex (OFC) and the supplementary motor area (SMA). These targets have been applied most commonly for treatment-resistant depression and obsessive-compulsive disorder. More recent evidence suggests that rTMS could be applied to the DLPFC and insula bilaterally to reduce food cravings in obese patients [41,42].
In a case study conducted by Ferrulli et al., a 23-year-old PWS patient with severe hyperphagia and obesity, as well as comorbid metabolic disorders, arterial hypertension, hypogonadism and obstructive sleep apnea syndrome, received a total of 15 treatment sessions of rTMS to bilateral PFC and insulae over the span of five weeks, and followed-up for 3 months [43]. The primary outcome measures of the study included weight changes and cognitive abilities as measured by the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MOCA) tests. Other outcomes which quantified the patient's desire for food, impulsiveness and behavior, self-esteem, and quality of life were also collected.
The patient lost 1 kg per week during the rTMS treatment, with a total weight loss of 4.4 kg at the end of a 5-week session and 5.9 kg at the 3-month follow-up. The MMSE scored improved from 22 to 29, while behavioral and psychiatric disturbances did not show improvement.
Deep brain stimulation (DBS)
Deep brain stimulation (DBS) is a neurosurgical procedure that involves the precise implantation of electrodes into specific brain regions to modulate neural activity with the intent to treat symptoms and behaviors [44]. Since its inception, DBS has been shown to be safe and effective in treating select movement disorders and in general the therapy has facilitated the understanding of brain circuits and their potential contributions to diseases [45, 46, 47]. For the past two decades, DBS has shown increasingly promising effectiveness for the treatment of neuropsychiatric conditions such as OCD, refractory depression, and addiction [48]. Several animal and human studies have investigated DBS as a treatment option for refractory obesity, most commonly targeting the hypothalamus for satiety and hunger control or alternatively the nucleus accumbens for reward/motivation circuitry [49,50]. Specific to the PWS population, there have been 2 studies with a total of 5 patients reported. In both studies, the DBS target investigated was bilateral lateral hypothalamus (LH).
Using local field potential (LFPs) recordings derived from the lateral hypothalamic area (LHA) in a PWS patient, Talakoub et al. reported the ability to distinguish hunger and satiety states [51]. The authors found significant increases in beta/low-gamma power and reduced theta activity when food images were presented to the patient in a hungry state. Neutral animal pictures increased alpha activity and decreased theta activity. However, in the satiated condition, food images induced a reduction in beta and theta band power while the animal images continued to induce increased alpha band activity. In order to test if the satiation could be induced by DBS, LH-DBS was set at 8 Hz, 90 μs and 3 V during the hunger state. The patient reported a sensation of fullness, although he continued to experience food craving, suggesting that cravings and satiety may be differentially regulated [51].
Following this case report, a small case series involving 4 PWS individuals aged between 18 and 28 years old was performed to investigate the effectiveness of continuous LH-DBS [52]. All 4 patients had comorbid clinical obesity and psychiatric disturbances, ranging from aggressiveness to skin-picking. Two had undergone bariatric surgeries and failed to achieve sustained weight control. These patients underwent LH-DBS with the target described as adjacent to the fornix, anterolateral to the mammillary bodies, and posterior to the optic tract. They first received low frequency stimulation (LFS) (3 mA, 210 μs, 40 Hz) for one month, followed by another month of high frequency stimulation (HFS) (2 mA, 91 μs, 130 Hz). The patients then received HFS/LFS for 6 months based on their individual subjective reports. The primary outcome was to test the safety of LH-DBS on PWS patients, and the secondary outcome was to measure the treatment effectiveness of chronic DBS [52]. Three of 4 patients were chronically stimulated with the HFS parameter. There were no significant changes in the anthropometric and calorimetric parameters following 6 months of DBS (mean 9.6% increase in weight, 5.8% increase in BMI). Hormonal levels and neuropsychological evaluations were also unchanged. Two patients developed stimulation-induced manic symptoms, and two patients developed infections with one infection in association with skin picking.
Discussion
In this systematic review, we summarized the literature on device-based neuromodulation therapies for the treatment of PWS. Although there was only a total of 9 studies that met the final inclusion and exclusion criteria, all were drawn from the past 8 years, highlighting the infancy of this field. PWS is increasingly considered a condition involving aberrant neural circuitries. Several of these studies provide promising preliminary results and may open new avenues for treatment. We will review the gaps in knowledge and potential future directions.
Aberrant neural circuitries in PWS
With increasing availability of advanced neuroimaging techniques, various groups have identified aberrant structural and functional imaging findings in PWS suggesting abnormalities in one or more brain networks. Many authors tested these circuits using rest conditions and conditions induced with cues. These affected brain regions can be broadly grouped into (a) the limbic system which is involved in motivation/reward (OFC, striatum, hippocampus/parahippocampus, amygdala and anterior insula), (b) the cortical inhibitory cognitive/emotional control circuit (DLPFC, MPFC, anterior cingulate cortex and insula) and (c) the hypothalamus [23]. Additionally, many structural changes in brain MRI in PWS individuals have been reported [23]. These imaging studies suggest that the local cortical gyrification index and cortical complexity in bilateral frontal, temporal, and parietal lobes are reduced when compared to healthy controls [53, 54, 55, 56]. Volume reduction in subcortical, limbic, brainstem and cerebellar structures has also been reported [57,58]. Positron emission tomography (PET) imaging with [11C] flumazenil has revealed that these regions may have a reduction in GABA-A receptors, suggesting potential correlation to underlying cognitive impairment and consequent poor impulse control [59].
