Abstract
Actinobacteria are one of the predominant groups that successfully colonize and survive in various aquatic, terrestrial and rhizhospheric ecosystems. Among actinobacteria, Nocardia is one of the most important agricultural and industrial bacteria. Screening and isolation of Nocardia related bacteria from extreme habitats such as endolithic environments are beneficial for practical applications in agricultural and environmental biotechnology. In this work, bioinformatics analysis revealed that a novel strain Nocardia mangyaensis NH1 has the capacity to produce structurally varied bioactive compounds, which encoded by non-ribosomal peptide synthases (NRPS), polyketide synthase (PKS), and post-translationally modified peptides (RiPPs). Among NRPS, five gene clusters have a sequence homology with clusters encoding for siderophore synthesis. We also show that N. mangyaensis NH1 accumulates both catechol- and hydroxamate-type siderophores simultaneously under iron-deficient conditions. Untargeted LC–MS/MS analysis revealed a variety of metabolites, including siderophores, lipopeptides, cyclic peptides, and indole-3-acetic acid (IAA) in the culture medium of N. mangyaensis NH1 grown under iron deficiency. We demonstrate that four CAS (chrome azurol S)-positive fractions display variable affinity to metals, with a high Fe3+ chelating capability. Additionally, three of these fractions exhibit antioxidant activity. A combination of iron scavenging metabolites produced by N. mangyaensis NH1 showed antifungal activity against several plant pathogenic fungi. We have shown that the pure culture of N. mangyaensis NH1 and its metabolites have no adverse impact on Arabidopsis seedlings. The ability of N. mangyaensis NH1 to produce siderophores with antifungal, metal-chelating, and antioxidant properties, when supplemented with phytohormones, has the potential to improve the release of macro- and micronutrients, increase soil fertility, promote plant growth and development, and enable the production of biofertilizers across diverse soil systems.
Keywords: Endolithic bacteria, Nocardia, Siderophores, Metabolome, NRPS, Antifungal activity, Biocontrol agent
Subject terms: Biotechnology, Microbiology, Plant sciences
Introduction
Modern agriculture confronts a range of complex challenges, including the degradation and exhaustion of agricultural land, the loss of biodiversity, the rise of phytopathogenic diseases and reduced crop productivity. Plants on degraded land suffer from elevated levels of salinity, drought and heavy metals, leading to stress and impaired growth. Iron deficiency is also a frequent occurrence in agricultural soil, in addition to adverse conditions1. Synthetic fertilisers are a beneficial approach to restoring degraded land and promoting plant nutrition2. However, they do not enhance soil fertility or microbial biodiversity despite their effectiveness in improving plant growth. Moreover, the high concentration of exogenous fertilizers reduces the solubility and availability of iron to plants in iron-deficient soils. An environmentally-friendly substitute is the application of organic fertilizers supplemented with plant growth-promoting (PGP) microorganisms3. PGP bacteria produce iron-chelating compounds (siderophores) that reduce iron deficiency and improve physiological and biochemical processes in plants in degraded soils. For agricultural applications, especially in organic farming systems, it is essential to screen microbial biological control agents from different habitats. Extreme environments provide an ecological niche for discovering novel microorganisms with immense metabolic potential, especially specialized secondary metabolites.
Actinobacteria are highly adaptable to different habitats and are used as biostimulants due to their ability to synthesize secondary metabolites, bioactive compounds, enzymes, plant phytohormones and agroactive chemicals4. In addition, Actinomycetes species have been found to increase the availability of essential metals in drylands and degraded soils, improve stress tolerance in plants, and protect against phytopathogens, ultimately benefiting major crop yields5. They are a prevalent bacterial group in rhizospheric environments, providing soluble phosphate to plants, fixing nitrogen, and producing 1-aminocyclopropane-1-carboxylate (ACC) deaminase along with phytohormones such as gibberellin, cytokinin, and indole acetic acid (IAA)5. Actinomycetes producing siderophores improve nutrient absorption in deteriorated agricultural soils, resulting in promotion of respiration, photosynthesis, and plant growth1.
Among all actinobacteria, Nocardia species are widely distributed in different aquatic, terrestrial and rhizhospheric ecosystems6–8. Morphologically, members of the Nocardia genus display distinct characteristics, such as the formation of fragmentary hyphae, short-chain spores, and pigmented rough or smooth irregular colonies6,9. Furthermore, the genomic features of Nocardia spp. possess valuable insights regarding the pool of biosynthetic novel natural products that may be useful in agriculture, especially for soil fertility, plant health maintenance, biofertilisers and biopesticides10. The genome of Nocardia strains contains NRPS, PKS or hybrid NRPS-PKS gene clusters, which are linked to the synthesis of a diverse array of secondary metabolites11–13.
NRPS genes synthesise siderophores, which act as natural ligands for the binding of iron. These facilitate the dissolution, sequestration and transport of iron from the environment, making it available to microorganisms or plants14. Bacterial siderophores bind iron and perform various functions in agriculture and the environment, such as hindering plant phytopathogens, improving nutrient absorption, and promoting plant growth15. Actinobacteria are widely known producers of siderophores, which can be distinguished according to their functional moieties, such as catecholate, hydroxamate, carboxylate and mixed types16. To combat iron deficiency, Nocardia species synthesise various siderophores, including nocobactins, nocardichelins, formobactin, amamistatins, asterobactin, brasilibactin, and nocardimicins17–23. The compounds studied have mainly shown antibacterial, cytotoxic, antineoplastic and antioxidant properties9,11. The aim of the present study was to determine the taxonomic classification of strain NH1 isolated from endolithic environments, and to investigate its biosynthetic capabilities and siderophore diversity in order to discover its agrobiotechnological and ecological potential.
Results
Physiological and genomic features of Nocardia mangyaensis NH1
The NH1 strain is a Gram-positive, aerobic, non-motile, catalase-positive, road-shaped bacterium (0.5–0.7 μm wide, 1–2 μm long) isolated from hydromagnesite found in the vent of the serpentinite rock (Khalilovsky massif, Russia)24. The NH1 strain was obtained from a colony on Luria agar (LA) by plating an aqueous rinse solution of crushed hydromagnesite rock onto the surface of the agar. It forms a substrate mycelium that is colored light orange or pink fragments into irregular rod-shaped elements. Among plant growth-promoting (PGP) activities, the secretion of siderophores and synthesis of phytohormones, such as indole acetic acid (IAA), have been determined.
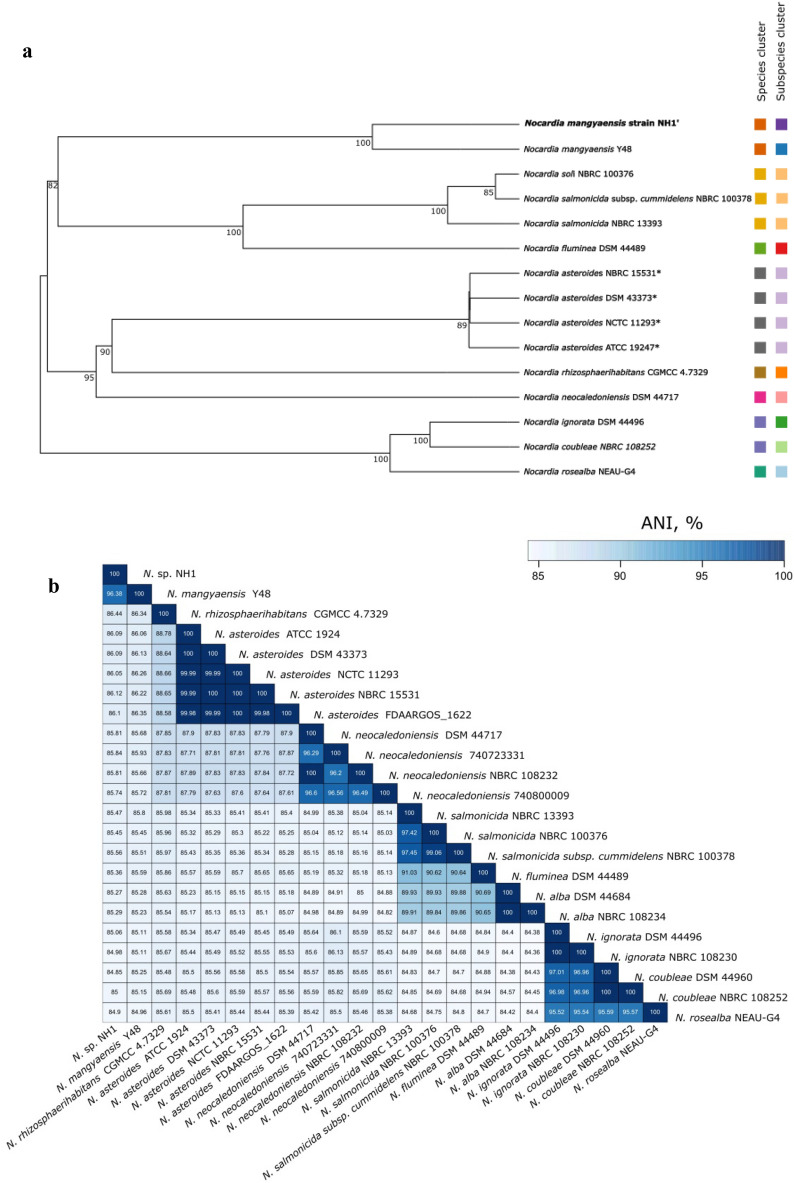
The size of a whole genome of strain NH1 is 6,743,715 bp. The DNA G + C content is 68%. The genomic analysis of NH1 resulted in a DNA-DNA hybridisation (DDH) value of 73.9%, demonstrating 100% similarity to Nocardia mangyaensis Y48 at the level of the subspecies cluster (Fig. 1a, see Supplementary Tables S1, S2). The ANI (average nucleotide identity) analysis showed that NH1 shares 96.38% nucleotide identity with N. mangyaensis Y48 isolated from oil contaminated soil, making it the closest specie25 (Fig. 1b). It also shares 86.44% similarity with Nocardia rhizospaerihabitans CGMCC 4.7329, Nocardia asteroides ATC 1924, DSM 43373, NCTC 11293 and NBRC 15531 (Fig. 1b, see Supplementary Table S1).
