Abstract
Despite improvements in imaging and treatment approaches, brain metastases (BMs) continue to be the primary cause of mortality and morbidity in about 20% of adult cancer patients. This research aimed to review the magnetic resonance imaging (MRI) and clinical characteristics of BMs resulting from breast cancer (BC). A systematic review of original research articles published from January 2000 to June 2023. We selected studies that reported MRI findings of BMs in BC patients. We excluded reviews, case reports, books/book chapters, animal studies and irrelevant records. We identified 24 studies that included 1580 BC patients with BMs. T1‐weighted (T1‐w) (pre‐ and postcontrast), T2‐weighted (T2‐w), fluid‐attenuated inversion recovery (FLAIR) and T2*‐weighted (T2*‐w) was used to measure the lesion size, shape and area. In other studies, advanced structural techniques including diffusion‐weighted imaging (DWI), diffusion tensor imaging (DTI) and susceptibility‐weighted imaging (SWI) were used to more precisely and sensitively evaluate the pathological area. Furthermore, functional and metabolic techniques like functional MRI (fMRI), magnetic resonance spectroscopy (MRS) and perfusion‐weighted imaging (PWI) have also been utilised. The MRI findings of BMs varied depending on the MRI technique, the BC subtype, the lesion size and shape, the presence of haemorrhage or necrosis and the comparison with other brain tumours. Some MRI findings were associated with prognosis, recurrence or cognitive impairment in BC patients with BMs. MRI detects, characterises and monitors BMs from BC. Findings vary by MRI technique, BC subtype, lesion characteristics and comparison with other brain tumours. More research should validate emerging MRI techniques, determine the clinical implications of findings and explore the underlying mechanisms and biology of BMs from BC. MRI is a valuable tool for diagnosis, targeted therapy and studying BC metastasis.
Keywords: Brain metastases, brain metastasis, breast cancer, magnetic resonance imaging
Magnetic resonance imaging (MRI) characterises and monitors brain metastases in breast cancer (BC), with findings varying by MRI technique, BC subtype, lesion features and comparison to other brain tumours. MRI is valuable for diagnosis, therapy and studying the mechanisms of BC brain metastasis.

Introduction
As a multi‐step process, metastasis involves the dissemination of cancer cells from the initial tumour to the target organ. 1 , 2 Brain metastases (BMs) remain the leading cause of death and morbidity in about 20% of adult cancer patients, despite advances in imaging and treatment modalities. 3 Having BMs is a disastrous consequence for patients with solid malignancies such as breast cancer (BC), lung cancer and melanoma, and is often linked with a poor prognosis and decreased quality of life. 1 , 4
Because of its specialised cells, anatomical characteristics, metabolic restrictions and immunological surroundings, the brain microenvironment exerts a vastly different selection pressure on tumour cells than extracranial lesions do, which in turn influences the metastatic process and treatment responses. 5
Approximately 15%–25% of patients with BC may develop central nervous system (CNS) metastases. 1 , 6 The ability of BC cells to metastasize to the brain is influenced by their receptor status. In fact, as compared to hormone receptor‐positive individuals, human epidermal growth factor receptor 2 (HER2)‐positive and triple‐negative BC (TNBC) subtypes had the highest association with CNS metastases, with the frequencies of BMs reaching values as high as 30–40%. 1 , 7 , 8
Accurate identification of the cause of BMs is critical for developing an appropriate treatment plan to enhance the patients prognosis. 9 Computed tomography (CT) scans and magnetic resonance imaging (MRI) scans are the most common imaging techniques used to diagnose brain malignancies. 10 , 11 , 12 , 13 , 14 , 15 The high signal‐to‐noise ratio (SNR), contrast‐to‐noise ratio (CNR), spatial resolution, contrast resolution and the abundance of MRI sequences available to define intracranial lesions allow MRI to give more detailed, localisation and characterisation of BMs. 16 , 17 To be more specific, T1‐weighted (T1‐w) and T2‐weighted (T2‐w) sequences outline the morphologic and anatomical de‐alignment of tissue brought about by the tumour. 18
The purpose of this systematic review was to document the utility of reported MRI techniques in imaging BC, as well as the MRI and clinical features of BMs from BC to aid in the diagnosis and treatment of similar individuals in the future.
