Abstract
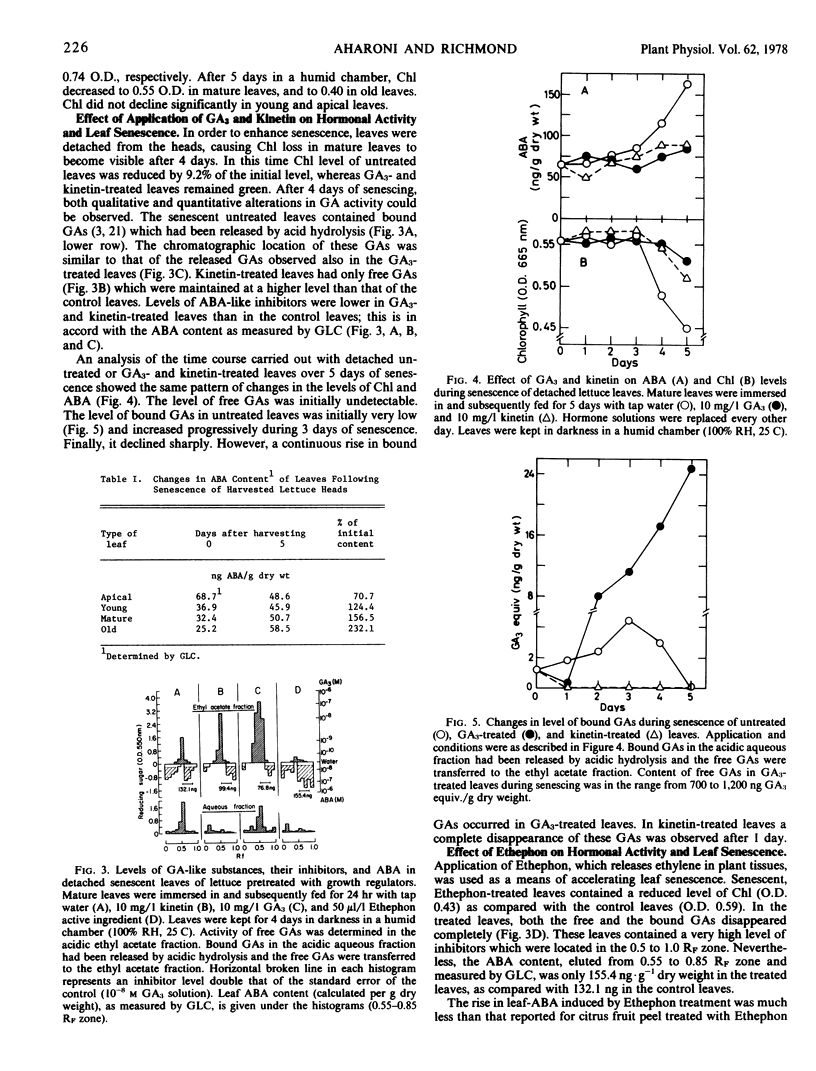
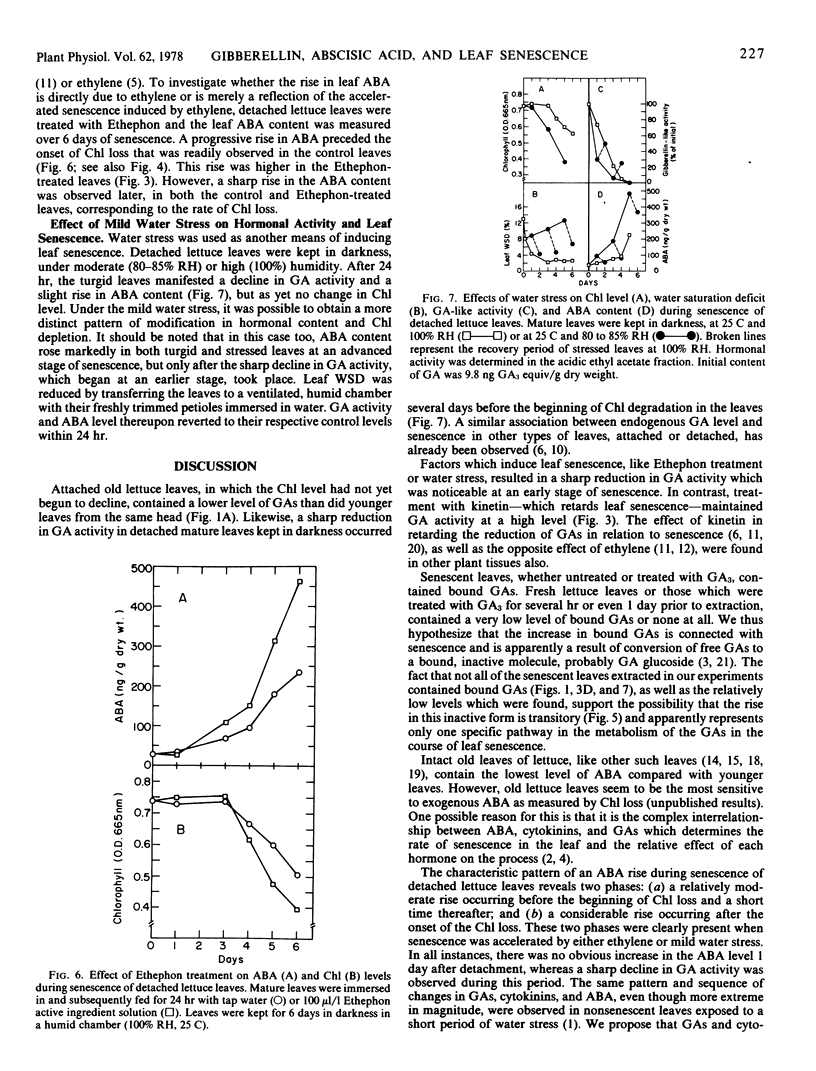
Levels of gibberillins (GAs) and of abscisic acid (ABA) in attached leaves of romaine lettuce (Lactuca sativa L.) declined as the leaf became older. The time course of changes in hormone levels, determined in detached lettuce leaves kept in darkness, revealed that a sharp decline in GAs accompanied by a moderate rise in ABA occurred before the onset of chlorophyll degradation. As senescence advanced, no GAs could be detected and a considerable rise of ABA was observed. A similar sequence of hormonal modifications, but more pronounced, was observed in the course of accelerated senescence induced by either Ethephon or water stress. When kinetin or GA3 was applied to detached leaves, the loss of chlorophyll and the rise in ABA were reduced. Bound GAs were detected in senescent leaves. They were not found in the kinetin-treated leaves, which contained a relatively high level of free GAs. The results suggest that senescence in detached romaine lettuce leaves is connected with a depletion of free GAs and cytokinins, which is thereafter followed by a great surge in ABA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Blumenfeld A., Richmond A. E. Hormonal activity in detached lettuce leaves as affected by leaf water content. Plant Physiol. 1977 Jun;59(6):1169–1173. doi: 10.1104/pp.59.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse G. W., Kende H., Lang A. Fate of radioactive gibberellin a(1) in maturing and germinating seeds of peas and Japanese morning glory. Plant Physiol. 1968 May;43(5):815–822. doi: 10.1104/pp.43.