Graphical abstract
Keywords: Derris elliptica, Streptozotocin, Antidiabetic, NMR
Highlights
-
•
No toxicity or mortality demonstrates the extract's safety.
-
•
D. elliptica methanolic leaf extract possesses significant antidiabetic activity.
-
•
Quercetin and ceramide were identified as potential phytoconstituents from the plant.
-
•
D. elliptica could be a promising alternative treatment for diabetes.
Abstract
Derris elliptica (Wall.) Benth, a native medicinal plant, has been used to treat diabetes for centuries; however, comprehensive documentation of its bioactive constituents and therapeutic effectiveness is lacking. In this study, we investigated the phytochemical profile and antidiabetic potential of D. elliptica methanolic leaf extract (DEME) in diabetic Sprague Dawley rats induced with streptozotocin (STZ). In normal rats, acute oral toxicity evaluations were conducted, and in STZ-induced rats, antidiabetic properties were investigated. 14 days of oral administration of standard glibenclamide and the extract at 200 and 400 mg/kg body weight to diabetic rodents. Assessed parameters included blood glucose levels, alterations in body weight, biochemical markers, and histological analysis of the pancreas, liver, and kidney. Numerous phytoconstituents were uncovered through qualitative phytochemical assays, 1H NMR, and 1H–13C HSQC screening. Quercetin was identified by 1H NMR characterization, and a ceramide analogue compound was isolated and partially characterized by 1H NMR. There were no indications of toxicity or mortality. The treatment with DEME significantly (p < 0.001) decreased body weight and had a remarkable hypoglycemic effect. Both 200 mg/kg and 400 mg/kg extract concentrations decreased total cholesterol levels significantly (p < 0.01 and p < 0.05, respectively). In addition, glibenclamide and the 400 mg/kg dose of extract increased serum insulin levels substantially (p < 0.05) and decreased total bilirubin, lactic acid dehydrogenase, aspartate aminotransferase, and alanine aminotransferase levels. In addition to glibenclamide, treatment with DEME has exhibited cytoprotective effects and increased insulin secretion, thereby exerting a potent antihyperglycemic effect. These results suggest that D. elliptica may have therapeutic potential for the treatment of diabetes mellitus.
1. Introduction
Diabetes is a major public health concern in Malaysia and the prevalence of type 2 diabetes has escalated to 20.8 % in adults above the age of 30, affecting 2.8 million individuals (Hussein et al., 2015). The National Health and Morbidity survey states 3.9 million people aged 18 years and above suffer from diabetes (Institute for Public Health, National Institutes of Health, 2020). Diabetes mellitus can become more costly when complications of the disease occur and dialysis treatment also contributes to long-term burden and health budgets (Crasto, 2021, Kane et al., 2021, Singh et al., 2022). Despite the availability of clinical therapeutic agents, patients may seek an option for alternative remedies (Sharma et al., 2022), such as natural products, to control these complications and improve glycemic control and glucose intolerance (Furman et al., 2020). Medicinal plants with hypoglycemic effects are used worldwide to treat diabetes with minimal side effects, a challenge for improving diabetes care (Aba & Asuzu, 2018). Studies involving diabetic patients and as well as preclinical studies were conducted in clinical settings and with an experimental animal model of the disease respectively. Nevertheless, it is still unclear how the majority of these plant-based compounds lower blood sugar levels in particular tissues or organs. The majority of the time, the active chemical or chemicals in question are also unknown. Owing to their high price and numerous adverse effects, researchers are looking for novel natural substances that may be utilised or produced into secure, affordable, and efficient anti-DM treatments. It's important to find an effective, natural and safe oral hypoglycemic agent to prevent, treat, and manage diabetes.
Derris elliptica (Wall.) Benth is a leguminous plant from the Fabaceae family (Derris elliptica (Wall.) (1860) and, locally known as akar tuba has been used traditionally by Malay and Iban communities in Sarawak to treat diabetes since a long time ago. It also treats several disorders like weakness, menorrhagia, headache, toothache and rheumatism (Chai, 2006). Ceramides isolated from the same plant family may be relevant in the lipotoxicity that causes diabetes, hepatic steatosis, and heart disease (Boon et al., 2013, Chaurasia et al., 2019, Lu and Liang, 2011, Sokolowska and Blachnio-Zabielska, 2019). The diversity of phytochemicals suggests that D. elliptica could serve as a natural source of traditional medicine for the treatment of various diseases or be able to maintain β-cell performance and decrease glucose levels in the blood (Jacob and Narendhirakannan, 2019, Do et al., 2014, Kooti et al., 2016). Pharmacological studies were carried out to evaluate the DEME on streptozotocin (STZ) diabetic-induced rats including toxicity, biochemical and histopathology with isolation phytochemicals from the extract.
2. Materials and methods
2.1. Chemicals and drugs
All chemicals were purchased from local suppliers (Merck, Fisher, Sigma, Chemsolute, Chem Elisa Kit and Pharmaniaga). The chemicals and reagents were n-Hexane (Hex), methanol (MeOH), chloroform (CHCl3) and ethyl acetate (EA), grade deuterated chloroform (CD3OD), formic acid, standard reference quercetin (Sigma). Streptozotocin was from Sigma Chemicals. Insulin Elisa Kit was obtained from Mercodia Inc., USA.
2.2. Plant material, extraction, and fractionation
The fresh leaves of Derris elliptica (D. elliptica) were collected from Tasek Bera (DD Coordinates 3.8166634 102.416665), Kuantan, Pahang Malaysia. The leaves were identified and authenticated by a botanist from Taman Botani, Putrajaya, Malaysia. A voucher specimen (HTBP 5119) was placed at herbarium Taman Botani Putrajaya, Malaysia. One (1) kg of leaves was washed, dried at 40 °C, and then crushed. The dried powder of D. elliptica was extracted with methanol. The methanol maceration took three days at room temperature with occasional shaking. Filtration and rotary vacuum evaporation removed excess solvent from the extract.
2.2.1. Phytochemical screening, isolation, and characterization
Standard procedures were used to conduct qualitative phytochemical screening utilizing specific reagents to identify the presence groups of compounds such as alkaloids, flavonoids, sugar, phytosterol, and amino acids. Preliminary profiling of the methanolic extract of D. elliptica (DEME) was conducted using 1H NMR in order to identify the main group of compounds in the extract. Proton (1H) nuclear magnetic resonance (NMR) and 1H,13C-HSQC spectra were recorded on a Bruker AVANCE III Ascend 600 spectrometer using a BBO probe with deuterated chloroform (CD3OD) as the solvent and tetramethylsilane (TMS) as an internal reference standard for the screening of extract. Following fractionation, the TLC fingerprint profile was developed using the stationary phase TLC silica gel 60 F254 plates (aluminium sheets, 20 cm × 20 cm, Merck). Different ratios of n-hexane, chloroform, and ethyl acetate were utilized to optimize the mobile phase until the best separation was achieved. For the derivatization procedure, the TLC plates were sprayed with anisaldehyde and ceric sulphate reagent, air-dried at room temperature and heated at 110 °C until clear spots appeared on TLC plate. The p-Anisaldehyde-sulfuric acid reagent was used to derivatize and visualize non-UV-active chemicals on the TLC plate.