Material and Methods
Search strategy
This systematic review followed the PRISMA statement for reporting systematic reviews and meta‐analyses. 19 The aim of this review was to synthesise the available evidence on BMs from BC using MRI. We searched PubMed and Scopus databases from January 2000 to June 2023 using the following combination of keywords: "MRI," "breast cancer," "brain metastases," and "brain metastasis." We restricted our search to articles published in English that reported MRI and clinical data on BMs in BC patients. The detailed search strategy for PubMed is provided in Table S1.
Study selection
We selected the titles and abstracts of the retrieved records for eligibility and excluded reviews (including literature reviews, systematic reviews and meta‐analyses), case reports, books and book chapters, animal studies and other irrelevant records. We obtained the full texts of the potentially eligible records and assessed their inclusion based on the following criteria: (1) original research article (2); MRI findings of BMs in BC patients; and (3) related BC findings associated with MRI. We excluded articles that did not meet these criteria or did not provide sufficient MRI data. We also checked the reference lists of included articles for additional relevant studies. After reviewing the full texts and removing any records not related to the MRI / BC findings, we identified 86 records that may meet our inclusion criteria.
Data extraction and quality assessment
Three independent reviewers (S.Gh., M.M. and S.M.) performed the study selection process and any disagreements were resolved by consensus or consultation. We extracted the following data from each included article: authors, year of publication, number of BC patients with BMs, MRI techniques used, anatomical locations of BMs and imaging findings. Table 1 summarises the characteristics and findings of the included studies. Other reviewers used Microsoft Excel (version 2016) to record the data. Two reviewers (S.Gh. and M.M.) independently extracted the data and cross‐checked for accuracy. Any discrepancies were resolved by discussion or consultation with a third reviewer (S.M.). The quality of the included studies was assessed using the Cochrane Risk of Bias tool. Figure 1 shows the PRISMA flow diagram of the study selection process.
Table 1.
Brain metastases from breast cancer MRI findings.
| First author (year) | Number of Patients | MRI Technique(s) | Anatomical Location(s) | MRI Findings |
|---|---|---|---|---|
| Xue (2023) 20 | 4 | T1‐w and T2‐w | Superficial parenchyma lesions and deep lesions |
|
| Young (2023) 21 | 34 | CE MRI | Frontal, temporal, perietal, occipital and cerebellum |
|
| Reibelt (2022) 22 | 15 | 3D T1‐w MPRAGE | L. cerebellum WM, R. pallidum, L. thalamus, L. choroid plexus, L. Lat. ventricle and total GM |
|
| Young (2021) 23 | 38 | T1‐w and T1‐w post | Frontal, temporal, perietal, occipital and cerebellum |
|
| Santos (2020) 24 | 147 | T1‐w, T1‐w post, T2‐w, DWI and T2*‐w | Hippocampal and hydrocephalus |
|
| Zhang (2019) 25 | 3 | T1‐w, T1‐w post, T2‐w, DWI, ADC and FLAIR | NA |
|
| Mayinger (2019) 26 | 851 | 3D T1‐w | Bilateral and central/median subcortical structures |
|
| Kniep (2019) 27 | 37 | T1‐w, T1‐w post and FLAIR | NA |
|
| Ortiz‐Ramón (2018) 28 | 17 | 3D T1‐w | NA |
|
|
Skogen (2018) 29 |
5 | DTI | Intracranial lesions without visible haemorrhage, multiple lesions and infratentorial lesions |