PWS individuals have also been reported to respond differently during fasted and satiated states. Compared to non-syndromic obese patients, PWS individuals demonstrated higher activity in the reward/limbic circuits while revealing hypoactivity in the prefrontal inhibitory cortices and hypothalamus in response to food images and when in a hungry condition. Post-meal, PWS individuals exhibited hyperactivation in the subcortical reward circuitry involving the hypothalamus, amygdala and hippocampus [60]. Early fMRI studies found significant delay (24 min) in activation in the hypothalamus, as well as reward-related brain regions such as the insula, vmPFC and nucleus accumbens [61,62]. When compared to healthy individuals, PWS individuals had hyperactivation of limbic and paralimbic brain regions (orbitofrontal cortex, medial PFC, insula, hippocampus, and parahippocampus) when presented with food images following eating [63]. Using a comparative fMRI study of PWS individuals and healthy siblings in a fasting state, a Dutch study found that viewing food pictures resulted in less activation of the brain regions involved in olfactogustatory sensation, emotional decision-making, and reward processing in PWS individuals. Amygdala activation was also positively associated with leptin levels, and was found to be related to reward circuitry in obese patients and negatively associated with glucose levels [64].
Subsequent investigations of resting state neural connectivity also revealed significant alterations in PWS, especially in networks involved in food and reward processing [65]. Building on prior knowledge of dysfunctional ventral prefrontal-basal ganglia connectivity in OCD patients, Pujol et al. reported overlapping hyperconnectivity between the prefrontal cortex and ventral striatum, which positively correlated with obsessive-compulsive behaviors [66]. In addition, self-picking was found to be associated with increased functional connectivity in the primary sensorimotor-putamen loop and obsessive eating behavior was associated with reduced coupling of the ventral striatum with limbic structures important for internal homeostasis (hypothalamus and amygdala) [66]. These findings suggest abnormal connections between limbic structures and the ventral frontostriatal systems related to reward, motivation, and satiety. Another group investigated this relationship using Granger causality analysis and identified elevated driving forces derived from the medial PFC and anterior cingulate cortex to the amygdala, which were reflective of impaired inhibition to food cues, as well as increased causal influences from the amygdala to the hypothalamus. These aberrant inputs possibly account for the override of normal homeostatic function in the hypothalamus in PWS [67]. Altered functional connectivity in the DLPFC was also positively correlated with the Autism-Spectrum Quotient [68]. Such resting aberrant neural circuitry was also found in a study using 18-F-FDG PET imaging in 16 PWS children, who exhibited decreased glucose metabolism in right superior temporal gyrus and left cerebellar vermis, regions that are associated with taste perception/food reward and emotional function respectively. Finally, metabolism was higher in regions involved in cognitive functions related to eating or obsessive-compulsive behavior [69].
While PWS has been postulated to be a disease model of hypothalamic dysfunction [7,10,70], it is evident that at least some of the manifestations of PWS involve more than the hypothalamus. As shown above, numerous functional imaging studies suggest dysfunction in brain regions that were connected to the hypothalamus, but not the hypothalamus itself. In an imaging study specifically investigating the hypothalamus, no difference in the volume of hypothalamus or mammillary bodies between children with PWS and healthy controls was found, although 50% smaller pituitary volumes were reported. The study revealed hyper-connectivity between the hypothalamus and the lateral occipital complex [71]. These findings suggest that the manifestation of hypothalamus dysfunction may be an end-result of aberrant and imbalance of neural inputs rather than a structural dysfunction. The afferent and efferent projections of the hypothalamus have been hypothesized to account for some of the behavioral and neuropsychiatric aspects of PWS [72,73]. In support of this concept, Blanco-Hinojo et al. conducted a fMRI study to investigate the mechanisms of indiscriminate eating in PWS [74]. They compared brain responses of PWS individuals to matched controls following viewing appetizing and disgusting food scenes. They found that the activation at the cortical level was similar, albeit less extensive, however subcortical limbic activation in the hypothalamus, amygdala/hippocampus, ventral striatum and peri-aqueductal gray were mostly absent. This finding was in distinct contrast to a normal temporal response that includes first activation of orbitofrontal and visual cortices, followed by engagement of periaqueductal gray and limbic structures. These observations collectively suggest multiple neural mechanisms that underlie indiscriminate eating and possibly multiple brain networks.