Next, the extract was fractionated by silica gel column chromatography packed with silica gel 60 (0.063–0.200 mm, 70–230 mesh ASTM, Merck). The crude extract was loaded on a column and eluted gradually with n-hexane, dichloromethane, and methanol to increase polarity. All fractions were dried in a rotary evaporator at 40 °C and the fractions with the same TLC profile were grouped together. For the isolation, the glass preparative TLC silica gel plate (60G F254 Merck, 20 cm x 20 cm) was developed in a mobile phase containing hexane and ethyl acetate (8:2). Pure compound bands were scraped, rinsed in methanol, and filtered. Pure compounds were characterized using 1H NMR on a Bruker 500 MHz with TMS internal standard and CD3OD as solvent. The raw data were processed with Bruker Software Topspin 2.1 and data was matched to a reference to determine the compound and potential plant functional groups.
2.3. Pharmacological studies
2.3.1. Experimental animals
A total number of 36 healthy Sprague Dawley (SD) male rats (age 9 to 10 weeks) weighing between 200 and 250 g were used in the study. The rats were obtained from, laboratory animal facility and management (LAFAM), F aculty of P harmacy, University Technology MARA and transported as per the animal ethics regulation. The rats were acclimatized for one week (7 days) at the pharmacology laboratory, School of Pharmacy KPJ Healthcare University College (KPJUC), Kota Seriemas, Negeri Sembilan. All experiments adhered to the ethical norms approved by Animal Ethics Committee, KPJUC (Approval no: KPJUC /CRI/LEC/EC/2015/09). They were housed in a polypropylene cage lined by corncob bedding with a change interval every 48 h. Each cage consists of 6 rats and provided standard environmental conditions at an ambient temperature of 25 to 27 °C with 12 h light–dark cycle. The rats were supplied with standard pellet food (702P Gold Coin, Limited, Malaysia) and water ad libitium.
2.3.2. Acute toxicity studies
An acute toxicity study was carried out in accordance with the Organization for Economic Co-Operation and Development guideline (OECD) (Section 4 Test No.423). Six male SD rats were used in the study (OECD, 2002). These standards establish uniform techniques for selecting doses and testing methodologies, ensuring the accuracy and replicability of the experimental outcomes. The animals were divided into two groups consisting of three animals in each group (n = 3). One group served as a control group, treated with vehicle distilled water (per oral, 0.8 ml), while the other group was given a dose of 2000 mg/kg DEME dissolved in distilled of which 2000 mg/kg equivalent dose was present in 0.8 ml. The rats were fasted for twelve hours before the study and weighed before administration with the extract. The acute toxicology dose was calculated in reference to the body weight of the rats. As per OECD guidelines, a single dose of 2000 mg/kg on the first day of 14 days of observation was administered orally (oral gavage, stainless steel, 3.00 mm ball diameter (16 G), Havard / USA) attached to 1 ml or 3 ml syringe) to three male rats in the treatment group, whereas control groups received only distilled water. The animals were observed for 30 min after dosing, then the animals were monitored hourly until 8 h. All the observations were visually compared with the control animals treated with distilled water. After 8 h, the animals are monitored further once daily during the 14 days to observe the changes for any toxicological symptoms including behaviour, neurological and autonomic profiles (OECD, 2002).
2.3.3. Drug treatment and experimental design for antidiabetic activity
The pharmacological evaluation was performed in 30 rats. The animals were divided into 5 groups, with 6 rats in each group (n = 6). The animals were treated with DEME by mouth (per oral (p.o) at a dose of 200 mg/kg and 400 mg/kg for 14 days. The doses for the treatment were selected according to the OECD 423 acute toxicity recommendation, which is verified by the acute toxicity test performed with the DEME. The DEME was assigned as non-toxic as no toxic signs and symptoms were found to be safe, and the median lethal dose (LD50) of 2000 mg/kg and the above is mentioned as unclassified. From these results of LD50 or the maximum tolerated dose (2000 mg/kg), 1/10th and 1/5th of LD 50 were taken as the minimum and maximum tolerated dose (200 mg/kg and 400 mg/kg) for the screening of various pharmacological activities. For the pharmacological study, the animals (n = 30) were fasted overnight (18 h). The animals were weighed and marked. The animals were divided into five groups of six.
Group I: Normal control rats receiving distilled water.
Group II: Negative control (diabetes induced), treated with STZ, injection and receiving distilled water orally.
Group III: Diabetic rats (induced with STZ) and treated with glibenclamide 10 mg/kg.
Group IV: Diabetic rats (induced with STZ) and treated with DEME 200 mg/kg.
Group V: Diabetic rats (induced with STZ) and treated with DEME 400 mg/kg.
The blood glucose level was measured for normal control rats (n = 6) and STZ-induced diabetes rat groups (n = 24). For STZ- induced diabetes group, the rats were administered intraperitoneally with STZ (45 mg/kg body weight). The STZ was dissolved in 0.1 M citrate buffer, pH 4.5) and freshly prepared (Hanalp et al., 2023, Ahmad et al., 2014). After the STZ injection, hyperglycemia was confirmed by measuring blood glucose levels after 72 h. The rats with a fasting plasma glucose ≥ 120 mg/dl were considered as diabetic and were used in this study. Then the drug treatment with DEME was started 72 h after STZ-induced diabetic rats. The treatment design follows as mentioned. The food and water intake were monitored daily. The body weights of each rat were measured and recorded with an animal weighing scale (Kent Scientific Scl-101) at day 1 and day 14 of the treatment. Weekly blood glucose monitoring was performed at day 7 and day 14 days of treatment for all groups. At the end of the 14 days treatment, blood samples were collected from overnight fasted rats by retro-orbital sinus puncture using a capillary tube under diethyl ether anaesthesia. The serum was separated by centrifuging (Centrifuges, Hettich Zentrifugen, EBA 200) the blood samples at 4000 rpm for 15 min and stored at −20 °C before biochemical analysis.