|
| Muto (2018) 30 | 13 | DSC | Temporal lobe |
|
| Kyeong (2017) 31 | 100 | 3D T1‐w | HER2‐positive type: occipital, temporal lobes and cerebellum / Luminal type: frontal, occipital lobes and cerebellum |
|
| Kesler (2017) 32 |
74 |
rsfMRI, T2*‐w GRE, HR3D IR, FSPGR GRE T1‐w and DTI | R. inferior parietal lobe, R. middle inferior orbital frontal gyrus, R. medial superior frontal gyrus, R. inferior and middle frontal gyri, bilateral postcentral gyri, R. precuneus, L. inferior temporal gyrus, L. middle occipital gyrus, R. parietal lobule, R. cuneus, R. superior temporal gyrus and R. inferior temporal gyrus. |
|
| Bette (2017) 33 | 13 | T2‐FLAIR | NA |
|
| Fan (2017) 34 | 13 | T1‐w FLAIR and DWI (before enhancement) and T1‐w FLAIR | R. occipital lobe |
|
| Franceschi (2016) 35 | 38 | T1‐w post and SWI | NA |
|
| Kesler (2015) 36 | 36 | DTI and T1‐w | L. corpus callosum, bilateral inferior longitudinal fasciculus, L inferior fronto‐occipital fasciculus, and bilateral temporal and frontal lobe white matter |
|
| Yeh (2015) 37 | 62 | T1‐w, T1‐w post, T2‐w fast spin echo (FSE), FLAIR and DWI | Parietal lobe, R. frontal lobe and L. cerebellum |
|
| Quattrocchi (2014) 38 | 42 | T1w, T1‐w post and T2‐w FLAIR | Parieto‐occipital lobes and cerebellum |
|
| Huang (2010) 39 | 17 | Multivoxel 2D‐CSI MRS and DCE (n = 21 BC) | NA |
|
| Hakyemez (2010) 40 | 5 | T2‐w and PWI for rCBV | NA |
|
| Takeda (2008) 41 | 13 | 2D T1‐w SE, 3D MPRAGE T1‐w post, 2D T1‐w SE post and 2D T2‐w SE | Posterior fossa, middle fossa and supratentorial |
|
| Kremer (2003) 42 | 2 | T1‐w, T2‐w, T2*‐w, R2* and DCE | NA |
|
| Geijer (2002) 43 | 1 | T1‐w, T2‐w, DWI and ADC | Lateral wall of the posterior horn of the L. lateral ventricle and falx cerebri |
|
2D T1‐w SE, two‐dimensional T1‐weighted spin echo; 2D T2‐w SE, two‐dimensional T2‐weighted spin echo; 3D T1‐w MPRAGE, 3D T1‐weighted magnetisation prepared rapid acquisition gradient echo; ADC, apparent diffusion coefficient; BC, breast cancer; BMs, brain metastases; CBV, cerebral blood volume; CE MRI, contrast‐enhanced MRI; CE, contrast‐enhanced; DCE, dynamic contrast‐enhanced; DSC, dynamic susceptibility contrast; DTI, diffusion tensor imaging; DWI, diffusion‐weighted imaging; EVR, enhanced volume ratio; FLAIR, fluid‐attenuated inversion recovery; FSPGR, fast spoiled gradient recalled; GBM, glioblastoma multiforme; HER2, human epidermal growth factor receptor 2; HIF‐1, hypoxia‐inducible factor‐1; HR3D IR high‐resolution 3D inversion recovery; L., Left; LAVA, liquid attenuation inversion recovery; LITT, laser interstitial thermal therapy; MPRAGE, magnetisation prepared rapid acquisition gradient echo; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; Multivoxel 2D‐CSI MRS, multivoxel two‐dimensional chemical shift Imaging magnetic resonance spectroscopy; PWI, perfusion‐weighted imaging; R., right; R2*, transverse relaxation rate; rCBV, relative cerebral blood volume; rsfMRI, resting‐state functional MRI; SWI, susceptibility‐weighted imaging; T1‐w post, T1‐weighted postcontrast; T1‐w, T1‐weighted; T2*‐w, T2‐star weighted; T2‐w, T2‐weighted; TNBC, triple‐negative breast cancer; WMH, white matter hyperintensities.
Hurst exponent measures a time series' memory, with values >0.5 showing trends and <0.5 indicating mean reversion.
Figure 1.
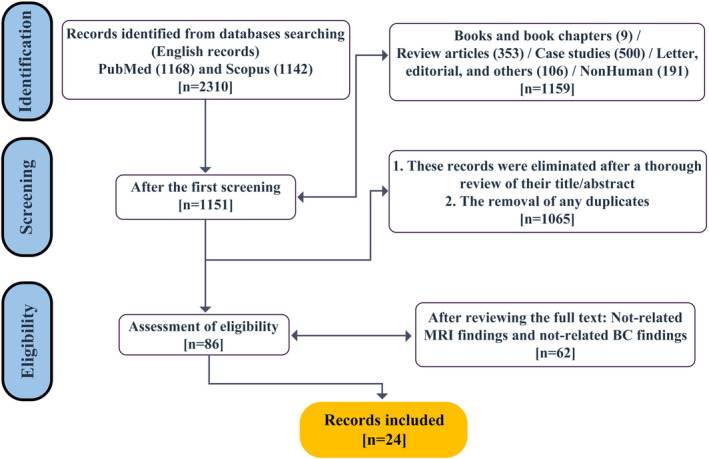
PRISMA flow diagram for systematic review.
