Abstract
Background
Resistance training (RT) and protein supplementation have beneficial effects on the human body. However, it is unknown if RT's health-promoting benefits are enhanced by food-borne protein, such as cheese supplements. This study investigated at how the body composition, lipid profile, muscle strength and intestinal microbiota changed following four weeks of RT combined with cheese supplementation.
Methods
Thirty-five male and untrained adults were divided into 4 groups [control group (CON), low-dose group (LG), medium-dose group (MG), and high-dose group (HG)] and underwent a 4-week RT (3 times/week) in combination with cheese supplementation. Participants received 108 g (LG), 216 g (MG), or 324 g (HG) of cheese on the day of RT, and each serving (108 g) of cheese contained 6.7 g of food-borne protein. The RT program was a whole-body program with movements such as chest presses, leg presses, seated rowing, knee extensions and triceps pushdown. The exercise consisted of 3 sets of 8–12 repetitions at 70%RM, with a 120-s break in between. Body parameters (body composition, lipid profile and muscle strength) were assessed at baseline and after the 4 weeks of the intervention. The feces sample was taken every weekend. A two-way (group × time) mixed-design ANOVA was used to examine the body parameters. Independent one-way ANOVA was used to analyze the differences between groups in baseline characteristics and different values of each parameter.
Results
HDL-C level was higher in MG than in LG. In comparison to LG, MG had lower levels of total cholesterol, low-density lipoprotein cholesterol, body weight, body mass index, body fat mass and body fat percentage. However, there was no difference in muscle strength between in the four groups. The abundance of Actinobacteria was higher in LG and Erysipelotrichaceae was lower in MG and HG.
Conclusion
The findings suggest that cheese could be a readily available food-borne protein supplement to enhance the beneficial effects of RT on health. It may improve body composition and lipid profile by altering the proportion of intestinal microbiota. During the 4-week RT intervention, 13.4 g of foodborne protein in the form of cheese 3 times per week was the ideal dosage.
Keywords: Resistance training, Foodborne protein, Body composition, Lipid profile, Intestinal microbiota
1. Introduction
Regular resistance training (RT) is considered one of the effective means to promote health.1,2 RT can not only enhance muscle mass,3,4 strength, and exercise performance5,6 but also effectively improve body composition and reduce body weight.7, 8, 9 The volume, intensity, ability, and number of muscle groups trained per training session are associated with optimal RT frequency. The American College of Sports Medicine (ACSM) states that for people with no training experience, the recommended training frequency is 3–4 days weekly, and the load corresponds to a repetition range of 8–12 maximum repetitions.10 Numerous studies have shown that 2–3 alternating days per week can induce greater strength gains in untrained individuals.11
In the sports field, whether it is the general public or professional athletes, they need to take some supplements. In particular, many sport people eat protein supplements to promote muscle hypertrophy and strength during RT.12 Protein supplementary is crucial to maintaining the net protein balance and enhancing muscle protein synthesis.13,14 It is reported that the ingestion of 20 g high-quality protein can increase muscle protein synthesis after exercise15 and a high dose of protein (>1.6 g/kg/d) seems to boost fat loss.16 Therefore, some nutritional supplements, such as branched-chain amino acids,17 leucine,18 and whey protein,19 are used in combination with RT to enhance the benefits. However, under normal dietary conditions, protein is mainly consumed in whole foods, not in whey protein or isolated milk. Whole foods have various multiple nutrients that can increase gastrointestinal digestion and absorption.20
Cheese is a nutritionally whole food21 that is rich in protein and amino acids.22, 23, 24 In addition, cheese also contains carbohydrates that allow for complete recovery of carbohydrate reserves after exercise and ensure an adequate supply before and during exercise.25 It is reported that cheese (20–30 g protein) supplementation could promote protein anabolism at rest and after resistance exercise through the mammalian target of rapamycin (mTOR) signaling pathway.22,24 High-cheese diets (approximately 96–120 g) could increase the high-density lipoprotein (HDL-C),26 improve serum lipids27, 28, 29 and relieve atherogenic than a low-fat, high-carbohydrate diet.30,31 On the other hand, regarding the application of cheese intake in the exercise field, a study showed that cheese intake can improve amino acid concentration and muscle protein synthesis rate at rest and during recovery from single-leg resistance exercise.22There are few studies and information focus on the effects of food-borne protein supplementation as RT supplements. Thus, more studies are needed to prove if cheese is a safe and effective food-borne protein supplement.
Intestinal microbiota, the essential microflora of the human body, play important role in regulating body function and health and can be influenced by daily diet32, 33, 34 and exercise.35,36 It was reported that moderate-intensity exercise can increase intestinal microbial diversity35 and lead to health promotion.37 Previously, few studies have evaluated the combined effects of foodborne protein supplements and RT, and the effects of foodborne protein in the form of cheese on human function have been unclear. Therefore, we conducted a 4 weeks intervention to investigate the dose-effect relationship of cheese combined with RT on body composition, lipid profile, muscle strength and intestinal microbiota. To provide the basis for the rational application of cheese as a food-borne protein and RT supplement.
2. Materials and methods
2.1. Participants and experimental design
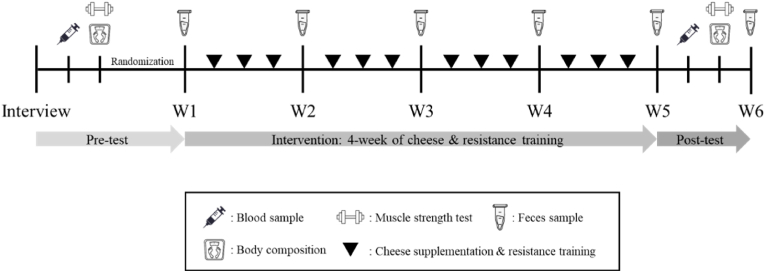
The research was a randomized and controlled trial, and it included 1 week of pre-test (body composition, lipid profile and muscle strength), 4 weeks of intervention (RT and cheese supplementation), and 1 week of post-test (Fig. 1). Because people who have no exercise habits can quickly gain muscle hypertrophy and muscle strength after resistance training. And previous studies have also used healthy men first as subjects when exploring new nutritional supplements. Therefore, healthy men with no exercise experience were used as participants in this study. Finally, thirty-five male and untrained participants were recruited for the research and randomly assigned to the control group or three groups of cheese treatment. Exclusion criteria were a diagnosis of diabetes mellitus, hypertension, cardiovascular disease, surgical history of bone injury, or regular exercise habits (30 min/times, 3 times/week). Persons with lactose intolerance, dairy protein allergies, or any diseases that may impact intestinal microbiota were not enrolled. A typical daily meal plan included 50–65% carbohydrate, 20–30% fat, and the rest of the protein. During the intervention, participants were asked to fill in the food record every day to ensure their daily intake of food, nutrients and energy (Table 1). They were also reminded not to consume dairy products and drink alcoholic beverages before the test to preserve the accuracy of the results. Participants had a suitable understanding of the intervention trial, agreed to voluntarily participate in the research, and signed the informed consent. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review committee of the National Institute of Sports Medicine, General Administration of Sport of China (202107).