2.3.4. Biochemical analysis
Serum aspartate amino transaminase (AST), Alanine amino transaminase (ALT), Lactate dehydrogenase (LDH), Total cholesterol (TC), Glucose (G) and Total bilirubin (T.Bil) were determined (Al-Attar & Alsalmi, 2019) using a semi-automated biochemical analyzer (BioLis 24i Premium) (Biorex Manheim Diagnostics) at Hematology and Clinical Biochemistry Lab, Faculty of Veterinary Medicine, Universiti Putra Malaysia. Serum insulin level was estimated using Mercodia Rat Insulin ELISA kit (Ghiasi et al., 2019). The serum sample was diluted with 50 µl Calibrator' 0′at ratio 5 µl: 50 µl. The enzyme conjugate was labelled 1X solution was prepared by mixing gently 1000 µl of enzyme conjugate 1X solution and 10 ml of enzyme conjugate buffer and the enzyme conjugate 1X was kept at 2–8 °C. The estimation of insulin in the test sample was determined according to the manufacturer’s direction with a standard calibration curve.
2.3.5. Histopathological study
After collection of the blood sample, rats were euthanized by cervical dislocation. Organs such as the pancreas, kidneys and livers were removed and rapidly fixed in a 10 % buffered formalin solution. The tissues were processed for paraffin sectioning by dehydrating in different concentrations of (70 % to 100 %) ethanol, cleared with xylene and embedded in paraffin wax. Sections cut at 5 µm (Micrometre) thick paraffin were made and stained with haematoxylin and eosin (H & E) staining (Slaoui & Fiette, 2011). Photomicrographs of the stained slides were taken using a light microscope (Olympus Optical) attached to a digital camera (Lumenera, 2.0).
2.3.6. Statistical analysis
Statistical evaluation was done using Graph Pad Prism 7. Analysis of variance (one-way ANOVA) was tested when more than 2 groups were involved. Post hoc comparisons were made using Turkey test and the test of homogeneity of variances. The data were expressed as mean ± Standard Error Mean (SEM). p value < 0.05 are considered statistically significant.
3. Results
3.1. Phytochemicals screening and compound characterization
Prior to commencing the pharmacological activity study, the phytochemical profile of the DEME was investigated. The results revealed that it contains alkaloids, reducing sugar, phytosterol, and flavonoids, but not proteins. Table 1 summarizes the results of the phytochemical qualitative screening of DEME.
Table 1.
Phytochemical qualitative screening of DEME.
| Phytochemical constituents | Chemical test | Observation (colour) | Result |
|---|---|---|---|
| Alkaloids | Mayer’s | Yellowish with cream precipitate | + |
| Reducing sugar | Fehling’s & Benedict’s | Red precipitate | + |
| Phytosterol | Salkowski’s | Reddish-brown | + |
| Flavonoids | Lead Acetate | White precipitate | + |
| Amino acids | Ninhydrin test | Bluish-purple | – |
*Definition: + indicates the presence; – indicates the absence of phytochemicals.
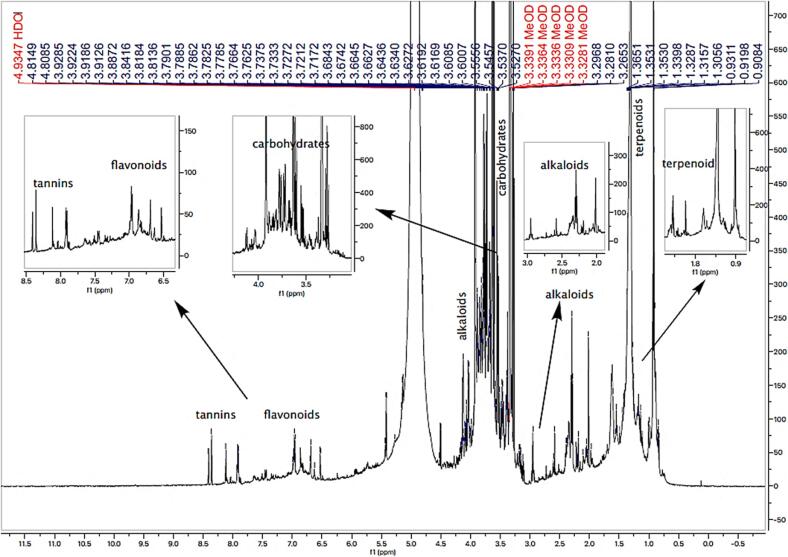
The high-resolution NMR spectrum (600 MHz, CD3OD) of crude extract supported the presence of phytosterols, terpenoids, alkaloids, tannins, carbohydrates, and several polar classes of compounds such as polyhydroxy and polyphenols in the extract (Fig. 1). The 1H NMR spectrum of the methanolic extract revealed characteristic resonances of phytosterols, terpenoids at the upfield region (δ 0.9–1.3) and OH-bearing methine proton resonated at δ 3.7. 1H NMR data of extract showed deshielded protons at δ 7.2–8.4, indicating a trisubstituted flavonoid. 1H–13C-HSQC correlations confirmed the presence of sugar moieties in the extract. 1H NMR spectra discovered polyphenolic contents in the methanolic extract with deshielded proton resonances. The 1H, 13C-HSQC ) technique was used to establish 1H–13C correlations. Correlations of aromatic protons with deshielded carbon atoms and OH-bearing methine proton with methine carbon resonated at δ 72.6 suggesting the presence of phytosterols (Fig. 1).
Fig. 1.
1H NMR spectrum of DEME showing the presence of phytochemicals in the extract.
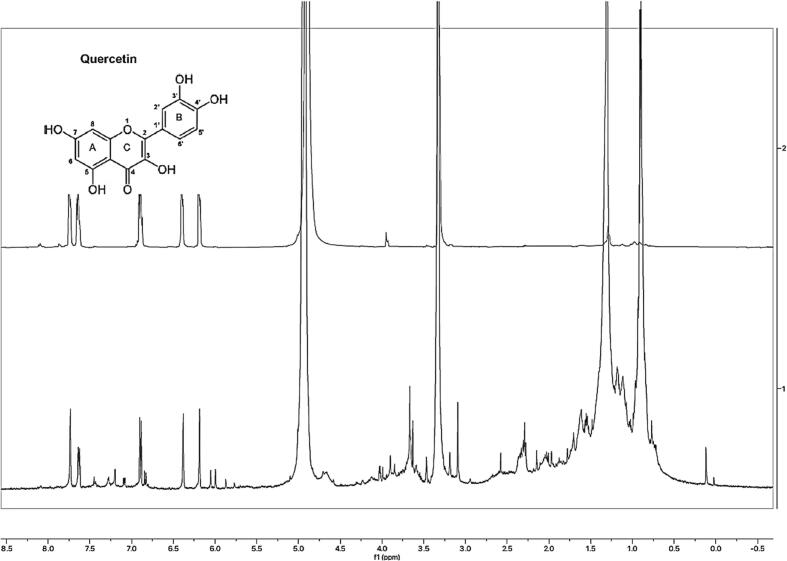
TLC analysis of the DEME gave the best separation with hexane and ethyl acetate (8:2) mobile phases. Some spots turned violet with an Rf of 0.59 when stained with ρ-Anisaldehyde-sulfuric acid, suggesting the extract contains phenolic compounds. Terpene-like compounds were also detected on the TLC profile with blue and greyish bands, while steroidal compounds had a green band. Analysis of the NMR spectra reveals that the major fraction of DEME exhibits signals resembling those of standard quercetin. (Fig. 2). The two aromatic protons on the benzopyrone of quercetin, at C6 and C8, resonated as a doublet at 6.17 ppm and 6.40 ppm, respectively; The C2′ (7.63 ppm) and C6′ (7.75 ppm) protons on the catechol substituent resonated as a doublet (J = 8.3 Hz) and singlet, respectively, whereas the C5′ proton only couples to a single neighbouring proton (C6′) and appeared as a doublet at 6.91 ppm. From the 1H NMR spectral data provided; it has been determined that the extract contains quercetin (Fig. 2).