Figure 1.
(a) A phylogenomic tree of the genus Nocardia based on in silico DNA-DNA hybridization (DDH) comparisons. The branch lengths are scaled in terms of GBDP distance formula d5. The numbers above branches are GBDP pseudo-bootstrap support values > 60% from 100 replications, with an average branch support of 81.3%. The tree was rooted at the midpoint. (b) Heat map of pairwise average nucleotide identity (ANI) among selected Nocardia genomes.
Specialized metabolite biosynthetic gene clusters
The mining of N. mangyaensis NH1 genome revealed a total of 11 NRPS gene clusters, including the predicted siderophore synthetase genes arranged across 5 clusters, 4 PKS and ribosomally-synthesized and posttranslationally-modified peptides (RiPPs) gene clusters. In addition to the predicted genes encoding the synthesis of siderophores, the genome of N. mangyaensis NH1 was found to contain a gene cluster responsible for the synthesis of aminopolycarboxylic acid metallophores, as well as an NRPS gene cluster involved in the synthesis of biosurfactants. Three adenylation domains of siderophore-like gene clusters 1.1, 6.1 and 14.1 had specificity for serine (Ser) suggesting the formation of catechol-type siderophores26 (Table 1). The transcription of two NRPSs and an aminopolycarboxylic acid metallophore is regulated by the iron-regulatory protein (DmdR1). The zinc uptake regulator (Zur), a member of the Fur (ferric uptake regulator) controls the transcription of three NRPSs. The nickel-responsive repressor (NuR) from the Fur family regulates the transcription of a single NRPS (Table 1). We found a association between siderophore-like gene clusters in N. mangyaensis NH1 and ABC (ATP-dependent pumps) and MFS (Major Facilitator Superfamily) transporters (Table 1). Additionally, the siderophore import protein FecCD was detected in clusters 1.1 and 6.2. The siderophore-reductase system-related transporter FhuF was identified in NRPS gene cluster 2.4 (Table 1).
Table 1.
AntiSmash prediction of siderophore-like gene clusters in the genome of N. mangyaensis NH1.
| Gene cluster | Type | Polymer prediction | Transcription factor binding site | Transporter genes |
|---|---|---|---|---|
| Cluster 1.1 | NRPS, NRP-metallophore | (X − D − Ser) + (OH − Orn) + (D − X) | Fe II | ABC transporter, FecCD domain |
| Cluster 2.4 | NRPS, NRP-metallophore, NI-siderophore* | (X) | Fe II | ABC transporter, Ferric iron reductase Fhu F-like transpoter |
| Cluster 6.1 | NRPS, NRP-metallophore | (Ser − Cys − Cys) + (Cys) | Zn, Ni | ABC transporter, MFS |
| Cluster 6.2 | NRPS, T1PKS** | (ccemal) | Zn | MFS, FecCD domain |
| Cluster 14.1 | NRPS | (Ser − Leu − X − Leu) | Zn | ABC transporter |
| Cluster 7.2 | Aminopolycarboxylic acid metallophore | Fe II | MATE |
*NRPS-independent, IucA/IucC-like siderophores.
**Type I PKS (Polyketide synthase).
X—Domain that is predicted to be inactive.
KEGG was used to map the genes involved in the IAA synthesis pathway in the N. mangyaensis NH1 genome (see Supplementary Fig. S2). Two primary gene groups responsible for the synthesis of 3-indoleacetic acid have been identified. The former group contains 14 aldehyde dehydrogenase genes, whereas the latter group consists of only two aliphatic amidases (see Supplementary Fig. S2). The LC–MS/MS analysis has verified the accumulation of IAA (32 ± 2 µg/mL) by N. mangyaensis NH1 in M9 minimal medium under Fe-deficient conditions (see Supplementary Fig. S3C).
Isolation and identification of siderophores from culture supernatant
Siderophore production by N. mangyaensis NH1 was evaluated using a chrome azurol S (CAS) agar plate assay. A clear zone around the bacterial colony was observed after 72 h, indicating the production of iron scavenging compounds (see Supplementary Fig. S5). N. mangyaensis NH1 was grown on M9 minimal medium with restricted iron supply to quantify the amount of catechol and hydroxamate type siderophores (see Supplementary Fig. S6). The Arnow test showed that a concentration of the catechol-type siderophores was reached 15.8 ± 2.9 μM after 72 h of growth. Similarly, the Atkin assay indicated the accumulation of hydroxamate siderophores to an amount of 87 ± 21 μM after 96 h of growth (see Supplementary Fig. S6). Accumulation of siderophores in the liquid medium occurred in a growth-phase-dependent manner of N. mangyaensis NH1. HPLC analysis detected four primary metabolites that were either absent or substantially suppressed in the presence of iron chloride III (see Supplementary Fig. S7).
To obtain a sufficient amount of iron-scavenging metabolites for screening of their activities, the bacterial culture of N. mangyaensis NH1 was scaled-up to a volume of 1L under conditions of limited iron, which promotes the production of siderophores. Metabolites were extracted from the culture supernatant after 72 h by using XAD16 resin. Untargeted LC–MS/MS analysis was used for the metabolic profiling of N. mangyaensis NH1 grown under iron-deficient conditions. Among the 1 553 unique metabolites with significant matches, the study detected the presence siderophores related to mycobactin (m/z 911.60, 897.58 and 869.55) and carboxymycobactin-like compounds (m/z 815.43, 773.38, 759.37, 829.45 and 897.58), which are produced by Mycobacterium (Table 2)27. The nocobactine-like compound (m/z 787.44) was identified in N. mangyaensis NH1 supernatant, commonly found in Nocardia species (Table 2)12. Additionally, siderophores produced by N. mangyaensis NH1 included loihichelin E from Halomonas sp. and desferribactin from Pseudomonas fluorescens ATCC 1352528,29.
Table 2.