PWS genotype-phenotype relationship
PWS, which is a condition with identified genetic causation provides us a potential model for understanding the role of genetic to phenotypic expression. The different genetic subtypes of PWS, especially between the UPD and DEL subtypes, have been shown to display differences in clinical manifestations, such as psychological features [3,75]. The DEL subtype has been associated with hypopigmentation, higher body mass index (BMI), higher prevalence of scoliosis in adulthood, and more “typical” PWS-related facial features [75, 76, 77, 78, 79, 80, 81]. In contrast, the UPD subtype was associated with greater psychotic episodes, more challenging behavioral disturbances as well as increased autism spectrum features [75, 76, 77, 78, 79]. It has been hypothesized that the increased prevalence of psychosis may be due to overexpression of the UBE3A gene in the UPD group, functioning as a possible ‘second-hit’ [3,82]. Intriguingly, different PWS subtypes may also manifest differences in neural circuitries. Using a fMRI study, Holsen et al. found that PWS individuals with the DEL subtype displayed hyperactivation of the food motivation network in the medial prefrontal cortex and amygdala, while the UPD group demonstrated greater activation in the inhibitory control circuits (dorsolateral prefrontal cortex and parahippocampal gyrus) [83]. It is currently unclear how the different genetic subtypes may lead to the above changes in clinical and neuroimaging manifestations. However, the different genetic subtypes may result in gene dosage differences for specific genes in the 15q11-13 region. One example is the haploinsufficiency of nonimprinted genes in individuals with the DEL subtype, and relative overexpression of maternally expressed, imprinted genes in individuals with the UPD subtype may result in different phenotypes and may be underpinned by different circuitries [82,83]. Understanding whether these gene dosage differences manifest as subtle alterations in neural circuitry may help us to elucidate the underpinnings of the core manifestations of PWS.
Limitations
In this systematic review, we comprehensively identified 9 studies reporting the use of neural stimulation therapies to treat PWS. Although some of these studies demonstrated promising results, all had small numbers (maximum of 12 participants) and had short follow-up duration. Only the 2 studies of VNS had follow-up periods of up to 1 year, and interestingly the authors reported significant improvement in outcomes only after 9 months of stimulation. Non-invasive stimulation therapies, including tDCS and rTMS, were applied for very short durations of time. While the authors reported clinical outcomes up to 3 months, the durability of these therapies in the long run was left uncertain. The importance of laterality of treatment target was also not addressed in these small studies. Additionally, whether repeated treatment is required, or may produce similar outcomes, remains unknown. Limitations of wearing off durability of neuromodulatory treatment is currently of concern to experts designing clinical applications [84]. It is possible that modulation of this neural circuitry may require more chronic stimulation to alter network connectivity, especially for the symptoms that are non-episodic and more pervasive [85].
The failure of DBS in PWS to reach a primary outcome could have been due to the brain target choice. In addition, failures could also be driven by management of medications and programming of the device. The presence of numerous important nuclei within the hypothalamus makes it difficult to isolate the therapeutic target for DBS without inadvertently affecting other hypothalamic functions. This lesson is best illustrated by adverse effects in the Franco's case series [52]. To date, greater therapeutic successes have been reported in the treatment of obesity using DBS targeting the nucleus accumbens [41,44,49,86]. However, very few patients have been implanted and the best target in PWS is still unknown. Recent translational work using MRI findings of PWS patients have identified a potential novel target in the cerebellum, which when stimulated in mice, increased striatal dopamine levels and led to reduced food consumption [87].
Future directions
There is growing appreciation of PWS as a brain disorder and neural circuitopathy, joining a myriad of neurological and neuropsychiatric disorders in the race to understand the underlying aberrant connectivity, potential biomarkers, brain target and optimal neuromodulation therapy [82]. However, the exact understanding of this condition remains elusive. While PWS is a rare disorder affecting a small population when compared to other neurological disorders, the absence of any effective therapy is an important clinical gap which negatively impacts the lives of PWS patients and their caretakers. The genetic basis of PWS facilitates a potential for investigating genotype-phenotype relationships, neurochemistry, and neural circuitry changes. Current evidence supports the possibility that we should consider a paradigm shift from a symptom-specific treatment approach to a network-based approach. Larger studies combining the use of neuroimaging, and a combination of non-invasive neuromodulation and invasive neuromodulation approaches will facilitate a more accurate depiction of this multi-system disorder. International collaborative efforts between specialist centers will be needed and possibly a registry given the rare prevalence of PWS. Improved understanding of PWS pathophysiology may also inform the understanding of overlapping disorders such as obsessive-compulsive disorders, various behavioral disorders, eating disorders and diseases of hypothalamic dysfunction.
In parallel with this growing research on neural stimulation therapies in PWS, concerns about neuroethical aspects of these clinical studies should be anticipated and proactively discussed [88,89]. For example, in vulnerable populations, such as individuals with PWS, special attention must be paid to ensuring adequate participant and caregiver understanding of study information to facilitate informed decisions regarding research participation [90]. Though a recent interview study of young individuals with PWS revealed that all were potentially interested in participating in clinical trials for treatments addressing hyperphagia or anxiety, it also raised questions about what constitutes meaningful understanding and consent for this population [91]. Future efforts should grow sustained partnerships between researchers, ethicists, and the PWS community so that future research can move in an ethically responsible manner.