Fig. 2.
1H NMR characterization quercetin in a major fraction of DEME.
Extensive research indicates that bioflavonoids, with quercetin as an illustrative example, have therapeutic potential for diabetes management. These natural compounds, which are produced by plants via the phenylpropanoid pathway, provide a safer alternative to conventional treatments due to their negligible or non-existent adverse effects. Quercetin's multi-targeted approach, which affects organs such as muscles, pancreas, liver, and small intestine, demonstrates its potential in regulating essential signalling pathways. Furthermore, the ability of quercetin to increase insulin secretion, protect pancreatic beta cells from oxidative stress, and strengthen cellular antioxidant defences demonstrates its capacity to treat not only hyperglycemia but also the macrovascular and microvascular complications of diabetes. Bioflavonoids such as quercetin offer a promising avenue for the development of novel and more holistic approaches to diabetes treatment and prevention as research in this field continues to advance (Dhanya, 2022).
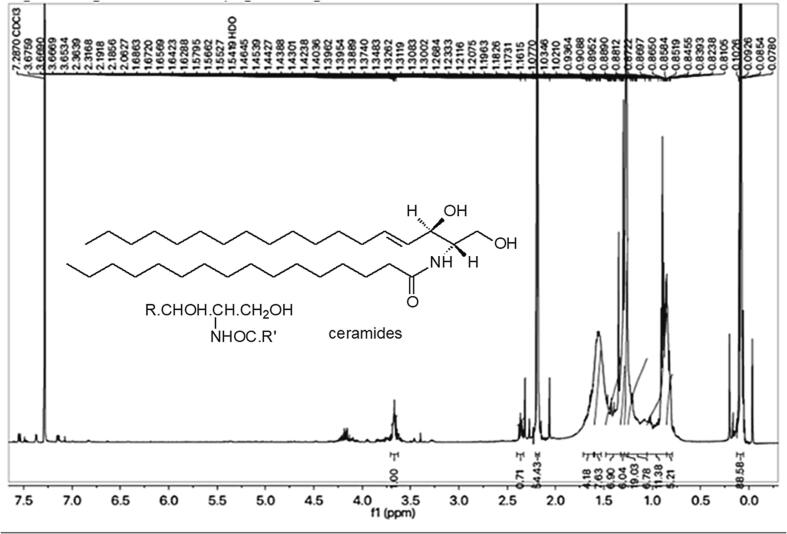
Subsequently, phytoconstituents were isolated through preparative TLC, resulting in a single major band yielding 3.5 mg for the isolate. 1H NMR was utilized for the characterization of this compound. Many methylene peaks in 1H NMR suggest a long aliphatic chain at 0.82 to 2.06 ppm. Multiple signals at 0.84 to 1.311 ppm indicate methyl groups. Broad signals at 3.65 ppm indicated a hydroxyl group, while signals between 4.12 and 4.2 ppm suggested methine protons. A 7.28 ppm doublet signal indicates a secondary amine group (NH). Chemical shifts of major 1H NMR functional groups were compared to published data, and the ceramide analogue structure was postulated (Fig. 3).
Fig. 3.
1H-NMR spectra and ceramide structure.
Recent research has identified a specific class of lipids, namely ceramides, as a significant factor in the onset and persistence of certain diseases. These ceramides, which are a subtype of sphingolipids, play a pivotal role in numerous sphingolipid pathways. It has been observed that elevated ceramide levels correlate with compromised cardiovascular and metabolic health. Moreover, it appears that the interaction between ceramides and adipokines, particularly adiponectin and leptin, contributes to the underlying mechanisms of these conditions. It appears that adiponectin mitigates the negative effects of elevated ceramide levels by activating the ceramidase function of its receptors. On the other hand, increased ceramides appear to exacerbate leptin resistance, a crucial pathophysiological phenomenon in obesity and metabolic syndrome (Field et al., 2020, Sokolowska and Blachnio-Zabielska, 2019).
3.2. Pharmacological studies
3.2.1. Acute toxicity study
The DEME was considered as non-toxic, as it does not exhibit any toxic signs or symptoms and no mortality was observed at the dose of 2000 mg/kg (oral) in mice. According to OECD- 423 guidelines, the LD50 of 2000 mg/kg and above is mentioned as unclassified and non-toxic.
3.2.2. Effect of DEME on body weight in STZ-induced diabetic rats
During the experiments, all rats' body weight, blood glucose, and insulin levels were monitored weekly. On day 14, normal, negative diabetics and treated diabetics had different body weights. The negative diabetic control group lost 60.9 g, while glibenclamide 10 mg/kg, extract 200 mg/kg, and extract 400 mg/kg gained 82.7 g, 63.6 g, and 35.1 g, respectively. The negative diabetic control group had a mean body weight of 151.3 ± 8.2 mg, while the normal group had 258 8.0 mg. Mean body weight was 297 ± 13.6 mg for glibenclamide, 274 ± 25.4 mg for 200 mg/kg, and 249.3 19.03 mg for 400 mg/kg. The negative diabetic control group lost weight (p < 0.001) compared to the normal group. DEME and glibenclamide-treated diabetic rats gained weight (p < 0.001) compared to the control group. The results of body weight difference are mentioned in Table 2.
Table 2.
Effect of DEME on Body Weight Level of STZ-induced rats.
| Groups |
Body Weight (g) in days |
||
|---|---|---|---|
| Day 1 | Day 14 | Change in Body weight | |
| Normal Control | 209.6 ± 2.08 | 258 ± 8.0 | 48.4 |
| Negative Control | 212.2 ± 1.9 | 151.3 ± 8.2a | −60.9 |
| Standard (Glibenclamide) | 215 ± 3.03 | 297.7 ± 13.6b | 82.7 |
| DEME 200 mg/kg | 210.4 ± 4.07 | 274 ± 25.4b | 63.6 |
| DEME 400 mg/kg | 214.2 + 4.15 | 249.3 ± 19.03b | 35.1 |
Values are mean ± S.E.M of six (6) animals. Superscript represents the significance by ANOVA, followed with Tukey’s multiple comparison test. a P < 0.001, is the significant induction of diabetes in the negative diabetic control group when compared to the normal control animal group. b P < 0.001, is the significant improvement from the diabetic conditions on comparison of negative control (STZ injected) animals with the treated animal groups such as standard drug, DEME 200 and 400 mg/kg, respectively.