Siderophore-like compounds from the cultural liquid of N. mangyaensis NH1 annotated by the Global Natural Products Social Molecular Networking (GNPS) Moldiscovery.
| Name of identified compound | Metabolite mass | m/z | RT | Adduct | Metabolite FDR* (%) |
|---|---|---|---|---|---|
| Carboxymycobactins-6 | 815.43 | 408.72 | 2241.25 | M + 2H | 0.00 |
| Carboxymycobactin-3 | 773.38 | 387.69 | 2189.47 | M + 2H | 0.00 |
| Carboxymycobactin_2 | 759.37 | 380.70 | 1571.62 | M + 2H | 0.12 |
| 759.37 | 380.69 | 2255.09 | M + 2H | 0.10 | |
| Carboxymycobactin-7 | 829.45 | 415.73 | 1989.07 | M + 2H | 0.07 |
| 897.58 | 449.80 | 2953.79 | M + 2H | 0.07 | |
| Mycobactin_(20,DB) | 911.60 | 456.81 | 3029.24 | M + 2H | 0.12 |
| Mycobactin_(19,DB) | 897.58 | 449.80 | 2953.79 | M + 2H | 0.07 |
| Mycobactin_P_(C18_alkyl) | 869.55 | 435.79 | 2671.56 | M + 2H | 0.10 |
| Loihichelin_E | 1113.56 | 557.79 | 2386.23 | M + 2H | 0.00 |
| Desferribactin_ATCC_13525 | 1177.57 | 589.79 | 1328.45 | M + 2H | 0.00 |
| Nocobactin_NA | 787.44 | 394.73 | 1374.45 | M + 2H | 0.08 |
*False discovery rate (FDR). The FDR below 0.9% was used as filters for processing of the final results: the highest quality annotations would require passing thresholds for both metrics.
The LC–MS/MS results revealed a variety of metabolites produced by N. mangyaensis NH1 in responce to Fe-limited conditions. These metabolites were identified as previously recognized lipopeptides such as bacillomycin (m/z 1034.53), agrastatin (m/z 1448.78), bacillopeptins (m/z 1034.53 and 1020.51), minutissamide (m/z 1151.57), and syringostatin (m/z 1194.59). These lipopeptides are commonly synthesized by representatives of the genera Bacillus, Pseudomonas, and Anabena (Table 3)30–34.
Table 3.
Lipopeptide-like compounds from the cultural liquid of N. mangyaensis NH1 annotated by the GNPS DEREPLICATOR + of Classic Molecular Networking V2/Moldiscovery.
| Name of identified compound | Metabolite mass | m/z | RT | Adduct | Metabolite FDR* (%) |
|---|---|---|---|---|---|
| Bacillomycin | 1034.53 | 518.27 | 3756.36 | M + 2H | 0.00 |
| Agrastatin_A | 1448.78 | 725.40 | 4407.16 | M + 2H | 0.00 |
| Bacillopeptin_B | 1034.53 | 518.27 | 3792.26 | M + 2H | 0.00 |
| Minutissamide_A_12ʹ-Chloro | 1151.57 | 576.79 | 2192.62 | M + 2H | 0.00 |
| Syringostatin B | 1194.59 | 598.30 | 1830.76 | M + 2H | 0.00 |
| Bacillopeptin-A | 1020.51 | 511.26 | 3588.17 | M + 2H | 0.00 |
*False discovery rate (FDR). The FDR below 0.9% was used as filters for processing of the final results: the highest quality annotations would require passing thresholds for both metrics.
The numbers of the cyclic peptides related to mycobacillin (m/z 1527.55) and mycosubtilin (m/z 1198.63) produced by members of Bacillus spp.; as well as mycoplanecins (m/z 1196.78) and peptidolipins (m/z 963.66 and 1089.80) originated from Actinoplanes and Nocardia genera, were found in the supernatant of N. mangyaensis NH1, when cultured under Fe-limited conditions (Table 4)35–38.
Table 4.
Cyclic peptide-like compounds from the cultural liquid of N. mangyaensis NH1 annotated by the GNPS Dereplicator.
| Name of identified compound | p-value | Peptide mass | SpecMass | RT | Adduct | PSM FDR (%) |
|---|---|---|---|---|---|---|
| Mycobacillin | 8.9e − 9 | 1527.55 | 774.39 | 2378.97 | M + 2H | 2.40 |
| Mycoplanecin_D | 1.1e − 9 | 1196.78 | 565.36 | 4047.87 | M + 2H | 2.17 |
| Mycoplanecin_C | 1.5e − 8 | 1196.78 | 411.93 | 3194.78 | M + 3H | 4.49 |
| Mycosubtilin | 8.9e − 7 | 1198.63 | 602.83 | 2376.23 | M + 2H | 2.50 |
| Peptidolipin NA | 6.7e − 14 | 963.66 | 502.74 | 1489.34 | M + 2H | 0.37 |
| Peptidolipin B | 4.4e − 12 | 189.80 | 502.74 | 1470.87 | M + 2H | 0.71 |
*Peptide-to-spectrum match (PSM) false discovery rate (FDR). Peptide FDR was maintained below 5% by requiring a minimum number of spectral counts for each peptide across the dataset.
Using molecular networking, metabolites with similar spectral features were grouped together. The resulting clusters were then visualized through Cytoscape software after exporting the outputs from GNPS-METABOLOMICS-SNETS-V2. The network of N. mangyaensis NH1 revealed one cluster combining siderophore- and lipopeptide-like compounds such as nocobactine NA, carboxymycobactin 2, bacillomycin, bacillopeptin A and B (Fig. 2). Metabolites in a given cluster are classified as belonging to the same molecular family and share analogous functions, particularly siderophore activity.
Figure 2.
Molecular networking of siderophore- and lipopeptide-like compounds produced by N. mangyaensis NH1 under Fe-limited conditions. Each node represents a distinct metabolite that needed to be present in two biological replicates. The known siderophore- and lipopeptide-like compounds from N. mangyaensis NH1 are indicated as red circles. Four separate families in one cluster can be recognized and include nocobactine NA, carboxymycobactin 2, bacillomycin, bacillopeptin A and B. For this network, nodes were manually positioned, and only chosen edges are displayed to enhance clarity of presentation. The numerical values in the nodes indicate the corresponding m/z value of metabolites (rounded to one digit). The software Cytoscape was used for visualization.
Activity of siderophores
After preparative HPLC, four fractions were collected with retention times of 3, 9–10, 11, and 14 min. These fractions were then tested for antioxidant activities, metal-binding and CAS assay.
Screening for metal-chelating activity
Fraction 3 was characterized by the appearance of an absorbance peak at 320 nm. By contrast, after incubation with metals, no 320 nm peak was detected. Nonetheless, the spectrum has been altered and displays a peak between 240 and 250 nm solely in the existence of iron (Table 5, see Supplementary Fig. S8A). The absorbance spectrum of the metal-free fraction 9–10 revealed peaks at 280 and 330 nm. Alongside the iron-related peaks at 240 and 250 nm, we identified a Cu2+-related peak at 310 nm and Ga3+-related peaks at 300 and 310 nm (Table 5, see Supplementary Fig. S8B). Fraction 11 exhibited an absorbance peak at 250 and 310 nm in the absence of metal, with an iron-related peak at 240 and 250 nm. Additionally, a new absorbance peak at 280 nm was observed in the presence of Ga3+ (Table 5, see Supplementary Fig. S8C). Among all CAS-positive fractions, fraction 14 with a unique peak at 270 nm revealed spectral alterations in the presence of examined metals. A new absorption peak at 400 nm was detected for all these metals except for iron, which generates an iron-associated peak at 240 and 250 nm (Table 5, see Supplementary Fig. S8D).
Table 5.
UV/Vis characteristics of CAS-positive HPLC-purified fractions of N. mangyaensis H1 in the presence of various metals.
| Metals | Fraction 3 | Fraction 9–10 | Fraction 11 | Fraction 14 |
|---|---|---|---|---|
| No metal | 320 nm | 280, 330 nm | 250, 310 nm | 270 nm |
| Co2+ | NM | NM | NM | 400 nm |
| Al3+ | NM | NM | NM | 400 nm |
| Ni2+ | NM | NM | NM | 400 nm |
| Fe3+ | 240–250 nm | 240–250 nm | 240–250 nm | 240, 250, 400 nm |
| Zn2+ | NM | NM | NM | 400 nm |
| Mn2+ | NM | NM | NM | 400 nm |
| Cu2+ | NM | 310 nm | NM | 400 nm |
| Ga3+ | NM | 300, 310 nm | 280 nm | 400 nm |
*NM—not modified. The UV/visible absorbance remains unaltered when compared to the spectrum of the metal-free fraction. Each of the fractions was dissolved in water.