Fig. 1.
Experimental design.
Table 1.
Daily energy and macronutrient intake.
| Nutrients | CON (n = 9) | LG (n = 9) | MG (n = 8) | HG (n = 9) | p-value |
|---|---|---|---|---|---|
| Energy (kcal) | 1703.9 ± 85.9 | 1774.6 ± 64.1 | 1774.6 ± 65.0 | 1749.6 ± 105.2 | 0.914 |
| Carbohydrate (g) | 212.4 ± 12.4 | 259.4 ± 6.5 | 262.5 ± 33.6 | 228.6 ± 19.8 | 0.371 |
| Carbohydrate (%) | 50.1 ± 5.4 | 58.6 ± 3.6 | 59.0 ± 5.4 | 52.2 ± 1.4 | 0.448 |
| Protein (g) | 72.8 ± 1.1 | 84.1 ± 12.5 | 74.5 ± 12.4 | 88.3 ± 3.0 | 0.601 |
| Protein (%) | 17.1 ± 0.6 | 18.9 ± 2.1 | 16.9 ± 3.4 | 20.2 ± 0.5 | 0.660 |
| Fat (g) | 59.1 ± 11.1 | 44.6 ± 4.5 | 47.4 ± 19.4 | 53.6 ± 34.0 | 0.439 |
| Fat (%) | 31.0 ± 4.3 | 22.5 ± 1.4 | 24.1 ± 2.0 | 27.6 ± 0.9 | 0.225 |
All data are expressed as the means ± SEM.
2.2. Supplementation protocol
The commercial and pudding-like cheese was made in Tianjin, China and per serving (108 g, 255 kcal) of cheese contained 6.7 g of food-borne protein. Other substances included 15.66 g carbohydrate, 18.58 g fat, 342.36 mg calcium and 465.48 mg sodium. Participants were given one (LG), two (MG), or three (HG) servings of cheese on the day of RT, respectively and CON received RT only. Avoiding eating all the cheese at once may cause discomfort, the consumption time of cheese was not the same among groups. MG and HG consumed cheese before and after exercise but LG only before exercise. LG, MG, and HG consumed firstly one serving of cheese (108 g) before training under the supervision of the staff. Then, the remaining doses of cheese in MG (108 g) and HG (216 g) were taken after training and we used phone calls or messages to follow up on the execution of supplementation.
2.3. Resistance training
According to the resistance training program of ACSM for people with no training experience, supervised and instructed RT was scheduled at 12:00–13:30. To increase muscle hypertrophy or muscle strength, training performed 3 times per week and allowed for at least one day in between training sessions. The RT program was a whole-body program which conducted with Technogym equipment (Cesena, Italy). Movements included chest press, leg press, seated row, knee extension and triceps pushdown. Under the guidance of professional and licensed instructors, exercise sessions began and ended with each 10 min of warm-up and stretching. Then, participants performed training of 8–12 repetitions at 70% repetition maximum (RM) and repeated for 3 times with 120 s rest between sets. In every training session, instructors adjusted the loads according to participants’ improvements and abilities. Regular exercise was defined as 30 min of exercise 3 times a week. Therefore, participants were asked not to exceed this level of physical activity and staff would call and message every three days for tracking and remaindering.
2.4. Body composition and lipid profile
Parameters of body composition (body weight, skeletal muscle mass, body fat mass, body lean mass, and body fat percentage) were measured by bioelectrical impedance analysis using Inbody 270 (Biospace, California, USA) at the beginning and end of the 4-week intervention trial. During 4 weeks intervention, all participants were asked to maintain their normal work and rest, including normal sleeping and balanced diet. Blood samples were collected at baseline and at the end of the intervention trial after an overnight fast (0:00–8:00). Participants were asked no food intake but moderate water intake during the fast. At 8:00 morning, blood samples were taken in a 5 ml vacutainer tube, were centrifuged at 4 °C over 10 min at 3500 rpm, and were kept at −20 °C until analysis. Total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and HDL-C were measured using an UniCel DxC 800 Synchron Clinical System (Beckman Coulter, Fullerton, USA).
2.5. Muscle strength test
We chose three tests commonly used to assess lower muscle strength, including one-repetition maximum (1-RM) of squat, isometric maximum voluntary contraction (MVC), and isokinetic total work. These tests were measured at the beginning and end of the 4-week intervention trial. To avoid the fatigue caused by other strength test, three tests were conducted on separate days. When participants first visited the laboratory and gym, staff explained matters needed attention and had participants performed light weight load to familiarize the procedure. Participants were also reminded to avoid doing high-intensity exercise and taking medicine three days before the tests to ensure the accuracy of exercise performance. Before tests, participants performed jogging and dynamic stretching to prevent sports injuries. Staff predicted each participant's 1-RM, and then participants performed a load of 70–80% on squat rack (Smith machine, New York, USA) at the first trial. After a successful finish, the load was increased in increments of 5–10 kg until only could complete one repetition. MVC at 60° of both legs was performed by dynamometer-based device (David Concept System, Helsinki, Finland). Participants performed 2 maximal exertions with a 2 min interval break. Each test included 3 s maximum contraction with a rest interval of 3 min and recorded the peak torque to determine the MVC. The isokinetic total work at 30°/s of the dominant leg was tested by using IsoMed 2000 isokinetic dynamometer (D. & R. Ferstl GmbH, Hemnau, Germany). Participants sat on the chair and the hip joint was 90° flexion, using straps to prevent torso movements. To precise the axis of rotation of the knee joint, we used the lateral femur condyle as anatomical orientation. Each test included 1 maximum flexion and 1 maximum extension (3 s for each contraction) and took three tests to determine the maximum value for data analysis.