3.2.3. Effect of DEME on biochemical parameters in STZ-induced diabetic rats
The weekly monitoring of blood glucose is regulated after treatment. In the negative control group, glucose levels were significantly higher (p < 0.001) after day 7 but decreased to 459.7 ± 38.95 mg/dl (p < 0.001) after day 14 when compared to the 7th-day level. The STZ-treated group had higher fasting blood glucose. After 14 days of treatment, the rat’s glucose levels were significantly controlled (Table 3). Diabetic control group concentrations were significantly higher (p < 0.001) than normal. Glucose levels were lower in glibenclamide-treated diabetic animals (p < 0.001). Total glucose in glibenclamide-treated animals reached near normal levels, while 200 mg/kg and 400 mg/kg of extracts reduced glucose and controlled diabetic conditions. After 14 days, diabetic animals administrated with 200 mg/kg and 400 mg/kg extract had better blood glucose control (p < 0.001). Table 4 represents the biochemical parameters. Negative diabetic control group insulin levels were significantly lower (p < 0.05) than normal. Diabetic rats treated with 10 mg/kg glibenclamide and 400 mg/kg extract had higher (p < 0.05) serum insulin levels. 200 mg/kg reduced insulin, but the diabetic control group did not affect it. Negative diabetic control group ALT levels were significantly higher (p < 0.001). Standard drugs and extracts (200 mg/kg and 400 mg/kg) decreased (p < 0.05) AST levels and had a protective effect in STZ-induced rats. Serum triglyceride was elevated (p < 0.001) in STZ-induced rats and was controlled by 200 mg/kg (p < 0.05) and 400 mg/kg (p < 0.001). 200 mg/kg extract lowered total cholesterol significantly (p < 0.01), while 400 mg/kg had the least effect (p < 0.05). Total bilirubin and LDH decreased at 200 mg/kg (p < 0.01). 400 mg/kg demonstrates a mild reduction (Table 4).
Table 3.
Effect of the DEME on Blood Glucose Level in STZ-Induced Diabetic Rats Weekly.
| Groups |
Blood glucose levels (mg/dl) |
||
|---|---|---|---|
| Day 1 | Day 7 | Day 14 | |
| Normal Control | 81.5 ± 3.93 | 83.5 ± 4.33 | 83 ± 3.74 |
| Negative Control | 376.3 ± 50.34 | 613 ± 89.23a | 459.7 ± 38.95a |
| Standard (Glibenclamide) | 293 ± 39.04 | 212.2 ± 28.22b | 163 ± 31.9b |
| DEME 200 mg/kg | 243.5 ± 26.15 | 151.3 ± 20.55b | 130.6 ± 14.84b |
| DEME 400 mg/kg | 275.7 ± 14.63 | 179.7 ± 26.32b | 147.0 ± 25.65b |
Values are mean ± S.E.M of six animals. Superscript represents the significance by ANOVA, followed by Tukey’s multiple comparison test. a P < 0.001, is the significant induction of diabetes in the negative diabetic control group when compared to the normal control animal group. b P < 0.001, is the significant improvement from the diabetic conditions on comparison of negative control (STZ injected) animals with the treated animal groups such as standard drug, DEME 200 and 400 mg/kg, respectively.
Table 4.
Effect of the DEME on biochemical parameters in STZ Induced Rats.
| Biochemical Markers (Unit) |
Normal Control |
Negative Control |
Standard drug (Glibenclamide) |
DEME 200mg/kg |
DEME 400mg/kg |
|---|---|---|---|---|---|
| AST (U/L) | 110.3 ± 2.7 | 261.2 ± 47.2a | 124.7 ± 11.97g | 150.7 ± 9.5h | 132.5 ± 38g |
| ALT (U/L) | 50.17 ± 3.4 | 239 ± 44a | 66.83 ± 6.3f | 76 ± 6.2f | 61.17 ± 6.9f |
| Glucose (mmol/L) | 6.37 ± 0.85 | 44.02 ± 5.19a | 20.37 ±4.18g | 24.8 ± 5.13h | 16.83 ± 3.14f |
| LDH (U/L) | 1334 ± 132 | 2222 ± 116.5b | 1357 ± 139.7g | 1435 ± 18h | 1441 ± 223h |
| TC (mm /L) | 66.28 ± 2.7 | 40.36 ± 27b | 59.7 ± 4.4h | 62.42 ± 4.04g | 59.20 ± 6.12h |
| T. Bil (µmol/L) | 0.587 ± 0.09 | 2.31 ± 0.34b | 0.9± 0.25g | 1.03 ± 0.27g | 0.983 ± 0.33h |
| Insulin µg/L | 2.71 ± 0.01 | 2.44 ± 0.001c | 3. 55 0.016h | 2.45 ± 0.004 | 3.53± 0.02h |
Values are mean ± S.E.M of six animals. Superscript represents the significant by ANOVA, followed with Tukey’s multiple comparison test, aP<0.001, bP<0.01, cP<0.05, is the significant induction of diabetes in the negative diabetic control group when compared to the normal control animal group. fP<0.001, gP<0.01, hP<0.05, is the significant improvement from the diabetic conditions on comparison of negative control (STZ injected) animals with the treated animal groups such as standard drug, DEME 200 and 400 mg/kg respectively.