Antioxidant activity
To determine the antioxidant activity, 2 µg/mL HPLC-purified CAS-positive fractions of N. mangyaensis NH1 were analysed using the AmplexRed assay based on hydrogen peroxide detection. The initial absorbance of a positive control (10 µM H2O2) started at 0.05 and progressively increased throughout the experiment (see Supplementary Fig. S9). The absorbance of fractions 3.0, 9.0–10.0 and 11.0 was lower than that of the positive control from the third minute of the experiment. The absorbance signal was remained stable for the next 3 h. A slight increase in absorbance was detected for fraction 14.0 compared to the positive control. The negative control was used water without H2O2 (see Supplementary Fig. S9).
Antifungal activity
A mixture of metabolites from N. mangyaensis NH1 grown under Fe-limited conditions was evaluated for its antifungal property against strong phytopathogenic strains including Colletotrichum coccodes MF 16-014, Rhizoctonia solani MFP 936011, Fusarium oxisporum PR 57 and Alternaria sp. obtained from potato tubers (Table 6, see Supplementary Fig. S10)39. The agar well diffusion method showed that iron-scavenging metabolites produced by N. mangyaensis NH1 had a strong inhibitory activity towards mycelial growth in all tested fungal strains. The inhibition zone reached above 40.0 mm for R. solani MFP 936011 and F. oxysporum PR 57; the minimal and maximal zones being observed for C. coccodes MF 16-014 and Alternaria sp., respectively (Table 6, see Supplementary Fig. S10). The inhibition zone of R. solani MFP 936011 represented a viscous mucus layer in comparison to the dried inhibition zones of other fungi. For all fungi tested, the duration of antifungal activity of the metabolites varied. Stability of antifungal activity of metabolites N. mangyaensis NH1 lasted for 14–21 days for R. solani MFP 936011, F. oxysporum PR 57 and C. coccodes MF 16-014 and 5 days for Alternaria sp.. Difenoconazole was used as a positive control.
Table 6.
Antifungal activity of metabolites produced by N. mangyaensis NH1 under Fe-limited conditions against phytopathogenic fungi.
| Fungal strains | Diameter clear zone (mm)* | |
|---|---|---|
| Metabolites of N. mangyaensis NH1 | Difenoconazole§ | |
| Colletotrichum coccodes MF 16-014 | 41.7 ± 7.6 | 41.6 ± 4.7 |
| Rhizoctonia solani MFP 936011 | 43.7 ± 7.1 | NIZ |
| Fusarium oxysporum PR 57 | 50.5 ± 5.2 | NIZ |
| Alternaria sp. | 62.7 ± 14.2 | NIZ |
* All experiments were performed in triplicates.
§A systematic triazole fungicide. The concentration of difenoconazole was 1.2 mg/mL.
NIZ no inhibition zone.
Phytotoxic activity
The study explored the impact of metabolites produced by N. mangyaensis NH1, under conditions of iron-deficiency, on the growth of Arabidopsis thaliana plant seedlings. The results demonstrated that iron-scavenging metabolites of N. mangyaensis NH1 did not impact the growth of Arabidopsis seedlings compared to the control groups (untreated and treated with DMSO) (see Supplementary Figs. S11–13). Metabolites produced by N. mangyaensis NH1 in the presence of iron chloride III did not hinder the growth and development of plants either. We noted that the color of plants treated with metabolites from N. mangyaensis NH1 was less intense compared to untreated plants. Arabidopsis seedlings were additionally exposed to N. mangyaensis NH1 cells (2.8 ± 0.53 × 10*11 CFU/mL) to evaluate phytopathogenic activity. The findings suggest that N. mangyaensis NH1 did not impede the growth and development of Arabidopsis seedlings.
Discussion
Endolithic systems are associated with environments permanently or seasonally exposed to various abiotic stresses, including desiccation/rehydration, temperature fluctuations and oligotrophy40. Despite severe environmental constraints, the microbial colonization of these systems by endoliths has significant evolutionary and ecological implications. Endolithic microbial communities are involved in the process of soil weathering, whereby they secrete metabolites into the soil surrounding minerals or rocks. Additionally, they play a role in the formation of soil aggregates and maintaining their structural stability41. Actinobacteria originating from endolithic environments have been described so far, including abundant genus Streptomyces and several rare genera Actinomadura, Amycolatopsis, Nocardiopsis, Nonomuraea, Saccharopolyspora and Saccharothrix42. It is well-known that Actinobacteria exhibit greater tolerance to extreme environments, such as arid, weathered or degraded soils. They have been found to bolster crop production under multiple stress conditions43.
Information on members of the genus Nocardia living in endolithic ecosystems is relatively limited. The cultivable Nocardia species isolated from stone ruins sampling in arid and semiarid climates in Tunisia were identified44. The adaptation strategy of actinobacteria to nutrient limitation and rock colonization was analyzed by studying the production of siderophores, hydrolytic enzymes, promoting plant growth and antimicrobial activity. Thus, endolithic habitats are a beneficial pool of potential sources for discovering unique Nocardia species that produce bioactive molecules including novel enzymes and secondary metabolites. This highlights the potential benefits of studying endolithic habitats as a rich resource for finding new species.
A significant number of biosynthetic gene clusters (BGCs) containing NRPS, PK or hybrid NRPS/PK enzymes encoding natural product production were found in Nocardia genomes12. A wide variety of gene clusters responsible for antibiotic synthesis by Nocardia species were demonstrated6,12. Despite this, a bioinformatic analysis revealed a highly conserved biosynthetic pathway leading to the formation of the siderophore nocobactin NA in both pathogenic and non-pathogenic Nocardia spp. Our findings reveal that the genome of N. mangyaensis NH1 comprises 5 gene clusters responsible for the production of siderophores. Additionally, nocobactine NA was confirmed by analysis via LC–MS/MS.
Non-targeted LC–MS/MS analysis showed that N. mangyaensis NH1 can synthesise membrane-bound mycobactins and non-membrane-bound carboxymycobactin-like compounds. Both types of siderophores were identified as siderophores that confer virulence to the genus Mycobacterium27. Considering that our strain was isolated from an endolithic environment, we hypothesize that these types of siderophores in Nocardia play significant physiological and ecological roles in enabling survival under extreme conditions. Beside the production of hydroxyphenyloxazoline/-oxazole type nocobactine and mycobactines, the strain N. mangyaensis NH1 was found to produce an amphiphilic peptide related to loihichelin-like siderophores. Loihichelins were initially identified and structurally characterized for the basalt weathering Halomonas strain LOB-529. The authors concluded that siderophores play a direct role in both Fe3+ sequestering and Fe2+ oxidising during rock weathering. We have also discovered that N. mangyaensis NH1 synthesises siderophores similar to desferribactin, in combination with other compounds. Desferriferribactin is known as a biogenetic precursor for the biosynthesis of pyoverdins by Pseudomonas fluorescens ATCC 1352545. It is well known that members of the genus Pseudomonas are leaders among PGP bacteria, secreting diffusible siderophores that induce systemic plant resistance and plant pathogen resistance46. Thus, N. mangyaensis NH1 expresses a large number of genes that encode siderophores. The diversity of siderophores produced by strain NH1 contributes to the retention metals in bioavailable form in endolithic environments and indirectly stimulates the maintenance of soil fertility for plant survival.
The siderophore metabolome profile of N. mangyaensis NH1 exhibited a range of lipopeptide-like compounds. Lipopeptides are a type of biosurfactant produced by many bacteria47. The unique chemical composition of a peptide core and lipid tail plays a vital role in the ecological functions of lipopeptides, including protecting cells in extreme endolithic conditions, forming biofilms and chelating metals48. Lipopeptides produced by the bacterial genera Bacillus and Pseudomonas are widely studied for their antimicrobial and antifungal properties. Bacillus species produce bacillomycin, which has an impact on iron metabolism, root colonization, biofilm formation, and antifungal activity. Bacillomycin facilitates iron acquisition and its subsequent intracellular accumulation has a direct impact on biofilm formation through KinB-dependent induction in B. velezensis SQR934. The antifungal activity of bacillomycins produced by B. subtilis FS 94-14 against the fungal plant pathogens Ophiostoma ulmi, Verticillium dahliae, Ceratocystis fagacearum and Cryphonectria parasitica has been demonstrated49. Similar to bacillomycins, bacillopeptins of B. subtilis FR-2 also well demonstrated antifungal and antimicrobial activities33.