2.6. Intestinal microbiota
Participants' feces samples were taken once on the weekend each week (pre-test, post-test and 4-week intervention trial). Samples were stored using the 1.5 ml centrifuge tube and kept at −80 °C until analysis. 210 samples were sent to QingLianBio for microbiome detection. DNA was extracted and quantified with OMEGA Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA), library preparation was performed with TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, USA), and 16S sequencing (2 × 250 bp PE V3–V4) was conducted with NovaSeq 6000 SP Reagent Kit (Illumina, San Diego, USA). Using the USEARCH to align the high-quality sequences to the reference database at 97% similarity and represent an operational taxonomic unit (OTU) as a genetically unique group. Based on the SILVA database, these OTUs were curated taxonomic labels. Then, the OTUs were used to examine the relative abundance through Metastats and lefse and compared the pathways and effects with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
2.7. Statistical analysis
Data are expressed as means (SEM) and analyzed with SPSS 25 and GraphPad Prism 10. Body composition, lipid profile, and muscle strength were evaluated using a two-way (group × time) mixed-design analysis of variance (ANOVA). Independent one-way ANOVA was used to analyze the differences among groups in the baseline characteristics and different values of each parameter. They were both followed with multiple comparisons by post hoc Tukey's test. The statistical level was set at p < 0.05.
Using G-Power 3.1 software to calculate the sample size. The following: F tests, ANOVA (repeated measures, between factors) and A priori are used. We used G*Power software to calculate the appropriate sample size and power of our research. After calculating, the total sample size for 4 groups needs at least 28 participants in this research, the overall effect size of 0.4, and the actual power is 80.7%. In the end, 35 participants finished this experimental trial. Using the sensitivity of G-power to calculate the effect size and the value was 0.29 (between mid-to-high standard).
3. Results
3.1. Participants
Our recruitment goal was thirty-six participants but one participant (from the MG group) withdrew due to time constraints. Therefore, thirty-five participants (CON = 9; LG = 9; MG = 8; HG = 9) had completed the study. Mean age of participants was 20.8 ± 0.5 years and body mass index (BMI) was 23.49 ± 0.64 kg/m2. Table 2 showed the baseline characteristics of participants in four groups, with no statistical differences observed.
Table 2.
Baseline characteristics and all parameters of the participants before and after the 4-week intervention.
| Parameter | CON (n = 9) | LG (n = 9) | MG (n = 8) | HG (n = 9) | Time p-value | Group p-value | Interaction p-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 21.8 ± 1.0 | 22.5 ± 1.2 | 19.5 ± 0.2 | 19.2 ± 0.3 | |||
| Height (cm) | 175.67 ± 1.80 | 173.17 ± 2.14 | 179.67 ± 1.40 | 176.50 ± 2.58 | |||
| Body composition | |||||||
| Body weight (kg) | |||||||
| pre | 76.15 ± 7.11 | 68.98 ± 1.82 | 74.18 ± 3.92 | 70.80 ± 3.90 | 0.354 | 0.717 | 0.000 |
| post | 76.33 ± 6.98 | 70.02 ± 1.94 | 73.22 ± 4.07 | 69.97 ± 4.04 | |||
| BMI (kg/m2) | |||||||
| pre | 24.75 ± 2.15 | 23.02 ± 0.65 | 22.97 ± 1.11 | 23.23 ± 0.95 | 0.522 | 0.731 | 0.001 |
| post | 24.78 ± 2.11 | 23.37 ± 0.68 | 22.65 ± 1.16 | 23.03 ± 0.89 | |||
| Skeletal muscle mass (kg) | |||||||
| pre | 33.43 ± 1.17 | 33.05 ± 0.96 | 32.67 ± 1.28 | 32.05 ± 1.75 | 0.115 | 0.820 | 0.110 |
| post | 33.78 ± 1.15 | 33.50 ± 1.21 | 32.93 ± 1.26 | 31.73 ± 1.80 | |||
| Body fat mass (kg) | |||||||
| pre | 10.45 ± 1.80 | 10.08 ± 1.49 | 12.65 ± 3.06 | 10.32 ± 2.33 | 0.021 | 0.908 | 0.006 |
| post | 10.35 ± 1.81 | 10.35 ± 1.42 | 11.55 ± 2.88* | 10.02 ± 2.06 | |||
| Body lean mass (kg) | |||||||
| pre | 58.35 ± 1.95 | 58.20 ± 1.66 | 58.23 ± 2.17 | 56.82 ± 2.89 | 0.148 | 0.894 | 0.108 |
| post | 58.88 ± 1.95 | 59.12 ± 2.09 | 58.53 ± 2.10 | 56.28 ± 2.94 | |||
| Body fat percentage (%) | |||||||
| pre | 16.90 ± 2.06 | 14.58 ± 1.95 | 16.95 ± 2.91 | 15.07 ± 3.20 | 0.023 | 0.926 | 0.032 |
| post | 16.52 ± 2.15 | 14.77 ± 1.80 | 15.60 ± 2.83* | 14.90 ± 2.83 | |||
| Lipid profile | |||||||
| TC (mmol/L) | |||||||
| pre | 4.19 ± 0.38 | 4.38 ± 0.47 | 4.11 ± 0.37 | 4.23 ± 0.46 | 0.119 | 0.718 | 0.006 |
| post | 4.38 ± 0.45 | 4.99 ± 0.50 | 4.10 ± 0.29 | 3.95 ± 0.38 | |||
| HDL-C (mmol/L) | |||||||
| pre | 1.22 ± 0.07 | 1.43 ± 1.18 | 1.34 ± 0.11 | 1.51 ± 0.13 | 0.009 | 0.361 | 0.011 |
| post | 1.32 ± 0.08* | 1.65 ± 0.11** | 1.44 ± 0.16 | 1.42 ± 0.11 | |||
| LDL-C (mmol/L) | |||||||
| pre | 2.58 ± 0.40 | 2.60 ± 0.48 | 2.53 ± 0.29 | 2.39 ± 0.33 | 0.370 | 0.713 | 0.000 |
| post | 2.74 ± 0.45 | 3.12 ± 0.50 | 2.28 ± 0.25 | 2.18 ± 0.30 | |||
| TG (mmol/L) | |||||||
| pre | 0.84 ± 0.10 | 0.74 ± 0.09 | 0.91 ± 0.12 | 0.89 ± 0.06 | 0.801 | 0.982 | 0.368 |
| post | 0.84 ± 0.06 | 0.88 ± 0.16 | 0.81 ± 0.09 | 0.80 ± 0.09 | |||
| Muscle strength | |||||||
| 1-RM (kg) | |||||||
| pre | 112.5 ± 13.1 | 125.0 ± 8.5 | 105.8 ± 7.2 | 99.2 ± 15.0 | 0.000 | 0.381 | 0.992 |
| post | 127.5 ± 10.7** | 140.8 ± 8.0** | 121.7 ± 7.8* | 115.8 ± 12.0* | |||
| MVC (Nm) | |||||||
| pre | 499.7 ± 25.3 | 451.5 ± 36.2 | 446.5 ± 39.0 | 448.7 ± 41.7 | 0.001 | 0.803 | 0.769 |
| post | 529.7 ± 34.0* | 514.7 ± 53.3 | 484.2 ± 39.7* | 500.8 ± 38.0 | |||
| Isokinetic total work (J) | |||||||
| pre | 384.3 ± 35.2 | 381.2 ± 23.6 | 378.7 ± 38.3 | 418.3 ± 45.3 | 0.000 | 0.856 | 0.430 |
| post | 441.7 ± 25.1** | 428.8 ± 25.7** | 413.0 ± 40.2** | 455.5 ± 41.3* | |||
All data are expressed as the means ± SEM. Significant difference between the pre-test and post-test of each group, *p < 0.05; **p < 0.01. Abbreviations: BMI: body mass index; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides; RM: repetition maximum; MVC: maximum voluntary contraction.