Results
Twenty‐four studies that investigated BMs from BC were included in the analysis (Table 1). The studies included a total of 1580 patients with BMs from BC and used various MRI techniques such as T1‐w, T2‐w, contrast‐enhanced (CE), diffusion‐weighted imaging (DWI), fluid‐attenuated inversion recovery (FLAIR), dynamic susceptibility contrast (DSC), resting‐state functional MRI (rsfMRI), susceptibility‐weighted imaging (SWI), magnetic resonance spectroscopy (MRS) and perfusion‐weighted imaging (PWI). The anatomical locations of BMs varied across studies, but some common regions were the frontal, temporal, parietal and occipital lobes, the cerebellum and the ventricles. The MRI findings of BMs also varied depending on the MRI technique, the BC subtype, the lesion size and shape, the presence of haemorrhage or necrosis and the comparison with other brain tumours such as glioblastoma multiforme (GBM). Some MRI findings were associated with prognosis, recurrence or cognitive impairment in BC patients with BMs. The table provides a comprehensive overview of the current state of knowledge on BMs from BC using MRI.
The MRI findings for BMs from BC based on the studies in Table 1 indicate that the most common location for metastases is in the cerebral hemispheres. The majority of metastases are solid‐enhancing masses with irregular or poorly defined margins. The number of patients in each study ranged from 1 to 851. The enhancement pattern of BMs was reported to be variable, with some studies reporting homogeneous enhancement, while others reported heterogeneous or rim enhancement.
Discussion
The aim of this systematic review was to summarise the MRI techniques, anatomical locations and MRI findings of BMs from BC using different MRI modalities. The MRI findings from these studies can be broadly categorised into lesion identification, characterisation and prediction of prognosis or response to treatment.
MRI is a useful and versatile tool for detecting, characterising and monitoring BMs from BC, as it can provide information on the lesion size, shape, location, composition, perfusion, metabolism and connectivity. 26 , 32 , 41 MRI techniques can vary in their sensitivity and specificity for BMs from BC, depending on the contrast agent, the sequence parameters, the image processing and the interpretation criteria. 21 , 34 , 35 Some MRI techniques such as CE MRI, DWI, ADC, DSC and SWI can help to differentiate BMs from BC from other brain tumours such as GBM or ischemic lesions. 29 , 30 , 34 , 35 , 40 , 42 Other MRI techniques such as postcontrast T1‐w and FLAIR can help to assess the response to treatment or the recurrence of BMs from BC. 33 , 34 , 35 Some MRI techniques such as DTI and rsfMRI can help to evaluate the cognitive impairment or the functional connectivity of BMs from BC. 29 , 32 , 36
MRI findings of BMs from BC can vary depending on the BC subtype, such as HER2‐positive, luminal or triple‐negative. Different BC subtypes can have different patterns of BM distribution in the brain, different lesion contours and compositions on MRI, and different associations with prognosis or imaging features. For example, HER2‐positive BMs tend to occur in the occipital lobe and cerebellum and have a smooth contour and a solid composition on MRI. Triple‐negative BMs tend to be evenly distributed in the brain and have a lobulated contour and a cystic necrotic composition on MRI. 21 , 23 , 31 , 37
MRI findings of BMs from BC can also vary depending on the lesion size and shape, the presence of haemorrhage or necrosis and the comparison with other brain tumours such as GBM or ischemic lesions. Larger BMs tend to have more haemorrhage and necrosis than smaller BMs. Haemorrhage is more prevalent in melanoma than in BC BMs. Necrosis is more common in GBM than in BMs. 30 , 35 ADC‐based texture analysis can help to distinguish between solitary BM and GBM by measuring the heterogeneity of the lesion. 25 rCBV can help to distinguish between metastatic lesions and gliomas by measuring the perfusion of the lesion and the surrounding oedema. 40 , 42
MRI techniques have also been used to predict prognosis and treatment response in BC BMs. 24 Xue et al. observed an increase in tumour volume on MRI scans following laser interstitial thermal therapy (LITT) and suggested that an enhanced volume ratio (EVR) greater than 40% on the 30‐day MRI could indicate a potential for tumour recurrence. 20 Reibelt et al. found that subcortical volume changes after radiotherapy were sensitive indicators of neuroanatomical modifications and brain atrophy, 22 while Muto et al. demonstrated the clinical utility of DSC in distinguishing between tumour recurrence, tumour necrosis and pseudoprogression in cerebral metastases. 30 These findings underscore the potential of MRI in monitoring treatment response and predicting outcomes in BC BM patients.
