Abstract
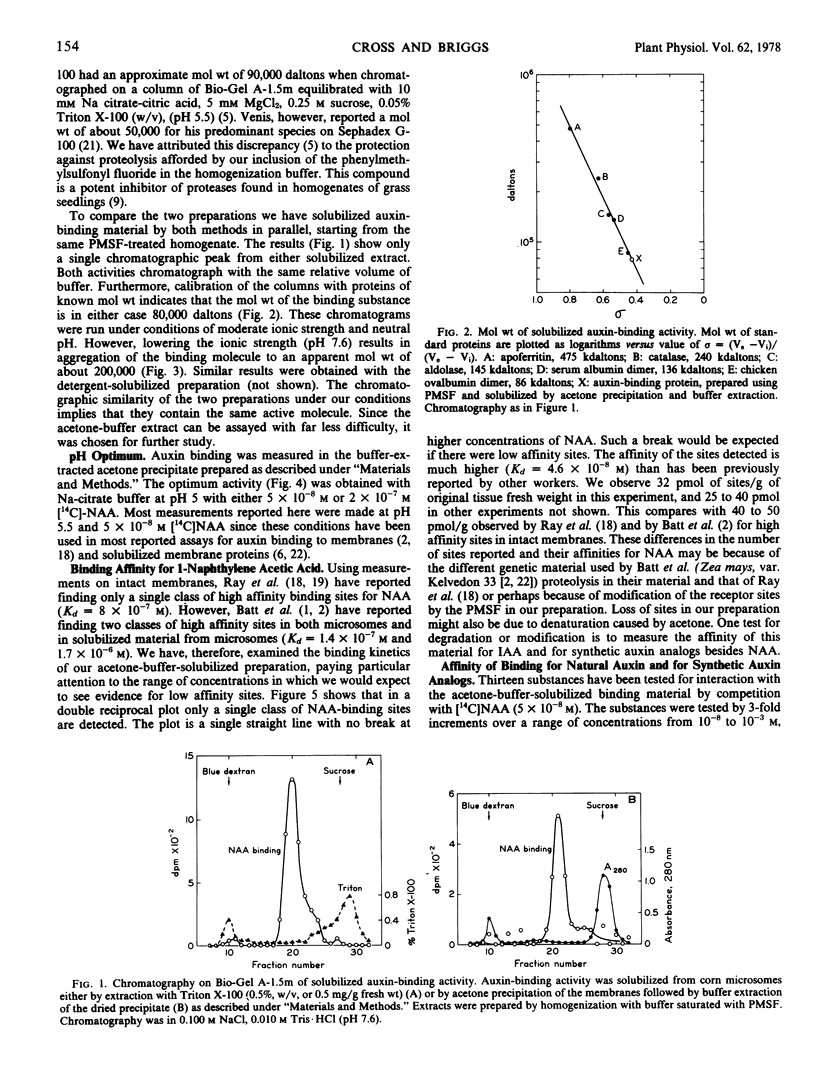
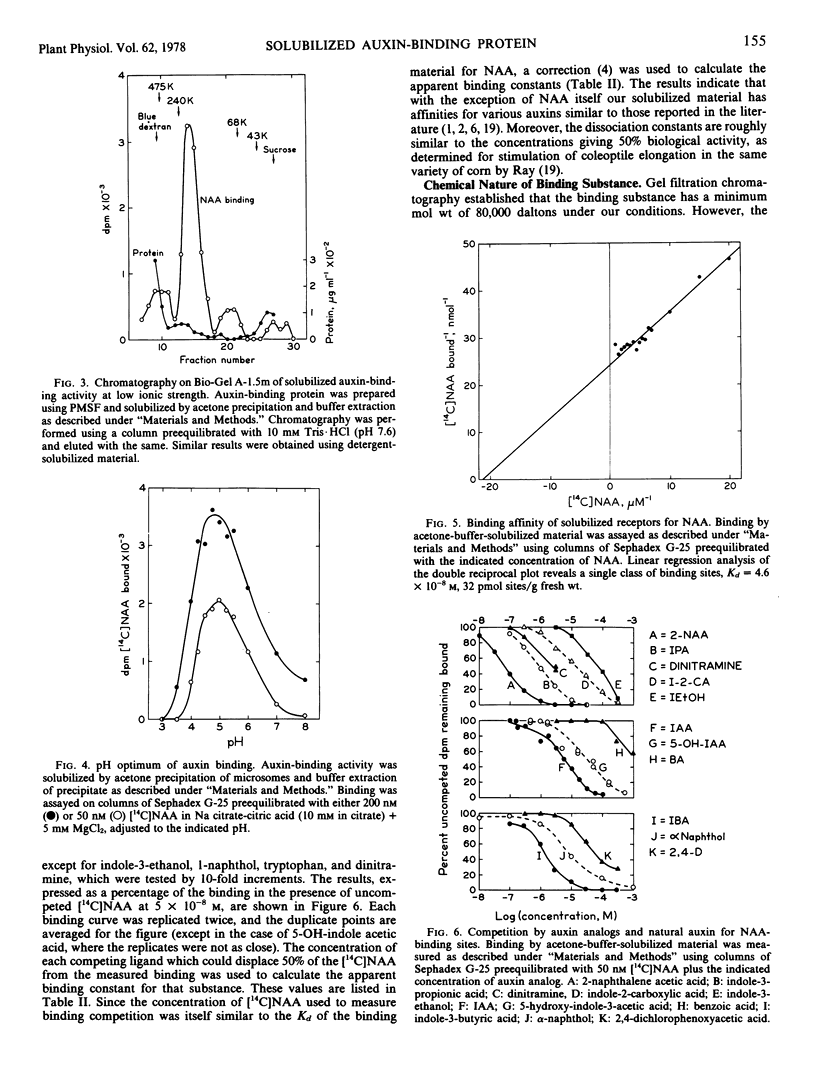
An auxin-binding protein can be solubilized from microsomal membranes of Zea mays using either Triton X-100 extraction of the membranes or buffer extraction of the acetone-precipitated membranes. This paper describes the properties of the binding protein solubilized by these two methods. The binding is assayed by gel filtration chromatography in the presence of naphthalene [2-14C]acetic acid. Binding is rapid and reversible with an optimum at pH 5. Both preparations show similar molecular weights by gel filtration (80,000 daltons) at pH 7.6 and 0.1 molar NaCl, and both aggregate at low ionic strength. They appear to be the same active molecular species. The binding activity is destroyed by trypsin, pronase or para-chloromercuribenzoic acid, but not significantly reduced by phospholipase C, DNase, RNase, or dithioerythritol. Since saturating amounts of naphthalene acetic acid protect the molecule from inhibition by para-chloromercuribenzoic acid, it is concluded that the binding protein has a sulfhydryl group at the binding site, or protects such a group in its binding conformation. The dissociation constant of the protein for naphthalene acetic acid is 4.6 × 10−8 molar with 30 picomoles of sites per gram of tissue fresh weight. Binding constants were estimated for 13 other natural and synthetic auxins by competition with naphthalene[2-14C]acetic acid. Their dissociation constants are in general agreement with published values