3.2. Body composition
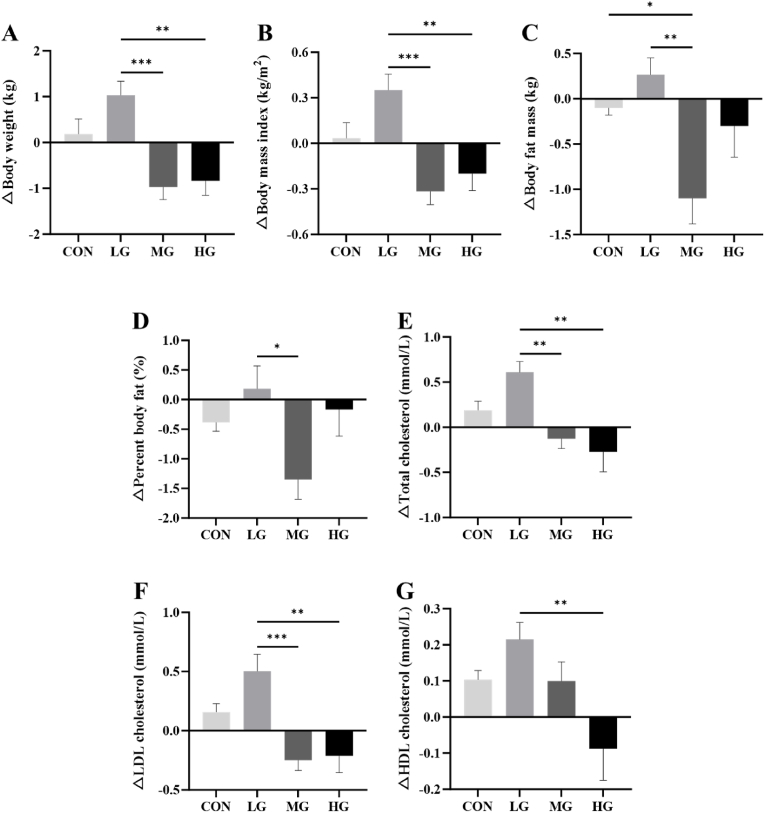
Four weeks of RT alone did not improve the body composition significantly (Table 2). But combined with cheese supplement, the variation trend of body composition changed. Compared with LG, the difference values of body weight (Fig. 2A) and BMI (Fig. 2B) of MG and HG, and the difference value of body fat mass (Fig. 2C) and body fat percentage (Fig. 2D) of MG were significantly lower (p < 0.05).
Fig. 2.
The difference values of body composition and lipid profile after 4 weeks of RT combined with cheese supplementation. (A) Body weight, (B) BMI, (C) Body fat mass, (D) Percent body fat, (E) Total cholesterol, (F) LDL-C, and (G) HDL-C. Data shown as means ± SEM. *, ** and ***: Significant differences among groups, at p < 0.05, 0.01, and 0.001, respectively.
3.3. Lipid profile
Four weeks of RT alone could only improve the HDL-C (Table 2). And combined with cheese supplement, the variation trend of serum lipids changed with the dose. Compared with LG, the difference values of TC (Fig. 2E) and LDL-C (Fig. 2F) of MG and HG, and the difference value of HDL-C (Fig. 2G) of HG was significantly lower (p < 0.05).
3.4. Muscle strength
After 4 weeks of intervention, the1-RM of the squat and isokinetic total work of each group increased significantly (p < 0.05), and the MVC of CON and MG also had a significant increase (Table 2). But the difference values of 1-RM of squat, MVC, and isokinetic total work did not differ between the four groups.
3.5. Intestinal microbiota
After 6 weeks of collection, including pre-test, post-test and 4 weeks of intervention, all samples were classified and expressed as an average of 6 weeks. They were aligned the high-quality sequences to the reference database at 97% similarity and represent an OTU. A total of 221891 sequences were analyzed in the samples. Sequence numbers in CON, LG, MG, and HG were 55687, 54874, 54739, and 56591, and the number of OTUs was 952, 1028, 980, and 1405, respectively.
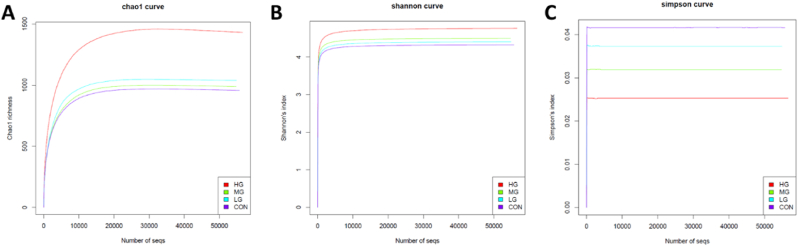
Alpha diversity curves were used to evaluate species richness in four groups (Fig. 3). The Chao1 richness (Fig. 3A), Shannon's index (Fig. 3B), and Simpson's index (Fig. 3C) were calculated. The curves pointed to an increasing trend in HG and these data showed the observed increase in microbial diversity in the effect of the high-dose cheese supplementation combined with RT.