In this systematic review, we summarized the literature and presented the neuromodulation techniques used for the experimental treatment of PWS, which is increasingly understood as a circuitopathy. Although several small studies present promising results, we will need to better understand the biology and neural networks as we embark on an era of brain stimulation for this population.
Authors contributions
Conceptualization: LQ, TVS, CHH; Data-collection, Analysis and Interpretation: LQ, AC, RM; Drafting and Revising Manuscript: LQ, AC, RM; Critical review and Approval of Manuscript: TVS, MSO, KDF, AW, AG, JM, CHH.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Aysegul Gunduz reports a relationship with Medtronic Inc that includes: funding grants. Casey H. Halpern reports a relationship with Boston Scientific Corp that includes: consulting or advisory and speaking and lecture fees. Casey H. Halpern reports a relationship with Insightec that includes: consulting or advisory and speaking and lecture fees. Casey H. Halpern reports a relationship with SynchNeuro Inc that includes: board membership and equity or stocks. Michael S. Okun reports a relationship with Parkinson's Foundation that includes: consulting or advisory and funding grants. Michael S. Okun reports a relationship with National Institutes of Health that includes: funding grants. Michael S. Okun reports a relationship with Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, UF Foundation that includes: funding grants. Casey H. Halpern has patent #USPTO serial number: 63/170,404 and 63/220,432 issued to Stanford University. Casey H. Halpern has patent #USPTO serial number: 63/210,472 pending to SynchNeuro. Michael S. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). M.S.O. is an associate editor for New England Journal of Medicine Journal Watch Neurology and JAMA Neurology. He has participated in CME and educational activities (past 12–24 months) on movement disorders sponsored by WebMD/Medscape, RMEI Medical Education, American Academy of Neurology, Movement Disorders Society, Mediflix and by Vanderbilt University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
No specific funding was received in the publication of this article.
References
- 1.Cassidy S.B., Schwartz S., Miller J.L., Driscoll D.J. Prader-Willi syndrome. Genet Med. 2012 Jan;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 2.Couper R., Couper J. Prader-Willi syndrome. Lancet. 2000 Aug 19;356(9230):673–675. doi: 10.1016/s0140-6736(00)02617-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg A.G.W., Wellink C.M., Tellez Garcia J.M., Pellikaan K., Van Abswoude D.H., Davidse K., et al. Health problems in adults with Prader-Willi syndrome of different genetic subtypes: cohort study, meta-analysis and review of the literature. J Clin Med. 2022 Jul 12;11(14):4033. doi: 10.3390/jcm11144033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S.J., Miller J.L., Kuipers P.J., German J.R., Beaudet A.L., Sahoo T., et al. Unique and atypical deletions in Prader–Willi syndrome reveal distinct phenotypes. Eur J Hum Genet. 2012 Mar;20(3):283–290. doi: 10.1038/ejhg.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horsthemke B., Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am J Med Genet. 2008 Aug 15;146A(16):2041–2052. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 6.Whittington J., Holland A. A review of psychiatric conceptions of mental and behavioural disorders in Prader-Willi syndrome. Neurosci Biobehav Rev. 2018 Dec 1;95:396–405. doi: 10.1016/j.neubiorev.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Polex-Wolf J., Lam B.Y.H., Larder R., Tadross J., Rimmington D., Bosch F., et al. Hypothalamic loss of Snord116 recapitulates the hyperphagia of Prader-Willi syndrome. J Clin Invest. 2018;128(3) doi: 10.1172/JCI97007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muscogiuri G., Barrea L., Faggiano F., Maiorino M.I., Parrillo M., Pugliese G., et al. Obesity in Prader–Willi syndrome: physiopathological mechanisms, nutritional and pharmacological approaches. J Endocrinol Invest. 2021 Apr 23;44(10):2057–2070. doi: 10.1007/s40618-021-01574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittington J., Holland A., Webb T., Butler J., Clarke D., Boer H. Academic underachievement by people with Prader-Willi syndrome. J Intellect Disabil Res. 2004 Feb;48(2):188–200. doi: 10.1111/j.1365-2788.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Tauber M., Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021 Apr 1;9(4):235–246. doi: 10.1016/S2213-8587(21)00002-4. [DOI] [PubMed] [Google Scholar]
- 11.Miller J.L., Lynn C.H., Driscoll D.C., Goldstone A.P., Gold J.A., Kimonis V., et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet. 2011 May;155(5):1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsthemke B. Mechanisms of imprint dysregulation. Am J Med Genet C Semin Med Genet. 2010 Aug 20;154C(3):321–328. doi: 10.1002/ajmg.c.30269. [DOI] [PubMed] [Google Scholar]