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisker H. E., Goldschmidt E. E., Goren R. Ethylene-induced Formation of ABA in Citrus Peel as Related to Chloroplast Transformations. Plant Physiol. 1976 Sep;58(3):377–379. doi: 10.1104/pp.58.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., Haimovich J., Fuchs Y. Immunological studies of plant hormones. Detection and estimation by immunological assays. Eur J Biochem. 1971 Feb 1;18(3):384–390. doi: 10.1111/j.1432-1033.1971.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E. E., Monselise S. P. Native growth inhibitors from citrus shoots. Partition, bioassay, and characterization. Plant Physiol. 1968 Jan;43(1):113–116. doi: 10.1104/pp.43.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan W. R., Brown K. W., Thomas J. C. Leaf Age as a Determinant in Stomatal Control of Water Loss from Cotton during Water Stress. Plant Physiol. 1975 Nov;56(5):595–599. doi: 10.1104/pp.56.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S., Halevy A. H. Cytokinin activity in rose petals and its relation to senescence. Plant Physiol. 1970 Oct;46(4):497–499. doi: 10.1104/pp.46.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne D. J., Jackson M. B., Milborrow B. V. Physiological properties of abscission accelerator from senescent leaves. Nat New Biol. 1972 Nov 22;240(99):98–101. doi: 10.1038/newbio240098a0. [DOI] [PubMed] [Google Scholar]
- Raschke K., Zeevaart J. A. Abscisic Acid Content, Transpiration, and Stomatal Conductance As Related to Leaf Age in Plants of Xanthium strumarium L. Plant Physiol. 1976 Aug;58(2):169–174. doi: 10.1104/pp.58.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]