3.2.4. Histopathological study
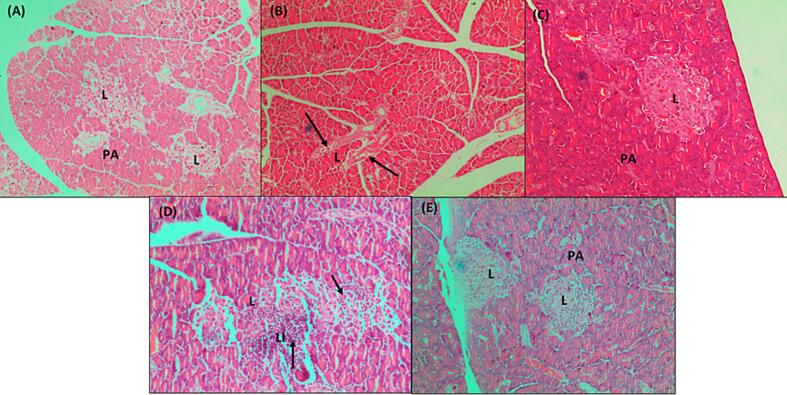
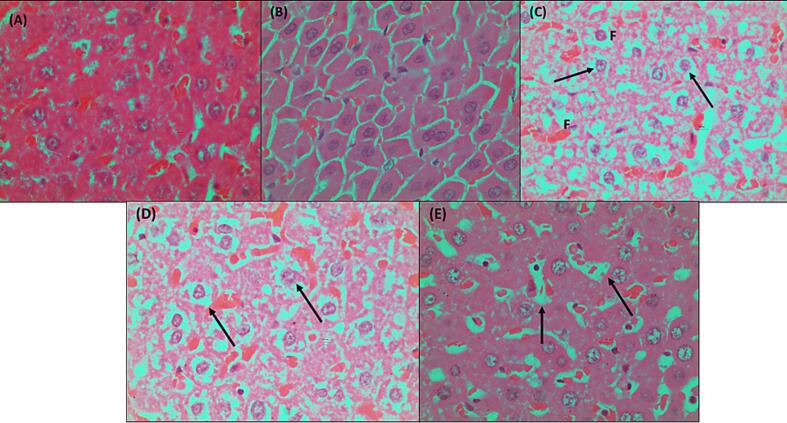
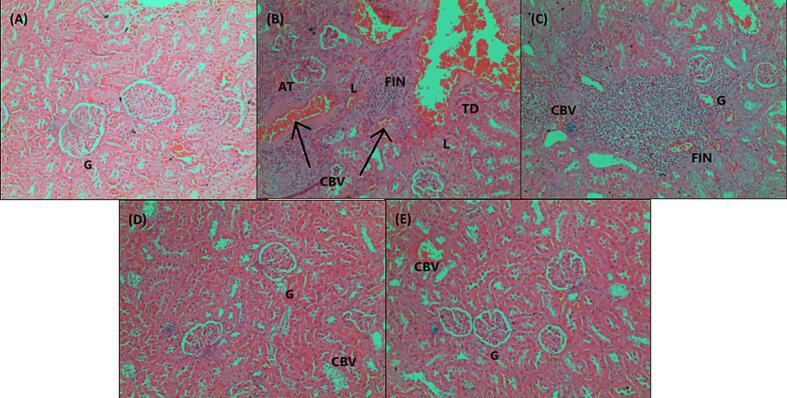
The histological investigation was conducted to assess the effect of extract (200 mg/kg and 400 mg/kg) on kidney, liver, and pancreatic histopathological changes in normal, diabetes negative control, and glibenclamide. The morphology of organs from the treated animals exhibits a protective effect and is found to reduce cell degeneration. The pancreas in the normal group was found to be relatively normal. Pancreatic acini (PA) with normal structure due to lack of complications from medications or chemically induced metabolic variables. A small number of blood vessels in the pancreatic group negative control (B) showed signs of congestion or necrotizing pancreatitis. Due to a decrease in venous channel flow caused by tissue debris circulation, proenzymes may have been activated and the blood became clogged. The connective tissue capsule exhibits deformity and enlargement. Islet of Langerhans contains a small number of cells displaying pyknotic nuclei (PN), which denotes both dead and regenerated cells in the standard drug group (Daonil). The pancreas was largely normal, however there were one or two Langerhans islets where a small number of cells had pyknotic nuclei, which is a sign of dead cells. Glibenclamide (10 mg/kg)-treated diabetic mice's islets of Langerhans display a return of the normal cellular population. A portion of the treated diabetic DEME 200 & 400 mg pancreas was found to be somewhat normal in the Isle of Langerhans, with few pyknotic cells. Normal animal liver sections demonstrated that the main blood arteries are not clogged. The liver's lobes exhibit a regular cubical form. The liver of a diabetic animal under negative control has modest congestion, with thrombi in the central veins and a small number of main blood arteries. Hydropic degeneration was observed in the hepatocytes around a portion of the thrombosed central vein. This degenerative change was observed in specific locations. The diabetic group that received treatment, both from the conventional and DEME animal groups, displayed normal blood vessels and restored hepatocytes. In the negative control plate, the kidneys displayed significantly clogged cortical blood arteries, some of which had thrombus. Severe congestion was present in certain locations at the cortico-medullary junction. Additionally, a small area of mild interstitial nephritis was present, containing a combination of lymphocytes and neutrophils. Severe blood vessel congestion was observed at the cortico-medullary junction in somewhat congested glomeruli. The medulla and cortex of the therapy group showed normal interstitial cells and no pathological alterations. The photomicrographs are shown in Fig. 4, Fig. 5, Fig. 6.
Fig. 4.
Photomicrographs of Pancreatic section
Fig. 5.
Photomicrographs of Liver sections
Fig. 6.
Photomicrographs of Kidney sections labelled
4. Discussion
Diabetes is considered a metabolic syndrome condition which triggers cardiovascular disease followed by neuropathy and nephropathy (Einarson et al., 2018). Treatments for diabetes and other complex metabolic diseases were the key objectives of traditional plant medicines (D. Singh & Singh, 2021). The DEME showed promise in controlling glycemia and biochemical regulation in STZ-induced diabetic rats. The DEME passed acute toxicity tests. STZ is used to induce diabetes in animal models and to study -cell dysfunction (Wu & Yan, 2015). In the STZ-induced diabetes model, many herbal formulations and orient drugs showed promising antidiabetic effect (Venkatachalam et al., 2021). STZ-induced pancreatic beta cell toxicity and diabetes activate poly ADP-ribosylation and alkylate DNA. Poly ADP-ribosylation depletes NAD + and ATP (Eleazu et al., 2013). Oxidative stress in diabetes involves glucose auto-oxidation, protein glycation, the polyol pathway, and lipid imbalance (Ether and Saif-Ur-Rahman, 2021, Juchli et al., 2021). During these processes, cytokine stimulation produces ROS that damage -cell diabetic rats (Zhang et al., 2016).