- 13.McCandless S.E. The committee on genetics. Health supervision for children with Prader-Willi syndrome. Pediatrics. 2011 Jan 1;127(1):195–204. doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 14.Bittel D.C., Butler M.G. Prader–Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expet Rev Mol Med. 2005 Jul 25;7(14):1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler M.G., Manzardo A.M., Heinemann J., Loker C., Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi syndrome association (USA) 40-year mortality survey. Genet Med. 2017 Jun;19(6):635–642. doi: 10.1038/gim.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grugni G., Sartorio A., Crinò A. Growth hormone therapy for Prader-Willi syndrome: challenges and solutions. Therapeut Clin Risk Manag. 2016 Jun:873. doi: 10.2147/TCRM.S70068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crinò A., Fintini D., Bocchini S., Grugni G. Obesity management in Prader–Willi syndrome: current perspectives. Diabetes, Metab Syndrome Obes Targets Ther. 2018;11:579–593. doi: 10.2147/DMSO.S141352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoud R., Kimonis V., Butler M.G. Clinical trials in Prader-Willi syndrome: a review. Int J Mol Sci. 2023 Jan 21;24(3):2150. doi: 10.3390/ijms24032150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S.Y.W., Wong S.K.H., Lam C.C.H., Ng E.K.W. Bariatric surgery for Prader-Willi syndrome was ineffective in producing sustainable weight loss: long term results for up to 10 years. Pediatr Obes. 2020;15(1) doi: 10.1111/ijpo.12575. [DOI] [PubMed] [Google Scholar]
- 20.Lavelle T.A., Crossnohere N.L., Bridges J.F.P. Quantifying the burden of hyperphagia in Prader-Willi syndrome using quality-adjusted life-years. Clin Therapeut. 2021 Jul;43(7):1164–1178.e4. doi: 10.1016/j.clinthera.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Tsai J.H., Scheimann A.O., McCandless S.E., Strong T.V., Bridges J.F.P. Caregiver priorities for endpoints to evaluate treatments for Prader-Willi syndrome: a best–worst scaling. J Med Econ. 2018 Dec 2;21(12):1230–1237. doi: 10.1080/13696998.2018.1528980. [DOI] [PubMed] [Google Scholar]
- 22.Kayadjanian N., Vrana-Diaz C., Bohonowych J., Strong T.V., Morin J., Potvin D., et al. Characteristics and relationship between hyperphagia, anxiety, behavioral challenges and caregiver burden in Prader-Willi syndrome. PLoS One. 2021 Mar 25;16(3) doi: 10.1371/journal.pone.0248739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z., Cai J. Progress in brain magnetic resonance imaging of individuals with Prader–Willi syndrome. J Clin Med. 2023 Jan;12(3):1054. doi: 10.3390/jcm12031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021 Mar 29;372 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. The risk of bias in non-randomized studies – of interventions (ROBINS-I) assessment tool.
- 26.Higgins J.P.T., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343(oct18 2) doi: 10.1136/bmj.d5928. d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning K.E., McAllister C.J., Ring H.A., Finer N., Kelly C.L., Sylvester K.P., et al. Novel insights into maladaptive behaviours in Prader-Willi syndrome: serendipitous findings from an open trial of vagus nerve stimulation. J Intellect Disabil Res. 2016 Feb 1;60(2):149–155. doi: 10.1111/jir.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland A., Manning K. t-VNS to treat disorders of behaviour in Prader-Willi Syndrome and in people with other neurodevelopmental conditions. Auton Neurosci Basic Clin. 2022 May 1:239. doi: 10.1016/j.autneu.2022.102955. [DOI] [PubMed] [Google Scholar]
- 29.Manning K.E., Beresford-Webb J.A., Aman L.C.S., Ring H.A., Watson P.C., Porges S.W., et al. Transcutaneous vagus nerve stimulation (t-VNS): a novel effective treatment for temper outbursts in adults with Prader-Willi Syndrome indicated by results from a non-blind study. Phan TG. PLoS One. 2019 Dec 3;14(12) doi: 10.1371/journal.pone.0223750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggio P.S., de Macedo E.C., Schwartzman J.S., Brunoni D., Teixeira M.C., Fregni F. Transcranial direct current stimulation: a novel approach to control hyperphagia in Prader-Willi syndrome. J Child Neurol. 2009;24(5):642–643. doi: 10.1177/0883073808322339. [DOI] [PubMed] [Google Scholar]
- 31.Rosa M.A., Lisanby S.H. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012 Jan;37(1):102–116. doi: 10.1038/npp.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fregni F., Orsati F., Pedrosa W., Fecteau S., Tome F.A.M., Nitsche M.A., et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008 Jul;51(1):34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefaucheur J.P., Antal A., Ayache S.S., Benninger D.H., Brunelin J., Cogiamanian F., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2017 Jan;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 34.Bravo G.L., Poje A.B., Perissinotti I., Marcondes B.F., Villamar M.F., Manzardo A.M., et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader-Willi syndrome. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2016 Mar;171B(2):266–275. doi: 10.1002/ajmg.b.32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azevedo C., Gomes J.S., Trevizol A.P., Dias Á.M., Cordeiro Q. At-home transcranial direct current stimulation in Prader-Willi syndrome with severe intellectual disability: a case study. J ECT. 2017 Sep;33(3):e29. doi: 10.1097/YCT.