Fig. 3.
(A) Chao1 richness, (B) Shannon's index, and (C) Simpson's index in four groups. A horizontal line marks the number of sequences and vertical axis marks each alpha diversity curve.
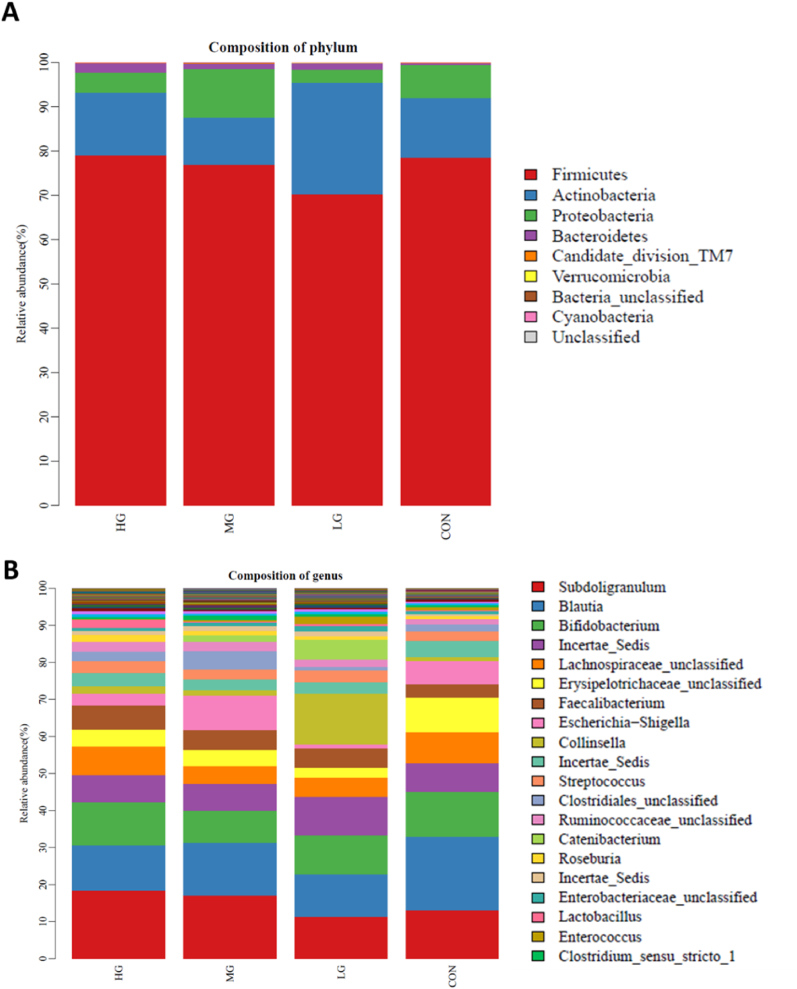
All taxa at the phylum and genus of the mean relative abundance were presented with broad taxonomic differences in four groups (Fig. 4). Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidales were the four most abundant bacterial phyla in the intestinal microbiota (Fig. 4A), and Subdoligranulum, Blautia, and Bifidobacterium were the three most abundant genera (Fig. 4B). For the phylum and genus levels, the average and relative proportion of intervention in each group is shown in Table 3. Compared with CON, the relative proportion of Firmicutes of LG was significantly lower. The relative proportion of Actinobacteria of LG was significantly higher than other groups, the level of Proteobacteria of MG was higher than LG and HG, and the level of Bacteroidales of HG was higher than other groups. Compared with LG, the relative proportion of the Subdoligranulum of HG was significantly higher. The proportion of Blautia of CON was significantly higher than LG and HG. As regards Erysipelotrichaceae, the proportion of CON was higher than in other groups.
Fig. 4.
Mean relative abundance (%) of (A) phylum and (B) genus in four groups.
Table 3.
The relative proportion of major microbiota for phylum or genus levels.
| Level (%) | CON (n = 9) | LG (n = 9) | MG (n = 8) | HG (n = 9) | p-value | |
|---|---|---|---|---|---|---|
| Phylum | Firmicutes | 80.49 | 72.66 | 78.93 | 79.08 | 0.100 |
| Actinobacteria | 12.05a | 22.48b | 9.50a | 13.19a | 0.000 | |
| Proteobacteria | 6.68ab | 2.77a | 9.65b | 4.22a | 0.006 | |
| Bacteroidales | 0.60a | 1.56a | 1.49a | 3.06b | 0.012 | |
| Genus | Subdoligranulum | 12.93ab | 10.85a | 15.94ab | 17.47b | 0.042 |
| Blautia | 19.05a | 10.95b | 13.33ab | 11.70b | 0.001 | |
| Bifidobacterium | 10.69 | 9.29 | 7.49 | 10.35 | 0.560 | |
| Erysipelotrichaceae | 8.66a | 2.44b | 4.40b | 4.20b | 0.000 | |
| Faecalibacterium | 3.42 | 4.65 | 4.78 | 6.04 | 0.186 | |
All data are expressed as the means. Different letters (a, b) in the same column of data are significantly different, p < 0.05. If any letter is the same, it means that the difference between groups is not significant.
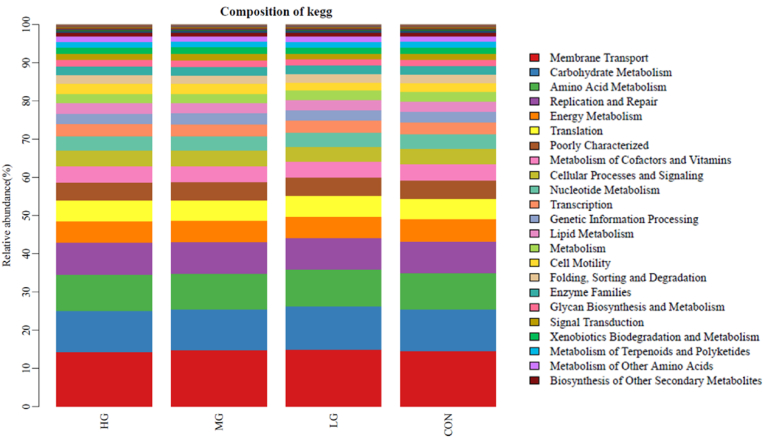
Based on the PCRUSI abundance table and KEGG database, 39 metabolic pathway predictions were obtained by calculating the 16S rRNA gene abundance values of each sample (Fig. 5). The data showed that Membrane Transport, Carbohydrate Metabolism, Amino Acid Metabolism, Replication and Repair, and Energy Metabolism are the five most dominant pathways. However, there were no significant differences in metabolic pathway predictions between groups.