The strengths of this review are that it provides a comprehensive overview of the current state of knowledge on BMs from BC using MRI. It covers a wide range of MRI techniques, anatomical locations and MRI findings that are relevant for clinical practice and research. It also identifies some knowledge gaps and limitations that need further investigation.
This review has some limitations that should be acknowledged and addressed in future research. The studies included in the analysis may have been subject to selection bias, as they were not randomly selected and may not represent the broader population of patients with BMs. The patients included in the studies varied in terms of tumour type, size and location, as well as demographic and clinical characteristics. This heterogeneity could introduce variability into the findings and limit the generalisability of the results. The studies used different MRI techniques and protocols, which could affect the accuracy and reliability of the findings. The studies used different criteria for defining and classifying MRI findings, which could lead to inconsistencies and difficulties in comparing results across studies. Some studies had limited follow‐up periods, which could limit the ability to assess longer‐term outcomes and the recurrence of BMs.
Based on the findings and limitations of this review, some recommendations for future research can be made. First, more studies are needed to validate some MRI techniques for BMs from BC, such as ADC‐based texture analysis, rCBV measurement, machine‐learning classifier, and structural and functional connectome analysis. These techniques have shown promising results in distinguishing BMs from BC from other brain tumours or predicting their prognosis or cognitive impairment, but they need further confirmation and refinement in larger and more diverse samples. Second, more studies are needed to explore the clinical implications of MRI findings for BMs from BC, such as their impact on treatment decision‐making, survival outcomes, quality of life or functional recovery. These studies can help to translate the MRI findings into meaningful and actionable information for patients and clinicians. Third, more studies are needed to investigate the underlying mechanisms and pathways of BMs from BC using MRI, such as their molecular and genetic characteristics, their interaction with the brain microenvironment, or their response to therapy. These studies can help to elucidate the pathophysiology and biology of BMs from BC and identify potential targets or biomarkers for diagnosis or treatment.
Conclusion
Diseases may be diagnosed earlier and more precisely with the use of medical imaging, especially MRI. This review shows that MRI is a valuable tool for detecting, characterising and monitoring BMs from BC using different MRI techniques. MRI can be used to examine the structure, function, metabolism and important MRI biomarkers. MRI can provide information on various aspects of BMs from BC such as their size, shape, location, composition, perfusion, metabolism and connectivity. MRI findings of BMs from BC can vary depending on the MRI technique, the BC subtype, the lesion size and shape, the presence of haemorrhage or necrosis, and the comparison with other brain tumours.
MRI can aid in the early diagnosis of tumours and their spread in the initial phases of their growth and metastasis. In addition to its better sensitivity, MR imaging's unique characteristics (such as its high SNR, CNR, spatial resolution and contrast resolution) make it a valuable tool for prognosis and early identification of disease, all of which are critical for implementing targeted therapy. Further studies are needed to validate some MRI techniques for BMs from BC and to explore their clinical implications.
Conflict of Interest
The authors declare no financial or other conflicts of interest.