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 36.Azevedo C.C., Trevizol A.P., Gomes J.S., Akiba H., Franco R.R., Simurro P.B., et al. Transcranial direct current stimulation for Prader-Willi syndrome. J ECT. 2021;37(1):58–63. doi: 10.1097/YCT.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 37.Poje A.B., Manzardo A., Gustafson K.M., Liao K., Martin L.E., Butler M.G. Effects of transcranial direct current stimulation (tDCS) on Go/NoGo performance using food and non-food stimuli in patients with Prader-Willi syndrome. Brain Sci. 2021;11(2) doi: 10.3390/brainsci11020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Mao Z., Ling Z., Yu X. Repetitive transcranial magnetic stimulation for cognitive impairment in Alzheimer's disease: a meta-analysis of randomized controlled trials. J Neurol. 2020 Mar;267(3):791–801. doi: 10.1007/s00415-019-09644-y. [DOI] [PubMed] [Google Scholar]
- 39.Kozel F.A. Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr Clin. 2018 Sep;41(3):433–446. doi: 10.1016/j.psc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Baeken C., Brem A.K., Arns M., Brunoni A.R., Filipčić I., Ganho-Ávila A., et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: current knowledge and future directions. Curr Opin Psychiatr. 2019 Sep;32(5):409–415. doi: 10.1097/YCO.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouveia F.V., Silk E., Davidson B., Pople C.B., Abrahao A., Hamilton J., et al. A systematic review on neuromodulation therapies for reducing body weight in patients with obesity. Obes Rev. 2021 Oct;22(10) doi: 10.1111/obr.13309. [DOI] [PubMed] [Google Scholar]
- 42.Ferrulli A., Macrì C., Terruzzi I., Massarini S., Ambrogi F., Adamo M., et al. Weight loss induced by deep transcranial magnetic stimulation in obesity: a randomized, double-blind, sham-controlled study. Diabetes Obes Metabol. 2019 Aug;21(8):1849–1860. doi: 10.1111/dom.13741. [DOI] [PubMed] [Google Scholar]
- 43.Ferrulli A., Cannavaro D., Macrì C., Luzi L. Repetitive transcranial magnetic stimulation: a potential therapeutic option for obesity in a patient with Prader-Willi syndrome. Diabetes Obes Metabol. 2022;24(12):2478–2481. doi: 10.1111/dom.14833. [DOI] [PubMed] [Google Scholar]
- 44.Contreras López W.O., Navarro P.A., Crispín S. Effectiveness of deep brain stimulation in reducing body mass index and weight: a systematic review. Stereotact Funct Neurosurg. 2022;100(2):75–85. doi: 10.1159/000519158. [DOI] [PubMed] [Google Scholar]
- 45.Neumann W.J., Horn A., Kühn A.A. Insights and opportunities for deep brain stimulation as a brain circuit intervention. Trends Neurosci. 2023 Jun 1;46(6):472–487. doi: 10.1016/j.tins.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Sironi V.A. Origin and evolution of deep brain stimulation. Front Integr Neurosci. 2011 Aug 18;5:42. doi: 10.3389/fnint.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019 Mar;15(3):148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graat I., Figee M., Denys D. The application of deep brain stimulation in the treatment of psychiatric disorders. Int Rev Psychiatr. 2017 Mar 4;29(2):178–190. doi: 10.1080/09540261.2017.1282439. [DOI] [PubMed] [Google Scholar]
- 49.Formolo D.A., Gaspar J.M., Melo H.M., Eichwald T., Zepeda R.J., Latini A., et al. Deep brain stimulation for obesity: a review and future directions. Front Neurosci. 2019 Apr 18;13:323. doi: 10.3389/fnins.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halpern C.H., Wolf J.A., Bale T.L., Stunkard A.J., Danish S.F., Grossman M., et al. Deep brain stimulation in the treatment of obesity: a review. J Neurosurg. 2008 Oct 1;109(4):625–634. doi: 10.3171/JNS/2008/109/10/0625. [DOI] [PubMed] [Google Scholar]
- 51.Talakoub O., Paiva R.R., Milosevic M., Hoexter M.Q., Franco R., Alho E., et al. Lateral hypothalamic activity indicates hunger and satiety states in humans. Ann Clin Transl Neurol. 2017 Dec 1;4(12):897–901. doi: 10.1002/acn3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franco R.R., Fonoff E.T., Alvarenga P.G., Alho E.J.L., Lopes A.C., Hoexter M.Q., et al. Assessment of safety and outcome of lateral hypothalamic deep brain stimulation for obesity in a small series of patients with Prader-Willi syndrome. JAMA Netw Open. 2018;1(7) doi: 10.1001/jamanetworkopen.2018.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manning K.E., Tait R., Suckling J., Holland A.J. Grey matter volume and cortical structure in Prader-Willi syndrome compared to typically developing young adults. NeuroImage Clin. 2018 Jan 1;17:899–909. doi: 10.1016/j.nicl.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukoshe A., Hokken-Koelega A.C., Van Der Lugt A., White T. Reduced cortical complexity in children with Prader-Willi syndrome and its association with cognitive impairment and developmental delay. Key A. PLoS One. 2014 Sep 16;9(9) doi: 10.1371/journal.pone.0107320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexis MJ, Barbosa DAN, Qiu L, Wong JK, Okun MS, Foote KD, et al. Defining a Deep Brain Stimulation Target for Hyperphagia in Prader-Willi Syndrome. In Los Angeles.