Fig. 5.
KEGG metabolic pathway predictions.
4. Discussion
This is the first study to investigate the effects of RT combined with different dose cheese supplementation on body composition, lipid profile, muscle strength, and intestinal microbiota. Results showed that 4 weeks of RT combined with medium-dose cheese supplementation had the best improvements, including the decrease of body weight, BMI, fat mass, fat percentage, and TC and LDL-C levels. It was possible that these effects were due to changes in intestinal microbiome caused by consuming cheese and medium was the more suitable dosage.
Numerous experimental research and system reviews have shown that RT is one of the effective methods for the improvement of body composition.38, 39, 40, 41, 42 For example, male adults performed short-term RT of 12 repetitions at 70–85%1RM and repeated 3 times were sufficient to increase the strength of squat.43,44 In the different populations, Kim et al.45 demonstrated that a 4-week intervention of 3 sets of 8 repetitions at 80%1RM and trained 3 times/week could also improve strength in college-aged women. On the other hand, RT has also been identified as a novel approach to improving blood lipid patterns.46, 47, 48 Son et al.49 reported that the level of HDL-C increased 5 mg/dl after conducting a 12-week of RT in women. And this positive influence was also supported by the study of Bemben et al.,50 which found that conducting RT 3 times a week for 16 weeks could improve HDL-C levels. As has been previously proposed, we observed a significantly greater gain in muscle strength and HDL-C levels after the 4-week intervention (Table 2). Too low exercise workload cannot induce changes51 and the intervention in this study was shorter than the above research. Therefore, our results suggested that the total volume, tri-weekly moderate-high intensity training, of our training program was enough to improve body states.
The worldwide trends of increasing nutrient strategies have increased the focus on understanding how different protein sources impact body composition and lipid profiles. Some studies have examined the positive effects of different protein supplements on body composition. For example, Teixeira et al.52 demonstrated that 8 weeks of supplementation of plant-based protein and whey protein both reduced fat mass in college-aged males. In the same population, Joy et al.53 also demonstrated that consuming rice protein or whey protein during RT decreased fat mass. The benefits of protein supplementation were also related to lipid profile. Soy protein was the most effective protein source to improve blood lipid and several studies have reported that the consumption of soy protein could decrease serum cholesterol and LDL-C.54,55 As has been previously proposed, we found that the 4-week intervention not only improved body weight (Fig. 2A) and body fat mass (Fig. 2B), but also decreased TC (Fig. 2A) and LDL-C (Fig. 2C) levels. Our results suggested that cheese had similar benefits to other protein supplements.
It was also worth mentioning that this study was the first time to investigate the effects of RT combined with cheese supplementation on blood lipids. And the improvement of lipid parameters observed in MG or HG groups may be correlated with the deterioration of lipid profile observed in LG group. We found the abundance of Actinobacteria in CON, MG and HG groups were decreased compared to LG group (Table 3). And Actinobacteria have the capacity to accumulating TAG under nitrogen limiting conditions.56 Furthermore, we also found the abundance of Erysipelotrichaceae was decreased after RT combined with cheese supplementation (Table 3) and there were the positive correlations between Erysipelotrichaceae and lipid metabolic disorders.57,58 Ingesting cheese had not only changed the proportion of Actinobacteria, it had also caused changes in other microbiota species such as Erysipelotrichaceae. And that may be why the MG and HG groups tended to improve blood lipids more than the CON group. Some bioactive metabolites may be produced after consuming cheese,59 and it changed the lipid metabolism and body parameters through altered relative proportion of microbiota.
Regarding the dose of protein consumption, the results of the present study showed that more cheese supplementation could significantly decrease body weight and fat mass when compared to the low dose. And consuming low-dose cheese did not induce differences when compared to the control. These suggested that the low protein ingestion may not have been a sufficient stimulus to alter the body parameters. However, there was no difference between medium- and high-dose of cheese supplementation. The phenomenon in which excess protein intake failed to gain more benefits was also supported by the study of Moore et al.60 They found that 20g protein intake after a single exercise resulted in a greater increase in muscle protein synthesis, while a double dose had no significant difference.
In addition to being related to body phenotype, the dose of protein consumption also affected the intestinal microbiota. Different doses of protein consumption influenced microbiotic diversity. For example, Mckenna et al.61 conducted a 3-week intervention of RT in 50 adults, and they had to ingest 15g or 30g of protein twice a day. This study found that the higher dose of protein could increase microbiotic diversity. A 6-week protein and RT intervention trial conducted by Cronin et al.62 also showed that 30 g/day dietary protein intake combined with exercise training could enhance the microbiota diversity compared to only protein or exercise intervention. Similar to previous studies, we found that RT combined cheese supplementation had an increasing trend on microbiotic diversity, and it existed a dose-effect association (Fig. 3). The possible explanation may be that amino acids produced by cheese decomposition could be used as an energy source to promote microbiota growth.59
There are some limitations may have influenced the results of this study. First of all, there was no adjustment according to body weight for cheese supplementation. This may lead to differences in absorption efficiency. Second, the study was also limited by cheese containing a few other substances, such as carbohydrate, fat or calcium. They may induce some additional changes alone or together to the results. In the present study, we primarily compared the effects of RT combined with different grams of food-borne protein in the form of cheese and found positive results at medium doses. The effects of intake patterns (did not standardized for body weight or at different time points) on the mTOR pathway needs to be explored in future studies. Furthermore, we only verbally asked participants to maintain the same physical activity every day, which should be monitored in the future using instruments such as accelerometers.
5. Conclusion
Consuming 3 times weekly of 13.4 g food-borne protein in the form of cheese combined with RT for 4 weeks improved the body composition, lipid profile and the diversity of the intestinal microbiota. Our findings provided the scientific basis that cheese could be an easily available food-borne protein supplement to boost the positive effects of RT on health.
Funding
This study was supported by the Herbalife Winter Sports Development Fund (KBL2021007) and the National Key Research and Development Program of China (2019YFF0301702-02-04).
Declaration of competing interest
All authors disclosed no relevant relationships.
