Abstract
A membrane-bound, monovalent cation-stimulated ATPase from Zea mays roots has been purified to a single band on sodium dodecyl sulfate gel electrophoresis. Microsomal preparations with K+ -stimulated ATPase activity were extracted with 1 m NaClO4, and the solubilized enzyme was purified by chromatography on columns of n-hexyl-Sepharose, DEAE-cellulose, and Sephadex G-100 Superfine. A 500-fold purification over the activity present in the microsomes was obtained. The K+ -stimulated activity shows positive cooperativity with increasing KCl concentrations. The purified enzyme shows K+ -stimulated activity with ATP, GTP, UTP, CTP, ADP, α + β-glycerophosphate, p-nitrophenyl phosphate, and pyrophosphate as substrates. Under most conditions ATP is the best substrate. Although dicyclohexyl carbodiimide and Ca2+ inhibit and alkylguanidines stimulate the K+ -ATPase while bound to microsomes, they have no effect on the purified enzyme.
Full text
PDF

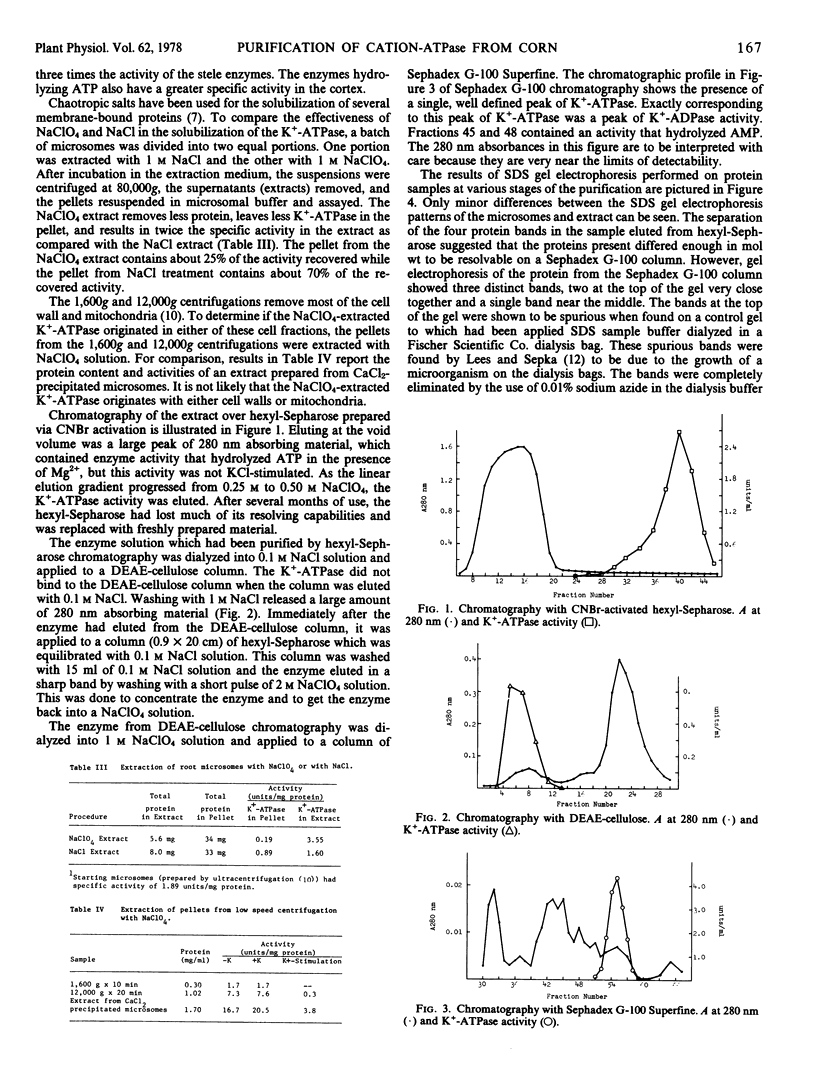

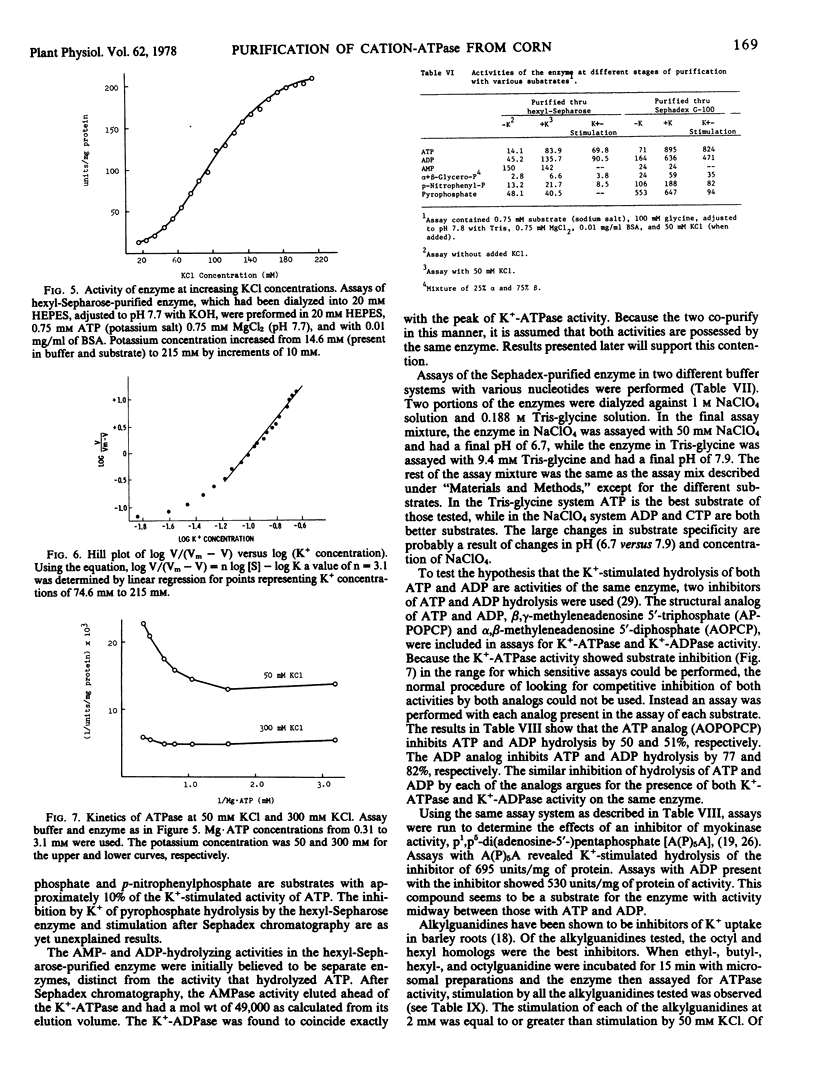



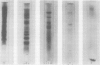
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Hilden S., Hokin L. E. Active potassium transport coupled to active sodium transport in vesicles reconstituted from purified sodium and potassium ion-activated adenosine triphosphatase from the rectal gland of Squalus acanthias. J Biol Chem. 1975 Aug 25;250(16):6296–6303. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Kandrach A., Racker E., Khorana H. G. Acetyl phosphatidylethanolamine in the reconstitution of ion pumps. J Biol Chem. 1975 Mar 10;250(5):1809–1813. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lees M. B., Sepka S. A. Artifacts during dialysis. Anal Biochem. 1976 Oct;75(2):686–686. doi: 10.1016/0003-2697(76)90132-9. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Williamson F. A., Jones R. G. Presence of Two Different Membrane-bound, KCl-stimulated Adenosine Triphosphatase Activities in Maize Roots. Plant Physiol. 1975 Apr;55(4):678–685. doi: 10.1104/pp.55.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Nagahashi G., Thomson W. W. Effect of lanthanum on ion absorption in corn roots. Plant Physiol. 1975 Mar;55(3):542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepe B. G., Avila E. J. Alkylguanidines as inhibitors of k transport in isolated barley roots. Plant Physiol. 1975 Oct;56(4):460–463. doi: 10.1104/pp.56.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A. H., Bailon P. Affinity purification methods. Improved procedures for cyanogen bromide reaction on agarose. Anal Biochem. 1975 Mar;64(1):268–275. doi: 10.1016/0003-2697(75)90428-5. [DOI] [PubMed] [Google Scholar]
- Shaltiel S. Hydrophobic chromatography. Methods Enzymol. 1974;34:126–140. doi: 10.1016/s0076-6879(74)34012-8. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Reconstitution of active ion transport by the sodium and potassium ion-stimulated adenosine triphosphatase from canine brain. J Biol Chem. 1975 May 25;250(10):4022–4024. [PubMed] [Google Scholar]
- Vanquelin G., Lacombe M. L., Hanoune J., Strosberg A. D. Stability of isoproterenol bound to cyanogen bromide-activated agarose. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1076–1082. doi: 10.1016/0006-291x(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]