- 56.Honea R.A., Holsen L.M., Lepping R.J., Perea R., Butler M.G., Brooks W.M., et al. The neuroanatomy of genetic subtype differences in Prader-Willi syndrome. Am J Med Genet B Neuropsychiatr Genet. 2012 Mar;159B(2):243–253. doi: 10.1002/ajmg.b.32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada K., Watanabe M., Suzuki K. Differential volume reductions in the subcortical, limbic, and brainstem structures associated with behavior in Prader–Willi syndrome. Sci Rep. 2022 Mar 23;12(1):4978. doi: 10.1038/s41598-022-08898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada K., Watanabe M., Suzuki K., Suzuki Y. Cerebellar volumes associate with behavioral phenotypes in Prader-Willi syndrome. Cerebellum Lond Engl. 2020 Dec;19(6):778–787. doi: 10.1007/s12311-020-01163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucignani G., Panzacchi A., Bosio L., Moresco R.M., Ravasi L., Coppa I., et al. GABAA receptor abnormalities in Prader–Willi syndrome assessed with positron emission tomography and [11C]flumazenil. Neuroimage. 2004 May;22(1):22–28. doi: 10.1016/j.neuroimage.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 60.Holsen L.M., Savage C.R., Martin L.E., Bruce A.S., Lepping R.J., Ko E., et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader–Willi syndrome vs simple obesity. Int J Obes. 2012 May;36(5):638–647. doi: 10.1038/ijo.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapira N.A. Satiety dysfunction in Prader-Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry. 2005 Feb 1;76(2):260–262. doi: 10.1136/jnnp.2004.039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller J.L., James G.A., Goldstone A.P., Couch J.A., He G., Driscoll D.J., et al. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader-Willi syndrome. J Neurol Neurosurg Psychiatry. 2007 Jun 1;78(6):615–619. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holsen L.M., Zarcone J.R., Brooks W.M., Butler M.G., Thompson T.I., Ahluwalia J.S., et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity. 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieuwpoort I.C.V., Slagboom T.N.A., Jakobsdóttir S., Deijen J.B., Veltman D.J., Curfs L.M.G., et al. Food-related brain activation measured by fMRI in adults with Prader–Willi syndrome. J Clin Med. 2021 Oct 31;10(21):5133. doi: 10.3390/jcm10215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Zhao H., Qiu S., Tian J., Wen X., Miller J.L., et al. Altered functional brain networks in Prader-Willi syndrome. NMR Biomed. 2013 Jun;26(6):622–629. doi: 10.1002/nbm.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pujol J., Blanco-Hinojo L., Esteba-Castillo S., Caixàs A., Harrison B.J., Bueno M., et al. Anomalous basal ganglia connectivity and obsessive–compulsive behaviour in patients with Prader Willi syndrome. J Psychiatry Neurosci. 2016 Jul 1;41(4):261–271. doi: 10.1503/jpn.140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Wang J., Zhang G., Zhu Q., Cai W., Tian J., et al. The neurobiological drive for overeating implicated in Prader–Willi syndrome. Brain Res. 2015 Sep;1620:72–80. doi: 10.1016/j.brainres.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Yamada K., Suzuki K., Watanabe M. Altered functional network architecture of the brain in Prader-Willi syndrome. Brain Connect. 2022 Mar;12(2):174–179. doi: 10.1089/brain.2020.0914. [DOI] [PubMed] [Google Scholar]
- 69.Kim SE, Jin DK, Cho SS, Kim JH, Hong SD, Paik KH, et al. Regional cerebral glucose metabolic abnormality in Prader–Willi syndrome: a 18F-FDG PET study under sedation. [PubMed]
- 70.Correa-da-Silva F., Fliers E., Swaab D.F., Yi C.X. Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome. J Neuroendocrinol. 2021 Jul 1;33(7) doi: 10.1111/jne.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lukoshe A., Van Dijk S.E., Van Den Bosch G.E., Van Der Lugt A., White T., Hokken-Koelega A.C. Altered functional resting-state hypothalamic connectivity and abnormal pituitary morphology in children with Prader-Willi syndrome. J Neurodev Disord. 2017 Feb 21;9(1) doi: 10.1186/s11689-017-9188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swaab D. Prader-Willi syndrome and the hypothalamus. Acta Paediatr. 1997 Nov 1;86(S423):50–54. doi: 10.1111/j.1651-2227.1997.tb18369.x. [DOI] [PubMed] [Google Scholar]