Acknowledgments
The authors are thankful to the participants for volunteering their time and efforts. Thanks to Beijing Sport University and Key Laboratory of Exercise and Physical Fitness (Beijing Sport University), Ministry of Education that provided the technology and equipment to this study.
Contributor Information
Xue-Han Li, Email: 2020110078@bsu.edu.cn.
Hao-Tian Zhao, Email: 2019210252@bsu.edu.cn.
Jian-Hao Chen, Email: chenjianhao@bsu.edu.cn.
Jia-Qi Li, Email: 2021210475@bsu.edu.cn.
Yi Yan, Email: yanyi@bsu.edu.cn.
References
- 1.Dieli-Conwright C.M., Courneya K.S., Demark-Wahnefried W., et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20(1):124. doi: 10.1186/s13058-018-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano-Maldonado A., Carrera-Ruiz A., Diez-Fernandez D.M., et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors: study protocol for the EFICAN randomized controlled trial. Medicine (Baltim) 2019;98(44) doi: 10.1097/MD.0000000000017625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfeld B.J., Contreras B., Krieger J., et al. Resistance training volume enhances muscle hypertrophy but not strength in trained men. Med Sci Sports Exerc. 2019;51(1):94–103. doi: 10.1249/MSS.0000000000001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchward-Venne T.A., Burd N.A., Phillips S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab. 2012;9(1):40. doi: 10.1186/1743-7075-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez P., Radaelli R., Taaffe D.R., et al. Resistance training load effects on muscle hypertrophy and strength gain: systematic review and network meta-analysis. Med Sci Sports Exerc. 2021;53(6):1206–1216. doi: 10.1249/MSS.0000000000002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenfeld B.J., Pope Z.K., Benik F.M., et al. Longer interset rest periods enhance muscle strength and hypertrophy in resistance-trained men. J Strength Condit Res. 2016;30(7):1805–1812. doi: 10.1519/JSC.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 7.Myers J., Kokkinos P., Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. doi: 10.3390/nu11071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedewa M.V., Spencer S.O., Williams T.D., Becker Z.E., Fuqua C.A. Effect of branched-chain amino acid supplementation on muscle soreness following exercise: a meta-analysis. Int J Vitam Nutr Res. 2019;89(5-6):348–356. doi: 10.1024/0300-9831/a000543. [DOI] [PubMed] [Google Scholar]
- 9.Morton R.W., Murphy K.T., McKellar S.R., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 11.Rhea M.R., Alvar B.A., Burkett L.N., Ball S.D. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc. 2003;35(3):456–464. doi: 10.1249/01.MSS.0000053727.63505.D4. [DOI] [PubMed] [Google Scholar]
- 12.Davies R.W., Carson B.P., Jakeman P.M. The effect of whey protein supplementation on the temporal recovery of muscle function following resistance training: a systematic review and meta-analysis. Nutrients. 2018;10(2):221. doi: 10.3390/nu10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton L.E., Wilson G.J., Moulton C.J., Layman D.K. Meal distribution of dietary protein and leucine influences long-term muscle mass and body composition in adult rats. J Nutr. 2017;147(2):195–201. doi: 10.3945/jn.116.231779. [DOI] [PubMed] [Google Scholar]
- 14.Mishra S., Goldman J.D., Sahyoun N.R., Moshfegh A.J. Association between dietary protein intake and grip strength among adults aged 51 years and over: what We Eat in America, National Health and Nutrition Examination Survey 2011-2014. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGlory C., Devries M.C., Phillips S.M. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Physiol. 2017;122(3):541–548. doi: 10.1152/japplphysiol.00613.2016. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonio J., Candow D.G., Forbes S.C., Ormsbee M.J., Saracino P.G., Roberts J. Effects of dietary protein on body composition in exercising individuals. Nutrients. 2020;12(6):1890. doi: 10.3390/nu12061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotkin D.L., Delcastillo K., Van Every D.W., Tipton K.D., Aragon A.A., Schoenfeld B.J. Isolated leucine and branched-chain amino acid supplementation for enhancing muscular strength and hypertrophy: a narrative review. Int J Sport Nutr Exerc Metabol. 2021;31(3):292–301. doi: 10.1123/ijsnem.2020-0356. [DOI] [PubMed] [Google Scholar]
- 18.Phillips S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr Metab. 2016;13:64. doi: 10.1186/s12986-016-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Liu F. Effect of whey protein supplementation during resistance training sessions on body mass and muscular strength: a meta-analysis. Food Funct. 2019;10(5):2766–2773. doi: 10.1039/c9fo00182d. [DOI] [PubMed] [Google Scholar]
- 20.Thorning T.K., Bertram H.C., Bonjour J.P., et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105(5):1033–1045. doi: 10.3945/ajcn.116.151548. [DOI] [PubMed] [Google Scholar]
- 21.Temme E.H., Toxopeus I.B., Kramer G.F., et al. Greenhouse gas emission of diets in The Netherlands and associations with food, energy and macronutrient intakes. Publ Health Nutr. 2015;18(13):2433–2445. doi: 10.1017/S1368980014002821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermans W.J.H., Fuchs C.J., Hendriks F.K., et al. Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: a randomized parallel-group trial. J Nutr. 2022;152(4):1022–1030. doi: 10.1093/jn/nxac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleman-Mateo H., Carreon V.R., Macias L., Astiazaran-Garcia H., Gallegos-Aguilar A.C., Enriquez J.R. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single-blind randomized clinical trial. Clin Interv Aging. 2014;9:1517–1525. doi: 10.2147/CIA.S67449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Hart N., Mahmassani Z.S., Reidy P.T., et al. Acute effects of cheddar cheese consumption on circulating amino acids and human skeletal muscle. Nutrients. 2021;13(2):614. doi: 10.3390/nu13020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargreaves M. Carbohydrates and exercise. J Sports Sci. 1991;9:17–28. doi: 10.1080/02640419108729864. Spec No. [DOI] [PubMed] [Google Scholar]
- 26.Thorning T.K., Raziani F., Bendsen N.T., Astrup A., Tholstrup T., Raben A. Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: a randomized crossover trial. Am J Clin Nutr. 2015;102(3):573–581. doi: 10.3945/ajcn.115.109116. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M.T., Nara T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 28.Nestel P.J., Chronopulos A., Cehun M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 2005;59(9):1059–1063. doi: 10.1038/sj.ejcn.1602211. [DOI] [PubMed] [Google Scholar]
- 29.Hjerpsted J., Leedo E., Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr. 2011;94(6):1479–1484. doi: 10.3945/ajcn.111.022426. [DOI] [PubMed] [Google Scholar]