Supporting information
Table S1. Search strategy and MeSH Terms on PubMed databases.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1. Custódio‐Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Biochim Biophys Acta Rev Cancer 2017; 1868: 132–147. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ‐specific colonization. Nat Rev Cancer 2009; 9: 274–284. [DOI] [PubMed] [Google Scholar]
- 3. Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol 2015; 33: 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Muñoz W, Kerbel RS. Preclinical approaches to study the biology and treatment of brain metastases. Semin Cancer Biol 2011; 21: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer 2020; 20: 4–11. [DOI] [PubMed] [Google Scholar]
- 6. Saha A, Ghosh SK, Roy C, Choudhury KB, Chakrabarty B, Sarkar R. Demographic and clinical profile of patients with brain metastases: A retrospective study. Asian J Neurosurg 2013; 8: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Witzel I, Oliveira‐Ferrer L, Pantel K, Müller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 2016; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010; 28: 3271–3277. [DOI] [PubMed] [Google Scholar]
- 9. Liu H, Chen J, Chen H, et al. Identification of the origin of brain metastases based on the relative methylation orderings of CpG sites. Epigenetics 2021; 16: 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence (NICE) . Evidence reviews for the clinical and cost‐effectiveness of routine MRI or CT of the brain in the management of people with lung cancer prior to therapy with curative intent: Lung cancer: diagnosis and management: Evidence review B [Internet]. National Institute for Health and Care Excellence (NICE), London, 2019. [cited 2023 Jun 12]. (NICE Evidence Reviews Collection). Available from. http://www.ncbi.nlm.nih.gov/books/NBK558780/. [PubMed] [Google Scholar]
- 11. Derks SHAE, van der Veldt AAM, Smits M. Brain metastases: the role of clinical imaging. Br J Radiol 2022; 95: 20210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghaderi S, Olfati M, Ghaderi M, et al. Neurological manifestation in COVID‐19 disease with neuroimaging studies. Am J Neurodegener Dis 2023; 12: 42–84. [PMC free article] [PubMed] [Google Scholar]
- 13. Ghaderi S, Mohammadi S, Heidari M, Sharif Jalali SS, Mohammadi M. Post‐COVID‐19 vaccination CNS magnetic resonance imaging findings: A systematic review. Can J Infect Dis Med Microbiol 2023; 2023: e1570830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghaderi S, Fatehi F, Kalra S, Batouli SAH. MRI biomarkers for memory‐related impairment in amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener 2023; 24: 1–17. [DOI] [PubMed] [Google Scholar]
- 15. Ghaderi S, Karami A, Ghalyanchi‐Langeroudi A, et al. MRI findings in movement disorders and associated sleep disturbances. Am J Nucl Med Mol Imaging 2023; 13: 77–94. [PMC free article] [PubMed] [Google Scholar]
- 16. Ghaderi S, Ghaderi K, Ghaznavi H. Using marker‐controlled watershed transform to detect Baker's cyst in magnetic resonance imaging images: a pilot study. J Med Signals Sens 2022; 12: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ocaña‐Tienda B, Pérez‐Beteta J, Villanueva‐García JD, et al. A comprehensive dataset of annotated brain metastasis MR images with clinical and radiomic data. Sci Data 2023; 10: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hadjipanteli A, Doolan P, Kyriacou E, Constantinidou A. Breast cancer brain metastasis: the potential role of MRI beyond current clinical applications. Cancer Manag Res 2020; 12: 9953–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 29: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue Z, Guan X, Yuan L, et al. Laser interstitial thermal therapy in the treatment of brain metastases: the relationship between changes in postoperative magnetic resonance imaging characteristics and tumor recurrence. Acta Neurochir 2023; 165: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 21. Young JR, Ressler JA, Mortimer JE, Schmolze D, Fitzgibbons M, Chen BT. Association of lesion contour and lesion composition on MR with HER2 status in breast cancer brain metastases. Magn Reson Imaging 2023; 96: 60–66. [DOI] [PubMed] [Google Scholar]
- 22. Reibelt A, Mayinger M, Borm KJ, Combs SE, Duma MN. Neuroanatomical changes seen in MRI in patients with cerebral metastasized breast cancer after radiotherapy. Tumori 2022; 108: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young JR, Ressler JA, Mortimer JE, Schmolze D, Fitzgibbons M, Chen BT. Performance of enhancement on brain MRI for identifying HER2 overexpression in breast cancer brain metastases. Eur J Radiol 2021; 144: 109948. [DOI] [PubMed] [Google Scholar]