We found that N. mangyaensis NH1 produces several cyclic peptides under Fe-deficient conditions. The metabolomic profile analysis revealed the presence of mycobacillin and mycosubtiline-like compounds. Isolated from B. subtilis species mycobacillin and mycosubtilin have the antifungal activity against plant pathogens35,37. Iron availability limitation was favorable for the synthesis of peptidolipins by N. mangyaensis NH1. For the first time, two peptidolipin NA derivatives were isolated from N. arthritidis IFM10035T co-cultivated with mouse macrophage cells36. Overcoming TRAIL-resistance and cytotoxic activities were found for N. arthritidis peptidolipins. Mycoplanecins-like moieties related to lipophilic cyclic peptide antibiotics with a specific mycobacterial activity were also annotated in a siderophore profile of N. mangyaensis NH138. These findings demonstrate that strain NH1 produces a variety range of chemically distinct compounds alongside siderophores in conditions where iron is scarce. These compounds, which are related to lipopeptides and cyclic peptides, serve more than just as iron scavengers; they actively combat pathogenic bacteria and fungi, participate in the bioavailability and sequestering of metals, and are necessary for the stabilization of plant growth and development.
To determine functional properties of siderophores, mixture of metabolites produced by N. mangyaensis NH1 under iron deficiency was separated via preparative HPLC. CAS analysis of the purified compounds identified only four fractions belonging to the siderophore group. Screening for metal-chelating activity revealed that all CAS-positive fractions could form the Fe (III) complex. There is a high specificity for Fe(III) in the siderophore present in fraction 3. Only the siderophore from fraction 14 chelated all of the metals tested in this study. This indicates that the siderophore binding site from fraction 14 has a wide range of metal specificity. Siderophores from fractions 9–10 and 11 reacted with various metals, including copper (II), gallium (III), and iron. Therefore, we investigated the ability of siderophores produced by N. mangyaensis NH1 to chelate their natural ligand Fe (III) as well as other metals. Iron acquisition through microbial siderophores has been demonstrated in numerous studies50. Microbial siderophores promote metal sequestration in endolithic systems, accelerating soil mineral weathering, maintaining metal mobility and bioavailability, and enhancing plant growth51. In addition, chelation of metals by siderophores reduces their toxicity and promotes the production of microbial auxins52. We identified that N. mangyaensis NH1 is capable of producing IAA in a minimal medium using the same conditions as those for siderophore production. Along with the Fe (III)-binding activity of siderophores, it has also been demonstrated that IAA forms a complex with ferric iron, resulting in the subsequent formation of bioavailable ferrous iron53. These findings support the involvement of siderophores and a microbial-secreted phytohormone in the promotion of plant growth activity by actinobacterial strains50.
This study demonstrates a decrease in reactive oxygen species (ROS) by purified siderophores. The metal-binding specificity of N. mangyaensis NH1 siderophores was found to be correlated with their antioxidant activity. The siderophore with broad metal specificity did not exhibit ROS-protective properties, however, siderophores with a narrow range of metal binding specificity were found to possess antioxidant activity. The redox nature of siderophores, which mediates radical scavenging activity, has been referred to as one of their non-classical functions14. The production of siderophores by Pseudomonas aeruginosa protects the bacterium against ROS and facilitates the induction of bacterial cooperation under various environmental stressors54. Detoxification of ROS by catechol-type siderophores is well established in bacterial species belonging to the genera Salmonella and Escherichia55,56. This study demonstrated that the diverse siderophores produced by N. mangyaensis NH1 confer benefits that could shield plants against ROS, particularly in severely stressful endolithic, arid or weathered soil environments.
The mixture of compounds from siderophore metabolome of N. mangyaensis NH1 showed antifungal activity against the phytopathogenic fungi F. oxysporum, R. solani, C. coccodes and Alternaria sp. Fungi are one of the dominated members in the endolithic microbial communities40. The presence of phytopathogenic fungi decreases the plant fitness in extreme conditions. Several studies have demonstrated the efficacy of siderophore-producing PGP bacteria in controlling fungal phytopathogens, but there are few reports detailing the antifungal activity of purified siderophores. For instance, an acinetobactin-like siderophore secreted by the wheat rhizospheric strain Acinetobacter calcoaceticus HIRFA32 inhibited F. oxysporum in vitro57. Pyoverdine produced by P. aeruginosa JAS-25 isolated from the saprophytic soil was found to inhibit the spore germination of F. oxysporum f. sp. ciceri, F. udum, and Aspergillus niger58. Thus, we have shown the direct antifungal activity of metabolites secreted by N. mangyaensis NH1 under Fe-deficient conditions as a valuable skill in the interactions between plant-microbial communities and the management of plant diseases.
In addition to the participation of N. mangyaensis NH1 siderophores in ROS protection, metal-binding, and antifungal activities, the effects of metabolome compounds on a model plant A. thaliana have been demonstrated. Plant seedlings cultivated in the iron-free medium with metabolites produced by N. mangyaensis NH1 were found to develop without any visually negative effects. However, the color of plants varied between treated and untreated plants. We propose that the alteration in color to a pale green hue in treated plants of A. thaliana could be ascribed to the chlorophyll content or chlorophyll a/b ratio in the foliage influenced by the availability of iron ions for plants.
Indeed, the N. mangyaensis NH1 siderophores exhibited a suppression of phytopathogenic fungi while lacking phytotoxicity. There is considerable evidence that microbial siderophores help promote plant growth, suppress disease and facilitate iron uptake59. However, only a limited number of bacterial siderophores have been purified and analyzed in plant experiments. The siderophore rhizoferrin produced by Rhizopus arrhizus serves as a source of Fe for barley (Hordeum vulgare L.) and corn (Zea mays L.)60. A positive influence of pseudobactin excreted by Pseudomonas putida strain WCS358 has been shown to serve on plant iron nutrition and chlorophyll synthesis61. Therefore, we propose that the siderophores of N. mangyaensis NH1 can be regarded as metabolites that provide a physiologically relevant amount of iron to plants under iron deficiency conditions.
Materials and methods
Genomic DNA from strain NH1 was extracted from a 96 h LA-grown culture using phenol–chloroform method. A paired-end DNA library was prepared using the NEBNextUltra II DNA Library Prep Kit (Illumina) and NEBNext Multiplex Oligos for Illumina (96 Unique Dual Index Primer Pairs) according to manufacturer guidelines. Whole-genome sequencing was carried out by NovaSeq6000 sequencing platform (Illumina) with 2 × 250 bp read length. The quality of raw sequence reads was evaluated by FastQC package (v0.11.3)62. The genome had an average 446.4X sequencing coverage. Overrepresented and low-quality sequences were removed using Trimmomatic (v0.36)63. The whole genome was assembled using SPAdes (v 3.12) software64. The genome assembly was evaluated using QUAST (v4.6.3) program65. Genome annotation was performed by RAST 2.0 server, Prokka (v1.14.6) annotation pipeline and the NCBI Prokaryotic Genomes Annotation Pipeline (PGAP) version 5.2 (see Supplementary Table S2)66.
16S rRNA gene analysis
Pairwise sequence similarities calculated using 16S rRNA genes available via the GGDC web server (http://ggdc.dsmz.de/) showed that strain NH1 clusters with the N. mangyaensis (see Supplementary Fig. S1).
The Average Nucleotide Identity (ANI) of the strain NH1 genome was analyzed using FastANI67.
Biosynthetic gene clusters were predicted to be present in the genome of strain NH1 by the antiSMASH software 7.068.
Indole-3-acetic acid (IAA) detection
The culture supernatant were mixed with methanol and centrifuged at 13,000 rpm, 30 min for complete protein precipitation. An aliquot of the resulting mixture was then used for LC–MS/MS analysis. Calibration curve of IAA (Acros) were constructed for 7.8, 15.6, 31.25, 62.5, 125, 250 and 500 ng/µL. The ABSciex QTRAP 6500 coupled with a Agilent 1290 Infinity (LC/MS/MS System), equipped with an electrospray ion source, and operated in the positive ion mode was used. A column Discovery HS C18 3 μm, 50 × 2.1 mm (Sigma-Aldrich, USA) with mobile phases A (5% acetonitrile and 0.1% formic acid in water) and B (5% water and 0.1% formic acid in acetonitrile) was used. The column temperature was set at 40 °C. Chromatographic separation was achieved by gradient elution at a flow rate of 0.4 mL/min. The gradient program was as follows: 0–1.0 min from 0 to 2% B; 1.0–6.0 min from 2 to 95% B; 6.0–7.0 min 95% B; 7.0–8.0 min from 95 to 2% B; and 8.0–9.0 min 2% column re-equilibration. The injection volume of 10 µL was used.