- 30.Hostmark A.T., Tomten S.E. The Oslo health study: cheese intake was negatively associated with the metabolic syndrome. J Am Coll Nutr. 2011;30(3):182–190. doi: 10.1080/07315724.2011.10719959. [DOI] [PubMed] [Google Scholar]
- 31.Hostmark A.T., Haug A. Does cheese intake blunt the association between soft drink intake and risk of the metabolic syndrome? Results from the cross-sectional Oslo Health Study. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong T.S., Luu K., Lagishetty V., et al. A high protein calorie restriction diet alters the gut microbiome in obesity. Nutrients. 2020;12(10):3221. doi: 10.3390/nu12103221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dao M.C., Clement K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med. 2018;48:18–24. doi: 10.1016/j.ejim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Isolauri E. Microbiota and obesity. Nestle Nutr Inst Workshop Ser. 2017;88:95–105. doi: 10.1159/000455217. [DOI] [PubMed] [Google Scholar]
- 35.Clarke S.F., Murphy E.F., O'Sullivan O., et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 36.Allen J.M., Mailing L.J., Niemiro G.M., et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 37.Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 38.Suchomel T.J., Nimphius S., Stone M.H. The importance of muscular strength in athletic performance. Sports Med. 2016;46(10):1419–1449. doi: 10.1007/s40279-016-0486-0. [DOI] [PubMed] [Google Scholar]
- 39.Cormie P., McGuigan M.R., Newton R.U. Developing maximal neuromuscular power: part 2 - training considerations for improving maximal power production. Sports Med. 2011;41(2):125–146. doi: 10.2165/11538500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.McQuilliam S.J., Clark D.R., Erskine R.M., Brownlee T.E. Free-weight resistance training in youth athletes: a narrative review. Sports Med. 2020;50(9):1567–1580. doi: 10.1007/s40279-020-01307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomas-Carus P., Ortega-Alonso A., Pietilainen K.H., et al. A randomized controlled trial on the effects of combined aerobic-resistance exercise on muscle strength and fatigue, glycemic control and health-related quality of life of type 2 diabetes patients. J Sports Med Phys Fit. 2016;56(5):572–578. [PubMed] [Google Scholar]
- 42.Robinson J.M., Stone M.H., Johnson R.L., Penland C.M., Warren B.J., Lewis R.D. Effects of different weight training exercise/rest intervals on strength, power, and high intensity exercise endurance. J Strength Condit Res. 1995;9(4):216–221. doi: 10.3390/ijerph18137201. [DOI] [Google Scholar]
- 43.Aube D., Wadhi T., Rauch J., et al. Progressive resistance training volume: effects on muscle thickness, mass, and strength adaptations in resistance-trained individuals. J Strength Condit Res. 2022;36(3):600–607. doi: 10.1519/JSC.0000000000003524. [DOI] [PubMed] [Google Scholar]
- 44.Androulakis-Korakakis P., Fisher J.P., Steele J. The minimum effective training dose required to increase 1RM strength in resistance-trained men: a systematic review and meta-analysis. Sports Med. 2020;50(4):751–765. doi: 10.1007/s40279-019-01236-0. [DOI] [PubMed] [Google Scholar]
- 45.Kim E., Dear A., Ferguson S.L., Seo D., Bemben M.G. Effects of 4 weeks of traditional resistance training vs. superslow strength training on early phase adaptations in strength, flexibility, and aerobic capacity in college-aged women. J Strength Condit Res. 2011;25(11):3006–3013. doi: 10.1519/JSC.0b013e318212e3a2. [DOI] [PubMed] [Google Scholar]
- 46.Boyden T.W., Pamenter R.W., Going S.B., et al. Resistance exercise training is associated with decreases in serum low-density lipoprotein cholesterol levels in premenopausal women. Arch Intern Med. 1993;153(1):97–100. [PubMed] [Google Scholar]
- 47.Fahlman M.M., Boardley D., Lambert C.P., Flynn M.G. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J Gerontol A Biol Sci Med Sci. 2002;57(2):B54–B60. doi: 10.1093/gerona/57.2.b54. [DOI] [PubMed] [Google Scholar]
- 48.Kelley G.A., Kelley K.S. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48(1):9–19. doi: 10.1016/j.ypmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Son W.M., Park J.J. Resistance band exercise training prevents the progression of metabolic syndrome in obese postmenopausal women. J Sports Sci Med. 2021;20(2):291–299. doi: 10.52082/jssm.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bemben D.A., Bemben M.G. Effects of resistance exercise and body mass index on lipoprotein-lipid patterns of postmenopausal women. J Strength Condit Res. 2000;14(1):80–85. [Google Scholar]
- 51.Wallace M.B., Moffatt R.J., Haymes E.M., Green N.R. Acute effects of resistance exercise on parameters of lipoprotein metabolism. Med Sci Sports Exerc. 1991;23(2):199–204. doi: 10.1249/00005768-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira F.J., Matias C.N., Faleiro J., et al. A novel plant-based protein has similar effects compared to whey protein on body composition, strength, power, and aerobic performance in professional and semi-professional futsal players. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.934438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joy J.M., Lowery R.P., Wilson J.M., et al. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr J. 2013;12:86. doi: 10.1186/1475-2891-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhan S., Ho S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81(2):397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds K., Chin A., Lees K.A., Nguyen A., Bujnowski D., He J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am J Cardiol. 2006;98(5):633–640. doi: 10.1016/j.amjcard.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez H.M. Triacylglycerol and wax ester-accumulating machinery in prokaryotes. Biochimie. 2016;120:28–39. doi: 10.1016/j.biochi.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Kaakoush N.O. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;5:84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen M.X., Wang S.Y., Kuo C.H., Tsai I.L. Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc. 2019;118(Suppl 1):S10–S22. doi: 10.1016/j.jfma.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Neis E.P., Dejong C.H., Rensen S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore D.R., Robinson M.J., Fry J.L., et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89(1):161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 61.McKenna C.F., Salvador A.F., Hughes R.L., et al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: a randomized control trial. Am J Physiol Endocrinol Metab. 2021;320(5):E900–E913. doi: 10.1152/ajpendo.00574.2020. [DOI] [PubMed] [Google Scholar]
- 62.Cronin O., Barton W., Skuse P., et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. 2018;3(3) doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]