- 24. Santos J, Arantes J, Carneiro E, et al. Brain metastases from breast cancer. Clin Neurol Neurosurg 2020; 197: 106150. [DOI] [PubMed] [Google Scholar]
- 25. Zhang G, Chen X, Zhang S, et al. Discrimination between solitary brain metastasis and glioblastoma multiforme by using ADC‐based texture analysis: A comparison of two different ROI placements. Acad Radiol 2019; 26: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 26. Mayinger M, Reibelt A, Borm KJ, et al. MRI based neuroanatomical segmentation in breast cancer patients: leptomeningeal carcinomatosis vs. oligometastatic brain disease vs. multimetastastic brain disease. Radiat Oncol 2019; 14: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kniep HC, Madesta F, Schneider T, et al. Radiomics of brain MRI: utility in prediction of metastatic tumor type. Radiology 2019; 290: 479–487. [DOI] [PubMed] [Google Scholar]
- 28. Ortiz‐Ramón R, Larroza A, Ruiz‐España S, Arana E, Moratal D. Classifying brain metastases by their primary site of origin using a radiomics approach based on texture analysis: a feasibility study. Eur Radiol 2018; 28: 4514–4523. [DOI] [PubMed] [Google Scholar]
- 29. Skogen K, Schulz A, Helseth E, Ganeshan B, Dormagen JB, Server A. Texture analysis on diffusion tensor imaging: discriminating glioblastoma from single brain metastasis. Acta Radiol 2019; 60: 356–366. [DOI] [PubMed] [Google Scholar]
- 30. Muto M, Frauenfelder G, Senese R, et al. Dynamic susceptibility contrast (DSC) perfusion MRI in differential diagnosis between radionecrosis and neoangiogenesis in cerebral metastases using rCBV, rCBF and K2. Radiol Med 2018; 123: 545–552. [DOI] [PubMed] [Google Scholar]
- 31. Kyeong S, Cha YJ, Ahn SG, Suh SH, Son EJ, Ahn SJ. Subtypes of breast cancer show different spatial distributions of brain metastases. PloS One 2017; 12: e0188542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kesler SR, Adams M, Packer M, et al. Disrupted brain network functional dynamics and hyper‐correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav 2017; 7: e00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bette S, Gempt J, Wiestler B, et al. Increase in FLAIR signal of the fluid within the resection cavity as early recurrence marker: also valid for brain metastases? Rofo 2017; 189: 63–70. [DOI] [PubMed] [Google Scholar]
- 34. Fan B, Li M, Wang X, et al. Diagnostic value of gadobutrol versus gadopentetate dimeglumine in enhanced MRI of brain metastases. J Magn Reson Imaging 2017; 45: 1827–1834. [DOI] [PubMed] [Google Scholar]
- 35. Franceschi AM, Moschos SJ, Anders CK, et al. Utility of susceptibility weighted imaging (SWI) in the detection of brain hemorrhagic metastases from breast cancer and melanoma. J Comput Assist Tomogr 2016; 40: 803–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging 2015; 36: 2429–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeh RH, Yu JC, Chu CH, et al. Distinct MR imaging features of triple‐negative breast cancer with brain metastasis. J Neuroimaging 2015; 25: 474–481. [DOI] [PubMed] [Google Scholar]
- 38. Quattrocchi CC, Errante Y, Mallio CA, et al. Inverse spatial distribution of brain metastases and white matter hyperintensities in advanced lung and non‐lung cancer patients. J Neurooncol 2014; 120: 321–330. [DOI] [PubMed] [Google Scholar]
- 39. Huang BY, Kwock L, Castillo M, Smith JK. Association of choline levels and tumor perfusion in brain metastases assessed with proton MR spectroscopy and dynamic susceptibility contrast‐enhanced perfusion weighted MRI. Technol Cancer Res Treat 2010; 9: 327–337. [DOI] [PubMed] [Google Scholar]
- 40. Hakyemez B, Erdogan C, Gokalp G, Dusak A, Parlak M. Solitary metastases and high‐grade gliomas: radiological differentiation by morphometric analysis and perfusion‐weighted MRI. Clin Radiol 2010; 65: 15–20. [DOI] [PubMed] [Google Scholar]
- 41. Takeda T, Takeda A, Nagaoka T, et al. Gadolinium‐enhanced three‐dimensional magnetization‐prepared rapid gradient‐echo (3D MP‐RAGE) imaging is superior to spin‐echo imaging in delineating brain metastases. Acta Radiol 2008; 49: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 42. Kremer S, Grand S, Berger F, et al. Dynamic contrast‐enhanced MRI: differentiating melanoma and renal carcinoma metastases from high‐grade astrocytomas and other metastases. Neuroradiology 2003; 45: 44–49. [DOI] [PubMed] [Google Scholar]
- 43. Geijer B, Holtås S. Diffusion‐weighted imaging of brain metastases: their potential to be misinterpreted as focal ischaemic lesions. Neuroradiology 2002; 44: 568–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy and MeSH Terms on PubMed databases.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