MRM (multiple reactions monitoring) transitions and mass spectrometry parameter optimization were performed by direct infusion using standard solution of IAA. The MRM transitions were: m/z 176.0 → 77.0, m/z 176.0 → 103.2, m/z 176.0 → 130.0. The daughter ion with m/z 130.1 used for quantification. The source parameters used in the study were: temperature—500 °C, capillary voltage—5200 V, curtain gas pressure—35 psi, nebulizer gas pressure—55 psi, auxiliary gas pressure—55 psi. The mass spectrometric data were analyzed using a MultiQuant 3.0.2 software (AB Sciex) (see Supplementary Fig. S3).
Isolation of siderophores from the culture supernatant. A preliminary CAS agar plate assay was conducted to assess the production of siderophores by strain NH1 (see Supplementary Fig. S5)69. N. mangyaensis NH1 was grown for 72 h in M9 medium under iron-limited conditions. Catecholate- and hydroxamate-type siderophores in solution were detected using the Arnow and Atkin assays, respectively70,71. All experiments were performed in triplicates.
After extraction of the culture supernatant with XAD16 resin, the adsorbed compounds were eluted with methanol and subjected to Orbitrap LC–MS/MS. Untargeted LC–MS/MS analysis was performed as described previously72. The data obtained from HPLC–MS/MS analysis were converted to .mzML format using the MsConvert software73. Data processing was performed by using the software MZMine 274. The data processing workflow included: (1) mass detection; (2) ADAP chromatogram builder48; (3) smoothing; (4) deconvolution; (5) deisotoping; (6) alignment; (7) gap filling. The LC–MS/MS processed and converted files (.mzML) were uploaded to the global natural products social molecular networking (GNPS) platform (https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp) with Dereplicator+, MolDiscovery and VarQuest (Analog Search) computational tools65,75,76. Network parameters were default. The networks were visualized using the Cytoscape network visualization tool/software 3.10.177.
Preparative HPLC (prep-HPLC)
The vacuum-dried metabolites, which were dissolved in methanol and directly injected into the column, were purified using the Puri Flash 450 Interchim HPLC instrument.. A C18-reversed phase silica gel column (15 µm, 40 g) was utilized in gradient elution mode (0 min water/acetonitrile = 100–0 to 60 min water/acetonitrile 0–100) at a flow rate of 5 ml/min. Absorbance at 256 and 340 nm was monitored during this procedure, resulting in the separation of the CAS-positive fractions (tR = 3.0, 9.0–10.0, 11.0 and 14.0 min).
Antioxidant activity
Hydrogen peroxide was detected in the presence of the CAS-positive fractions (2 µg/mL HPLC-purified CAS-positive fractions) using the Amplex™ Red Hydrogen Peroxide/Peroxidase Assay Kit, following the manufacturer’s protocol (Invitrogen). A positive control of 10 µM hydrogen peroxide and a negative control of water were employed.
Metal-binding activity
All metals, CoCl2 × 6H2O, AlCl3, NiSO4 × 7H2O, FeCl3 × 6H2O, ZnSO4, MnSO4, CuSO4 and GaBr3 were prepared in 50 mM concentration in 0.5N HCl. The final concentration of the tested metals was 100 µM. For each metal tested, 2 µg/mL of the HPLC-purified CAS-positive fraction was added. The siderophores and metals were mixed and left to incubate at room temperature overnight. The absorbance spectra were observed within the wavelength range of 230–700 nm.
Antifungal activity
The spores of Fusarium oxysporum PR57, Colletotrichum coccodes MF 16-014, Rhizoctonia solani MFP 936011 and Alternaria sp. (1 × 108 CFU/mL) were spread on the Czapek's agar (CZA) plates. 10 µL of the siderophore mixture (1.2 mg/ml) was added to the well in the centre of the plate. A control plate was inoculated with fungal spores alone, while another plate was inoculated with 10 µL of methanol as the negative control. The positive control was a mixture of metabolites (1.2 mg/mL) secreted by N. mangyaensis NH1 in the presence of FeCl3. The plates were incubated for 3–5 days at 30 °C until fungal mycelia fully covered the agar surface in the control plate. The diameter of the inhibition zone was measured in mm. A solution of commercial difenoconazole (250 g/L, Raek, KE®, Avgust Crop Protection, Russia) was used as a positive control (Line 504–505). The 0.312 mg/mL working solution has been tested as recommended for agricultural practice (State Catalogue of Pesticides and Agrochemicals, 2023) for the treatment of tomatoes and potatoes against phytopathogens78 and 1.2 mg/mL, which is equivalent to a tested mixture of metabolites secreted by N. mangyaensis NH1. All experiments were performed in triplicates.
Plant treatment assay
The influence of metabolites produced by N. mangyaensis NH1 under iron-deficiency conditions on plants was performed using Arabidopsis thaliana plants. A. thaliana (L.) Heynh. wild-type accession Col-0 (CS6673) seeds were purchased from the Arabidopsis Biological Resource Center (ABRC, https://abrc.osu.edu/). Seeds were surface sterilized using 50% bleach and plated on ½ strength Murashige and Skoog medium with 2% sucrose and 0.7% agar. Seeds were incubated for 7 days in a growth chamber under a 16-h light/8-h dark cycle at 20 °C. Cotyledon-formed seedlings were transferred to new plates with the same medium and grown for 7 days under the standard conditions before reaching 6–7 true leaves.
Nocardia mangyaensis NH1 metabolites produced under iron-deficiency and iron-enriched conditions were dissolved in DMSO in concentrations of 1.2 mg/mL and 0.6 mg/mL. Then 5 uL of metabolites were applied on a plant under the leaf rosette. DMSO-treated and non-treated plants were used as a negative control. Plates with plants were incubated for 10 days under standard conditions. Plant phenotypes (leaf rosette size, leaf color (chlorophyll content), root branching) were monitored every day and characterized on the 10th day of incubation.
Plant infection assay
Plant infection assay was performed as it was described previously72. N. mangyaensis NH 1 strain was cultured in LB medium at 30 °C on an orbital shaker with 250 rpm agitation for 96 h. All treatments of A. thaliana seedlings by NH1 strain were performed in triplicates.
All methods were carried out in accordance with relevant guidelines and regulations. All necessary permissions for planting and investigating this cultivar were obtained from South China Agricultural University, and the collection and research of this cultivar have complied with the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Conclusions
We conducted a detailed study of a 'rare' endolithic bacterium isolated from hydromagnesite and identified as Nocardia mangyaensis NH1. Genomic analysis of strain NH1 showed a highly diverse biosynthetic capacity for the production of natural products. N. mangyaensis NH1 demonstrates the secretion of siderophores and the synthesis of indole acetic acid. Here, we present a detailed annotation of the siderophore metabolome of the bacterium. A significant amount of metabolites belonging to the categories of siderophores, lipopeptides and cyclic peptides produced by N. mangyaensis NH1 during cultivation under Fe-limited conditions was discovered. Metal-binding screening of N. mangyaensis NH1 siderophores revealed their high, narrow and broad specificity to metals. Siderophores with high and specific metal chelating activity have antioxidant properties. We have shown that the siderophore metabolites of N. mangyaensis NH1 exhibit antifungal activity while having no deleterious effects on the model plant A. thaliana. The study concludes that by inoculating soil, particularly in degraded and arid regions, with the endolithic bacterium N. mangyaensis NH1, the agronomic effectiveness can be improved through enhanced mineral availability. Furthermore, soil fertility can be increased and crops can be protected against phytopathogens and oxidative stress.
Supplementary Information
Acknowledgements
The work is carried out in accordance with the Strategic Academic Leadership Program "Priority 2030" of the Kazan Federal University of the Government of the Russian Federation. We would like to thank Dr. Aliya Suleymanova for providing us with the phytopathogenic strains of Colletotrichum coccodes MF 16-014 and Rhizoctonia solani MFP 936011. Dr. Guzel Lutfullina kindly provided phytopathogenic fungi Fusarium oxysporum PR57 and Alternaria sp. for this study. Dr Nikita V. Shtyrlin is gratefully acknowledged for their assistance with preparative HPLC analysis. Sequencing was performed in the Genomics Core Facility of Skolkovo Institute of Science and Technology.