In this study, the extract controlled diabetic conditions by improving the level of insulin and liver enzymes. DEME has the potential for antidiabetic action in STZ-induced diabetic rats and the effect was found to be more similar to the reference drug glibenclamide. These findings support the claim that the DEME showed antidiabetic activities by improving the body weight in diabetic rats. The extract at a higher dose (400 mg/kg) was found to be more effective than a lower dose (200 mg/kg) after 14 days of treatment. As a result, it has been discovered that high-dose extract is more efficient and has a similar therapeutic effect to standard glibenclamide (10 mg/kg). The antidiabetic effect may be due to the increased insulin release from the existing β-cells of the pancreas. The rise in plasma insulin levels that have been observed indicates that the extract stimulates insulin secretion. It is also effective in increasing body weight and reducing the blood glucose level in the chemically induced diabetic in small animal models. DEME exhibited the improvement in insulin, AST, ALT, TP, TG, LDH, T Bil and TC. The loss of body weight and high blood glucose levels are the most prominent symptoms observed in diabetes patients. The current study demonstrated that the administration of DEME exhibited an antihyperglycemic effect and balanced body weight in STZ-induced diabetic rats. Many previous studies also claimed that the administration of the natural product could potentially reduce diabetic effects in rats (Bakac et al., 2023). Insulin deficiency contributes to derangements of lipid metabolism in DM. Dyslipidemia found in diabetic rats is characterized by elevated levels of TC. These changes in the lipid profile can represent a risk factor for cardiovascular disease (Wu et al., 2020). It is well established that lipoprotein lipase (LPL) activity plays a central role in serum triglyceride-rich lipoprotein particles and HDL levels. On the other hand, in the diabetic state, this enzyme is poorly activated due to insulin deficiency. As a result, the lack of LPL activity led to hypertriglyceridemia. In addition, this lack of insulin may account for dyslipidemia, as insulin inhibits hydroximethylglutaryl coenzyme A (HMG-CoA) reductase, a rate-limiting enzyme for the metabolism of cholesterol-rich LDL particles. Furthermore, insulin decreases VLDL formation by regulating the fatty acid levels in plasma and suppresses the VLDL1 production in the liver, independent of the availability of fatty acids (Singh et al., 2020). The transmembrane proteins responsible for transporting glucose are called glucose transporters (GLUT). Their activities are either glucose- or insulin-dependent, glucose-sensory, or sodium-dependent. One of the most significant isoforms found in skeletal muscles and adipocytes is glucose transporter-4 (GLUT-4), which has 12 membrane-spanning regions with intracellular amino and carboxyl termini, and which enables the entry of glucose to hexose-utilizing cells. GLUT-4 is sufficiently expressed in the cell, however insufficient GLUT-4 recruitment to the plasma membrane is linked to insulin resistance. Angiogenesis, inflammation, glucose metabolism, and adipogenesis are all impacted by PPAR-γ. Glitazone-class antidiabetic medicines and fibrate-class antidyslipidemic medications are examples of ligands that act on PPAR-γ and PPAR-γ, respectively, which in turn enhances glucose oxidation, lowers free fatty acid levels, and improves insulin resistance in type 2 diabetes mellitus. The extract from DEME may have lipid-lowering effects because it has insulin-like action, which enhances insulin sensitivity in people with diabetes and contributes to the effects. Moreover, the administration of STZ induced hepatic damage, which was associated with increased plasmatic levels of AST and ALT. These increased levels were caused by oxidative dysfunction associated with an imbalance in insulin secretion. The hepatic dysfunction in diabetic conditions as indicated by elevated levels of AST, ALT, TP, Glucose, LDH, and total bilirubin (Kotb El-Sayed et al., 2020). The extract-based intervention was able to bring the increased liver enzyme levels back to normal. The results of the histological studies showed that normal pancreatic, liver, and kidney functions are among its antidiabetic mechanisms, along with regeneration and restoration. This might be due to its ability to raise insulin secretion in STZ-induced diabetic rats, which has an anti-hyperglycaemic effect with a high dose of extract 400 mg/kg. The ceramide analogue compound isolated from the plant extract, which has been previously reported in terms of its pharmacological significance, could be important for managing the antidiabetic effect.
5. Conclusion
In conclusion, this research reveals the therapeutic benefits of Derris elliptica and its potential effects as anti-diabetic. Phytochemical analyses identified quercetin and a ceramide analogue compound, and the extract positively attenuated the lipid profiles, decreased total cholesterol levels, increased insulin secretion, and lowered levels of metabolic indicators, suggesting a potential role in protecting pancreatic function and enhancing metabolic health. The potential cytoprotective properties and the ability to increase insulin secretion delineate the strong antihyperglycemic potential effect. While these findings suggest that DEME possess a promising approach for managing diabetes, further research in molecular aspects of mitogen-activated protein kinase (MAPK) associated with the nuclear factor erythroid 2- related factor/heme oxygenase-1 (Nrf2/HO-1) for insulin transduction, as well as clinical trials, will strengthen its pivotal role as an antidiabetic agent.
CRediT authorship contribution statement
Rassheda Abd Rahman: Conceptualization, Methodology, Investigation, Project administration, Writing – original draft, Reviewing. Hanish Singh Jayasingh Chellammal: Conceptualization, Methodology, Investigation, Project administration, Writing – original draft, Reviewing, Experimental studies and review the draft paper. Rozaini Mohd Zohdi: Experimental evaluations and pharmacology works. Syed Adnan Ali Shah: Acquisition and interpretation of 1H NMR data. Dhani Ramachandran: Histopathological evaluations and interpretations. The section is amended in the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Acknowledgements
The authors acknowledge the Faculty of Pharmacy and Research and Innovation Department of Universiti Teknologi MARA (UiTM), Malaysia, an impact publication funding schemean impact publication funding scheme, for funding the research through impact publication funding scheme and well as research facility support. The authors also thank the School of Pharmacy, KPJ Healthcare University, Malaysia for their support in research facilities.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2024.102016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aba P.E., Asuzu I.U. Mechanisms of actions of some bioactive anti-diabetic principles from phytochemicals of medicinal plants: A review. Indian J. Nat. Prod. Resour. 2018 [Google Scholar]
- Ahmad W., Khan I., Khan M.A., Ahmad M., Subhan F., Karim N. Evaluation of antidiabetic and antihyperlipidemic activity of Artemisia indica linn (aerial parts) in Streptozotocin induced diabetic rats. J. Ethnopharmacol. 2014;151(1):618–623. doi: 10.1016/j.jep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Al-Attar A.M., Alsalmi F.A. Influence of olive leaves extract on hepatorenal injury in streptozotocin diabetic rats. Saudi J. Biol. Sci. 2019;26(7):1865–1874. doi: 10.1016/j.sjbs.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaç M.S., Dogan A., Yılmaz M.A., et al. Ameliorative effects of Scutellaria Pinnatifida subsp. pichleri (Stapf) Rech.f. Extract in streptozotocin-induced diabetic rats: chemical composition, biochemical and histopathological evaluation. BMC Complement. Med. Ther. 2023;23:410. doi: 10.1186/s12906-023-04252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon J., Hoy A.J., Stark R., Brown R.D., Meex R.C., Henstridge D.C., Schenk S., Meikle P.J., Horowitz J.F., Kingwell B.A., Bruce C.R., Watt M.J. Ceramides Contained in LDL Are Elevated in Type 2 Diabetes and Promote Inflammation and Skeletal Muscle Insulin Resistance. Diabetes. 2013;62(2):401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, P. (2006). Medicinal Plants of Sarawak. Publisher, Paul Chai P.K., 2006. ISBN, 9834325517, 9789834325510. Pages; 212.
- Chaurasia B., Tippetts T.S., Mayoral Monibas R., Liu J., Li Y., Wang L., Wilkerson J.L., Sweeney C.R., Pereira R.F., Sumida D.H., Maschek J.A., Cox J.E., Kaddai V., Lancaster G.I., Siddique M.M., Poss A., Pearson M., Satapati S., Zhou H., Summers S.A. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386–392. doi: 10.1126/science.aav3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasto, W. (2021). P re v e n t i o n o f M i c ro v a s c u l a r Complications of Diabetes. 50, 431–455.