for their binding to intact membranes and their biological activity, although several exceptions were noted. A supernatant factor from the same tissue changes the apparent affinity of the protein for naphthalene acetic acid. This factor may be the same one as has been previously reported to alter the affinity of intact microsomes for auxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Auxin receptors of maize coleoptile membranes do not have ATPase activity. Plant Physiol. 1978 Apr;61(4):581–584. doi: 10.1104/pp.61.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Omran R. G. The direct involvement of hydrogen peroxide in indoleacetic acid inactivation. Biochem Biophys Res Commun. 1977 Oct 10;78(3):970–976. doi: 10.1016/0006-291x(77)90516-2. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Kemper D. L., Ragland W. L. The relationship of molecular weight to electrophoretic mobility of fluorescamine-labeled proteins in polyacrylamide gels. Biochem Biophys Res Commun. 1974 Mar 25;57(2):482–487. doi: 10.1016/0006-291x(74)90957-7. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Specificity of Auxin-binding Sites on Maize Coleoptile Membranes as Possible Receptor Sites for Auxin Action. Plant Physiol. 1977 Oct;60(4):585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Pinkus L. M., Meister A. Inhibition by dithiothreitol of the utilization of glutamine by carbamyl phosphate synthetase. Evidence for formation of hydrogen peroxide. J Biol Chem. 1974 Mar 25;249(6):1915–1921. [PubMed] [Google Scholar]