Author contributions
Conceptualization, I.K.V. and M.R.S.; formal analysis, I.K.V., M.I.M., L.R.V., T.M.I., E.I.S., A.V.L., A.A.E. and E.S.B.; investigation, I.K.V., M.I.M., L.R.V., T.M.I., E.I.S., A.V.L., A.A.E., E.S.B. and G.L.; resource, I.K.V. and M.R.S.; writing—original draft preparation, I.K.V. and M.R.S.; writing—review and editing, I.K.V. and M.R.S.; funding acquisition, M.R.S.
Funding
This work was funded by the Russian Science Foundation project # 22-16-00138.
Data availability
This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAUMIP000000000. The data sets exhibited in this research can be located within digital repositories available online (GNPS). The metadata can be found on https://massive.ucsd.edu/, MSV000093024.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54095-9.
References
- 1.Singh P, et al. Mechanistic insights and potential use of siderophores producing microbes in rhizosphere for mitigation of stress in plants grown in degraded land. Front. Microbiol. 2022 doi: 10.3389/fmicb.2022.898979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh, D., Thapa, S., Geat, N., Mehriya, M. L. & Rajawat, M. V. S. in Biofertilizers (eds Amitava Rakshit et al.) 151–166 (Woodhead Publishing, 2021).
- 3.El-Saadony MT, et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022 doi: 10.3389/fpls.2022.923880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva GDC, Kitano IT, Ribeiro IADF, Lacava PT. The potential use of actinomycetes as microbial inoculants and biopesticides in agriculture. Front. Soil Sci. 2022 doi: 10.3389/fsoil.2022.833181. [DOI] [Google Scholar]
- 5.Oyedoh OP, et al. Rare rhizo-Actinomycetes: A new source of agroactive metabolites. Biotechnol. Adv. 2023;67:108205. doi: 10.1016/j.biotechadv.2023.108205. [DOI] [PubMed] [Google Scholar]
- 6.Luo Q, Hiessl S, Steinbüchel A. Functional diversity of Nocardia in metabolism. Environ. Microbiol. 2014;16:29–48. doi: 10.1111/1462-2920.12221. [DOI] [PubMed] [Google Scholar]
- 7.Nouioui I, Ha S-M, Baek I, Chun J, Goodfellow M. Genome insights into the pharmaceutical and plant growth promoting features of the novel species Nocardia alni sp. nov. BMC Genom. 2022;23:70. doi: 10.1186/s12864-021-08257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang B-Z, et al. Nocardia aurea sp. Nov., a novel actinobacterium isolated from a karstic subterranean environment. Int. J. Syst. Evol. Microbiol. 2019;69:159–164. doi: 10.1099/ijsem.0.003122. [DOI] [PubMed] [Google Scholar]
- 9.Dhakal D, Rayamajhi V, Mishra R, Sohng JK. Bioactive molecules from Nocardia: Diversity, bioactivities and biosynthesis. J. Ind. Microbiol. Biotechnol. J. Ind. Microbiol. Biot. 2019;46:385–407. doi: 10.1007/s10295-018-02120-y. [DOI] [PubMed] [Google Scholar]
- 10.Shah A, et al. PGPR in agriculture: a sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021 doi: 10.3389/fsufs.2021.667546. [DOI] [Google Scholar]
- 11.Dhakal, D. et al. in Pharmaceuticals from Microbes: Impact on Drug Discovery (eds. D. Arora, C. Sharma, S. Jaglan, & E. Lichtfouse) 49–74 (Springer International Publishing, 2019).
- 12.Männle D, et al. Comparative genomics and metabolomics in the genus Nocardia. mSystems. 2020 doi: 10.1128/mSystems.00125-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komaki H, et al. Genome based analysis of type-I polyketide synthase and nonribosomal peptide synthetase gene clusters in seven strains of five representative Nocardia species. BMC Genom. 2014;15:323. doi: 10.1186/1471-2164-15-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone TC, Nolan EM. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. (Cambridge, England: 2003). 2015;44:6320–6339. doi: 10.1039/c4dt03559c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed E, Holmström SJ. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014;7:196–208. doi: 10.1111/1751-7915.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Qiu Z, Tan H, Cao L. Siderophore production by actinobacteria. BioMetals. 2014;27:623–631. doi: 10.1007/s10534-014-9739-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino Y, et al. Identification of nocobactin NA biosynthetic gene clusters in Nocardia farcinica. J. Bacteriol. 2011;193:441–448. doi: 10.1128/jb.00897-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda Y, Furumai T, Igarashi Y. Nocardimicins G, H and I, siderophores with muscarinic M3 receptor binding inhibitory activity from Nocardia nova JCM 6044. J. Antibiot. 2005;58:566–572. doi: 10.1038/ja.2005.77. [DOI] [PubMed] [Google Scholar]
- 19.Murakami Y, et al. Formobactin, a novel free radical scavenging and neuronal cell protecting substance from Nocardia sp. J. Antibiot. 1996;49:839–845. doi: 10.7164/antibiotics.49.839. [DOI] [PubMed] [Google Scholar]
- 20.Nemoto A, et al. Asterobactin, a new siderophore group antibiotic from Nocardia asteroides. J. Antibiot. 2002;55:593–597. doi: 10.7164/antibiotics.55.593. [DOI] [PubMed] [Google Scholar]
- 21.Schneider K, et al. Nocardichelins A and B, siderophores from Nocardia strain acta 3026. J. Nat. Prod. 2007;70:932–935. doi: 10.1021/np060612i. [DOI] [PubMed] [Google Scholar]
- 22.Suenaga K, Kokubo S, Shinohara C, Tsuji T, Uemura D. Structures of amamistatins A and B, novel growth inhibitors of human tumor cell lines from an actinomycete. Tetrahedron Lett. 1999;40:1945–1948. doi: 10.1016/S0040-4039(99)00050-7. [DOI] [Google Scholar]
- 23.Tsuda M, et al. Brasilibactin A, a cytotoxic compound from actinomycete Nocardia brasiliensis. J. Nat. Prod. 2005;68:462–464. doi: 10.1021/np0496385. [DOI] [PubMed] [Google Scholar]
- 24.Khilyas IV, et al. Microbial diversity and mineral composition of weathered serpentine rock of the Khalilovsky massif. PloS One. 2019;14:e0225929. doi: 10.1371/journal.pone.0225929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang RQ, et al. Nocardia mangyaensis sp. nov., a novel actinomycete isolated from crude-oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2019;69:397–403. doi: 10.1099/ijsem.0.003159. [DOI] [PubMed] [Google Scholar]
- 26.Crits-Christoph A, Bhattacharya N, Olm MR, Song YS, Banfield JF. Transporter genes in biosynthetic gene clusters predict metabolite characteristics and siderophore activity. Genome Res. 2020;31:239–250. doi: 10.1101/gr.268169.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madigan CA, et al. Lipidomic discovery of deoxysiderophores reveals a revised mycobactin biosynthesis pathway in Mycobacterium tuberculosis. PNAS. 2012;109:1257–1262. doi: 10.1073/pnas.1109958109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohlneicher U, Hartmann R, Taraz K, Budzikiewicz H. Pyoverdin, ferribactin, azotobactin -a new triade of siderophores from Pseudomonas chlororaphis ATCC 9446 and its relation to Pseudomonas fluorescens ATCC 13525. Z. Naturforsch. C. 1995;50:337–344. doi: 10.1515/znc-1995-5-602. [DOI] [Google Scholar]
- 29.Homann VV, et al. Loihichelins A−F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J. Nat. Prod. 2009;72:884–888. doi: 10.1021/np800640h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrić S, Meyer T, Ongena M. Bacillus responses to plant-associated fungal and bacterial communities. Front. Microbiol. 2020;11:1350. doi: 10.3389/fmicb.2020.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fewer DP, et al. Chemical diversity and cellular effects of antifungal cyclic lipopeptides from cyanobacteria. Physiol. Plant. 2021;173:639–650. doi: 10.1111/ppl.13484. [DOI] [PubMed] [Google Scholar]
- 32.Fukuchi N, et al. Structure and stereochemistry of three phytotoxins, syringomycin, syringotoxin and syringostatin, produced by Pseudomonas syringae pv. syringae. J. Chem. Soc. Perkin Trans. 1992;1:1149–1157. doi: 10.1039/P19920001149. [DOI] [Google Scholar]
- 33.Kajimura Y, Sugiyama M, Kaneda M. Bacillopeptins, new cyclic lipopeptide antibiotics from Bacillus subtilis FR-2. J. Antibiot. 1995;48:1095–1103. doi: 10.7164/antibiotics.48.1095. [DOI] [PubMed] [Google Scholar]
- 34.Xu Z, et al. Antibiotic Bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr-mediated FeuABC-dependent pathway. Cell Rep. 2019;29:1192–1202.e1195. doi: 10.1016/j.celrep.2019.09.061. [DOI] [PubMed] [Google Scholar]
- 35.Duitman EH, et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: A multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. PNAS. 1999;96:13294–13299. doi: 10.1073/pnas.96.23.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara Y, et al. Isolation of Peptidolipin NA derivatives from the culture of Nocardia arthritidis IFM10035T in the presence of mouse macrophage cells. Heterocycles. 2021;104:185–190. doi: 10.3987/COM-21-14567. [DOI] [Google Scholar]
- 37.Majumdar SK, Bose SK. Mycobacillin, a new antifungal antibiotic produced by B. subtilis. Nature. 1958;181:134–135. doi: 10.1038/181134a0. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima M, et al. Mycoplanecins, novel antimycobacterial antibiotics from Actinoplanes awajinensis subsp. mycoplanecinus subsp. nov. II. Isolation, physico-chemical characterization and biological activities of mycoplanecin A. J. Antibiot. 1983;36:961–966. doi: 10.7164/antibiotics.36.961. [DOI] [PubMed] [Google Scholar]
- 39.Akosah YA, et al. Induced expression of CYP51a and HK1 genes associated with penconazole and fludioxonil resistance in the potato pathogen Fusarium oxysporum. Microorganisms. 2023 doi: 10.3390/microorganisms11051257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pointing SB, Belnap J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012;10:551–562. doi: 10.1038/nrmicro2831. [DOI] [PubMed] [Google Scholar]
- 41.Belnap, J., Weber, B. & Büdel, B. in Biological Soil Crusts: An Organizing Principle in Drylands (eds. B. Weber, B. Büdel, & J. Belnap) 3–13 (Springer International Publishing, 2016).