- 73.Averbeck B.B., Murray E.A. Hypothalamic interactions with large-scale neural circuits underlying reinforcement learning and motivated behavior. Trends Neurosci. 2020 Sep 1;43(9):681–694. doi: 10.1016/j.tins.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blanco-Hinojo L., Pujol J., Esteba-Castillo S., Martínez-Vilavella G., Giménez-Palop O., Gabau E., et al. Lack of response to disgusting food in the hypothalamus and related structures in Prader Willi syndrome. NeuroImage Clin. 2019;21 doi: 10.1016/j.nicl.2019.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butler M.G., Bittel D.C., Kibiryeva N., Talebizadeh Z., Thompson T. Behavioral differences among subjects with Prader-Willi syndrome and Type I or Type II deletion and maternal disomy. Pediatrics. 2004 Mar 1;113(3):565–573. doi: 10.1542/peds.113.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shepherd D.A., Vos N., Reid S.M., Godler D.E., Guzys A., Moreno-Betancur M., et al. Growth trajectories in genetic subtypes of Prader–Willi syndrome. Genes. 2020 Jul 2;11(7):736. doi: 10.3390/genes11070736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Webb T., Whittington J., Clarke D., Boer H., Butler J., Holland A. A study of the influence of different genotypes on the physical and behavioral phenotypes of children and adults ascertained clinically as having PWS. Clin Genet. 2002 Oct 1;62(4):273–281. doi: 10.1034/j.1399-0004.2002.620404.x. [DOI] [PubMed] [Google Scholar]
- 78.Hartley S.L., MacLean W.E., Butler M.G., Zarcone J., Thompson T. Maladaptive behaviors and risk factors among the genetic subtypes of Prader-Willi syndrome. Am J Med Genet. 2005 Jul 15;136A(2):140–145. doi: 10.1002/ajmg.a.30771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butler M.G. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990 Mar;35(3):319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogels A., Hert M.D., Descheemaeker M.J., Govers V., Devriendt K., Legius E., et al. Psychotic disorders in Prader-Willi syndrome. Am J Med Genet. 2004 Jun 15;127A(3):238–243. doi: 10.1002/ajmg.a.30004. [DOI] [PubMed] [Google Scholar]
- 81.Fox R., Yang G.S., Feurer I.D., Butler M.G., Thompson T. Kinetic form discrimination in Prader-Willi syndrome. J Intellect Disabil Res. 2001 Aug;45(4):317–325. doi: 10.1046/j.1365-2788.2001.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holland A., Manning K., Whittington J. The paradox of Prader-Willi syndrome revisited: making sense of the phenotype. eBioMedicine. 2022 Apr 1:78. doi: 10.1016/j.ebiom.2022.103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holsen L.M., Zarcone J.R., Chambers R., Butler M.G., Bittel D.C., Brooks W.M., et al. Genetic subtype differences in neural circuitry of food motivation in Prader-Willi syndrome. Int J Obes. 2009 Feb;33(2):273–283. doi: 10.1038/ijo.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senova S., Cotovio G., Pascual-Leone A., Oliveira-Maia A.J. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 2019;12(1):119–128. doi: 10.1016/j.brs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Siddiqi S.H., Khosravani S., Rolston J.D., Fox M.D. The future of brain circuit-targeted therapeutics. Neuropsychopharmacology. 2023 Jul 31:1–10. doi: 10.1038/s41386-023-01670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shivacharan R.S., Rolle C.E., Barbosa D.A.N., Cunningham T.N., Feng A., Johnson N.D., et al. Pilot study of responsive nucleus accumbens deep brain stimulation for loss-of-control eating. Nat Med. 2022 Sep;28(9):1791–1796. doi: 10.1038/s41591-022-01941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Low A.Y.T., Goldstein N., Gaunt J.R., Huang K.P., Zainolabidin N., Yip A.K.K., et al. Reverse-translational identification of a cerebellar satiation network. Nature. 2021 Dec;600(7888):269–273. doi: 10.1038/s41586-021-04143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pisapia J.M., Halpern C.H., Muller U.J., Vinai P., Wolf J.A., Whiting D.M., et al. Ethical considerations in deep brain stimulation for the treatment of addiction and overeating associated with obesity. AJOB Neurosci. 2013 May;4(2):35–46. doi: 10.1080/21507740.2013.770420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grant R.A., Halpern C.H., Baltuch G.H., O'Reardon J.P., Caplan A. Ethical considerations in deep brain stimulation for psychiatric illness. J Clin Neurosci Off J Neurosurg Soc Australas. 2014 Jan;21(1):1–5. doi: 10.1016/j.jocn.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Hendriks S., Grady C., Ramos K.M., Chiong W., Fins J.J., Ford P., et al. Ethical challenges of risk, informed consent, and posttrial responsibilities in human research with neural devices: a review. JAMA Neurol. 2019 Dec 1;76(12):1506–1514. doi: 10.1001/jamaneurol.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dykens E.M., Roof E., Hunt-Hawkins H. ‘The cure for us is a lot of things’: how young people with Prader-Willi syndrome view themselves and future clinical trials. J Appl Res Intellect Disabil JARID. 2022 Mar 1;35(2):460–470. doi: 10.1111/jar.12950. [DOI] [PubMed] [Google Scholar]