- Derris elliptica (Wall.) Benth. J. Proc. Linn. Soc., Bot. 4 (Suppl.): 111 (1860), https://www.worldfloraonline.org/taxon/wfo-0000186905.
- Dhanya R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022;146(December 2021) doi: 10.1016/j.biopha.2021.112560. [DOI] [PubMed] [Google Scholar]
- Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17(1):1–19. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleazu C.O., Eleazu K.C., Chukwuma S., Essien U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013;12(1):60. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ether S., Saif-Ur-Rahman K.M. A systematic rapid review on quality of care among non-communicable diseases (NCDs) service delivery in South Asia. Public Health in Practice. 2021;2(July) doi: 10.1016/j.puhip.2021.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field B.C., Gordillo R., Scherer P.E. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front. Endocrinol. 2020;11(October):1–14. doi: 10.3389/fendo.2020.569250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman B.L., Candasamy M., Bhattamisra S.K., Veettil S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2020;247(May 2019) doi: 10.1016/j.jep.2019.112264. [DOI] [PubMed] [Google Scholar]
- Ghiasi S.M., Dahlby T., Andersen C.H., Haataja L., Petersen S., Omar-Hmeadi M., Yang M., Pihl C., Bresson S.E., Khilji M.S., Klindt K., Cheta O., Perone M.J., Tyrberg B., Prats C., Barg S., Tengholm A., Arvan P., Mandrup-Poulsen T., Marzec M.T. Endoplasmic reticulum chaperone glucose-regulated protein 94 is essential for proinsulin handling. Diabetes. 2019;68(4):747–760. doi: 10.2337/db18-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanalp H.C., Dogan A., Saygi T.K., Donmez F., Battal A. Exploring phytochemical constituents of Achillea arabica Kotschy. ethanolic flower extract by LC-MS/MS and its possible antioxidant and antidiabetic effects in diabetic rats. Zeitschrift Für Naturforschung C. 2023;78(5–6):189–199. doi: 10.1515/znc-2022-0082. [DOI] [PubMed] [Google Scholar]
- Hussein Z., Taher S.W., Gilcharan Singh H.K., Chee Siew Swee W. Diabetes Care in Malaysia: Problems, New Models, and Solutions. Ann. Glob. Health. 2015;81(6):851–862. doi: 10.1016/j.aogh.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Institute for Public Health, National Institutes of Health, M. of H. M. (2020). Key findings. In Non-communicable diseases, healthcare demand, and health literacy: Key Findings; National Health and Morbidity Survey (NHMS) Malaysia (p. 11). https://doi.org/10.18356/be4d1601-en.
- Jacob B., Narendhirakannan R.T. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2019;9(1):4. doi: 10.1007/s13205-018-1528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juchli F., Zangger M., Schueck A., von Wolff M., Stute P. Chronic Non-Communicable Disease Risk Calculators – An Overview, Part II. Maturitas. 2021;143(September 2020):132–144. doi: 10.1016/j.maturitas.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Kane J.P., Pullinger C.R., Goldfine I.D., Malloy M.J. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr. Opin. Pharmacol. 2021;61:21–27. doi: 10.1016/j.coph.2021.08.013. [DOI] [PubMed] [Google Scholar]
- Kooti W., Farokhipour M., Asadzadeh Z., Ashtary-Larky D., Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron. Physician. 2016;8(1):1832–1842. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb El-Sayed M.I., Al-Massarani S., El Gamal A., El-Shaibany A., Al-Mahbashi H.M. Mechanism of antidiabetic effects of Plicosepalus Acaciae flower in streptozotocin-induced type 2 diabetic rats, as complementary and alternative therapy. BMC Complem. Med. Therap. 2020;20(1):1–15. doi: 10.1186/s12906-020-03087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.-Y., Liang J.-Y. A Novel Ceramide from the Roots of Derris elliptica. Chin. J. Nat. Med. 2011 doi: 10.3724/SP.J.1009.2011.00094. [DOI] [Google Scholar]
- OECD. (2002). Test No. 423: Acute Oral Toxicity - Fixed Dose Procedure. In OECD Guideline for Testing of Chemicals (Issue December). OECD. https://doi.org/10.1787/9789264070943-en.
- Sharma P., Hajam Y.A., Kumar R., Rai S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomed. Plus. 2022;2(1) doi: 10.1016/j.phyplu.2021.100188. [DOI] [Google Scholar]
- Singh J., Sehrawat A., Mishra J., Singh I., Reddy P.H. Free Radical Biology and Medicine Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184(1):114–134. doi: 10.1016/j.freeradbiomed.2022.03.019. [DOI] [PubMed] [Google Scholar]
- Singh H., Sharma A.K., Gupta M., Singh A.P., Kaur G. Tinospora cordifolia attenuates high fat diet-induced obesity and associated hepatic and renal dysfunctions in rats. Pharma Nutrition. 2020;13(March) doi: 10.1016/j.phanu.2020.100189. [DOI] [Google Scholar]
- Singh D., Singh S. Phytomedicine: Alternative safe vehicles on the pathway of diabetes mellitus. Ann. Phytomed. Int. J. 2021;10(1):114–122. doi: 10.21276/ap.2021.10.1.12. [DOI] [Google Scholar]
- Slaoui, M., & Fiette, L. (2011). Histopathology procedures: from tissue sampling to histopathological evaluation. Methods in Molecular Biology (Clifton, N.J.), 691(January), 69–82. https://doi.org/10.1007/978-1-60761-849-2_4. [DOI] [PubMed]
- Sokolowska E., Blachnio-Zabielska A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019 doi: 10.3389/fendo.2019.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam, T., Chitra, M., Kalaiselvi, P., Chitra, A., Sumathi, K., Babu, C. M. S., Senthilkumar, N., & Sattanathan, K. (2021). Phytochemical screening and antidiabetic potentiality of Pavetta indica L. (Angiosperms: Rubiaceae) methanol extract on streptozotocin induced diabetic mice. Ann. Phytomed. Int. J. 10 (2), 292–297. https://doi.org/10.21276/ap.2021.10.2.39.
- Wu, J., & Yan, L. J. (2015). Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. In Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. https://doi.org/10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed]
- Wu W.-C., Wei J.-N., Chen S.-C., Fan K.-C., Lin C.-H., Yang C.-Y., Lin M.-S., Shih S.-R., Hua C.-H., Hsein Y.-C., Chuang L.-M., Li H.-Y. Progression of insulin resistance: A link between risk factors and the incidence of diabetes. Diabetes Res. Clin. Pract. 2020;161 doi: 10.1016/j.diabres.2020.108050. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016;2016:1–18. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.