- 42.Sayed AM, et al. Extreme environments: Microbiology leading to specialized metabolites. J. Appl. Microbiol. 2020;128:630–657. doi: 10.1111/jam.14386. [DOI] [PubMed] [Google Scholar]
- 43.Qin S, Xing K, Jiang JH, Xu LH, Li WJ. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011;89:457–473. doi: 10.1007/s00253-010-2923-6. [DOI] [PubMed] [Google Scholar]
- 44.Saadouli I, et al. Diversity and adaptation properties of actinobacteria associated with Tunisian stone ruins. Front. Microbiol. 2022 doi: 10.3389/fmicb.2022.997832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linget C, et al. Bacterial siderophores: The structure of a desferriferribactin produced by Pseudomonas fluorescens ATCC 13525. Tetrahedron Lett. 1992;33:3851–3854. doi: 10.1016/S0040-4039(00)74802-7. [DOI] [Google Scholar]
- 46.Cornelis, P. & Matthijs, S. in Microbial siderophores (eds. A. Varma & S. B. Chincholkar) 193–203 (Springer Berlin Heidelberg, 2007).
- 47.Hamley IW. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015;51:8574–8583. doi: 10.1039/C5CC01535A. [DOI] [PubMed] [Google Scholar]
- 48.Gutiérrez-Chávez C, Benaud N, Ferrari BC. The ecological roles of microbial lipopeptides: Where are we going? CSBJ. 2021;19:1400–1413. doi: 10.1016/j.csbj.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eshita SM, Roberto NH, Beale JM, Mamiya BM, Workman RF. Bacillomycin Lc, a new antibiotic of the iturin group: Isolations, structures, and antifungal activities of the congeners. J. Antibiot. 1995;48:1240–1247. doi: 10.7164/antibiotics.48.1240. [DOI] [PubMed] [Google Scholar]
- 50.Saha M, et al. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016;23:3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 51.Barton LE, Quicksall AN, Maurice PA. Siderophore-mediated dissolution of hematite (α-Fe2O3): Effects of nanoparticle size. Geomicrobiol. J. 2012;29:314–322. doi: 10.1080/01490451.2011.558566. [DOI] [Google Scholar]
- 52.Dimkpa CO, et al. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere. 2008;74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 53.Kamnev AA, Shchelochkov AG, Perfiliev YD, Tarantilis PA, Polissiou MG. Spectroscopic investigation of indole-3-acetic acid interaction with iron(III) J. Mol. Struct. 2001;563–564:565–572. doi: 10.1016/S0022-2860(00)00911-X. [DOI] [Google Scholar]
- 54.Jin Z, et al. Conditional privatization of a public siderophore enables Pseudomonas aeruginosa to resist cheater invasion. Nat. Commun. 2018;9:1383. doi: 10.1038/s41467-018-03791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogomolnaya LM, Tilvawala R, Elfenbein JR, Cirillo JD, Andrews-Polymenis HL. Linearized siderophore products secreted via macab efflux pump protect Salmonella enterica Serovar Typhimurium from Oxidative Stress. mBio. 2020 doi: 10.1128/mBio.00528-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peralta DR, et al. Enterobactin as part of the oxidative stress response repertoire. PloS One. 2016;11:e0157799. doi: 10.1371/journal.pone.0157799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maindad DV, et al. Characterization and fungal inhibition activity of siderophore from wheat rhizosphere associated Acinetobacter calcoaceticus strain HIRFA32. Indian J. Microbiol. 2014;54:315–322. doi: 10.1007/s12088-014-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sulochana MB, Jayachandra SY, Kumar SKA, Dayanand A. Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25. J. Basic Microbiol. 2014;54:418–424. doi: 10.1002/jobm.201200770. [DOI] [PubMed] [Google Scholar]
- 59.Crowley, D. E. in Iron Nutrition in Plants and Rhizospheric Microorganisms (eds L.L. Barton & J. Abadia) 169–198 (Springer Netherlands, 2006).
- 60.Yehuda Z, et al. The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol. 1996;112:1273–1280. doi: 10.1104/pp.112.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duijff BJ, De Kogel WJ, Bakker PAHM, Schippers B. Influence of pseudobactin 358 on the iron nutrition of barley. Soil Biol. Biochem. 1994;26:1681–1688. doi: 10.1016/0038-0717(94)90321-2. [DOI] [Google Scholar]
- 62.Wingett SW, Andrews S. FastQ screen: A tool for multi-genome mapping and quality control. F1000Research. 2018;7:1338. doi: 10.12688/f1000research.15931.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England) 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bankevich A, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurevich A, et al. Increased diversity of peptidic natural products revealed by modification-tolerant database search of mass spectra. Nat. Rev. Microbiol. 2018;3:319–327. doi: 10.1038/s41564-017-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tatusova T, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blin K, et al. AntiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualization. Nucleic Acids Res. 2023 doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 70.Arnow LE. Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. JBC. 1937;118:531–537. doi: 10.1016/S0021-9258(18)74509-2. [DOI] [Google Scholar]
- 71.Atkin CL, Neilands JB, Phaff HJ. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J. Bacteriol. 1970;103:722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khilyas IV, et al. Genomic and metabolomic profiling of endolithic Rhodococcus fascians strain S11 isolated from an arid serpentine environment. Arch. Microbiol. 2022;204:336. doi: 10.1007/s00203-022-02955-1. [DOI] [PubMed] [Google Scholar]
- 73.Adusumilli R, Mallick P. Data conversion with proteowizard msconvert. Methods Mol. Biol. (Clifton, NJ). 2017;1550:339–368. doi: 10.1007/978-1-4939-6747-6_23. [DOI] [PubMed] [Google Scholar]
- 74.Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao L, et al. MolDiscovery: Learning mass spectrometry fragmentation of small molecules. Nat. Commun. 2021;12:3718. doi: 10.1038/s41467-021-23986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohimani H, et al. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018;9:4035. doi: 10.1038/s41467-018-06082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.State Catalogue of Pesticides and Agrochemicals . State Catalogue of Pesticides and Agrochemicals Approved For the Use in Russian Federation in 2023. Ministry of Agriculture of Russian Federation; 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAUMIP000000000. The data sets exhibited in this research can be located within digital repositories available online (GNPS). The metadata can be found on https://massive.ucsd.edu/, MSV000093024.