Abstract
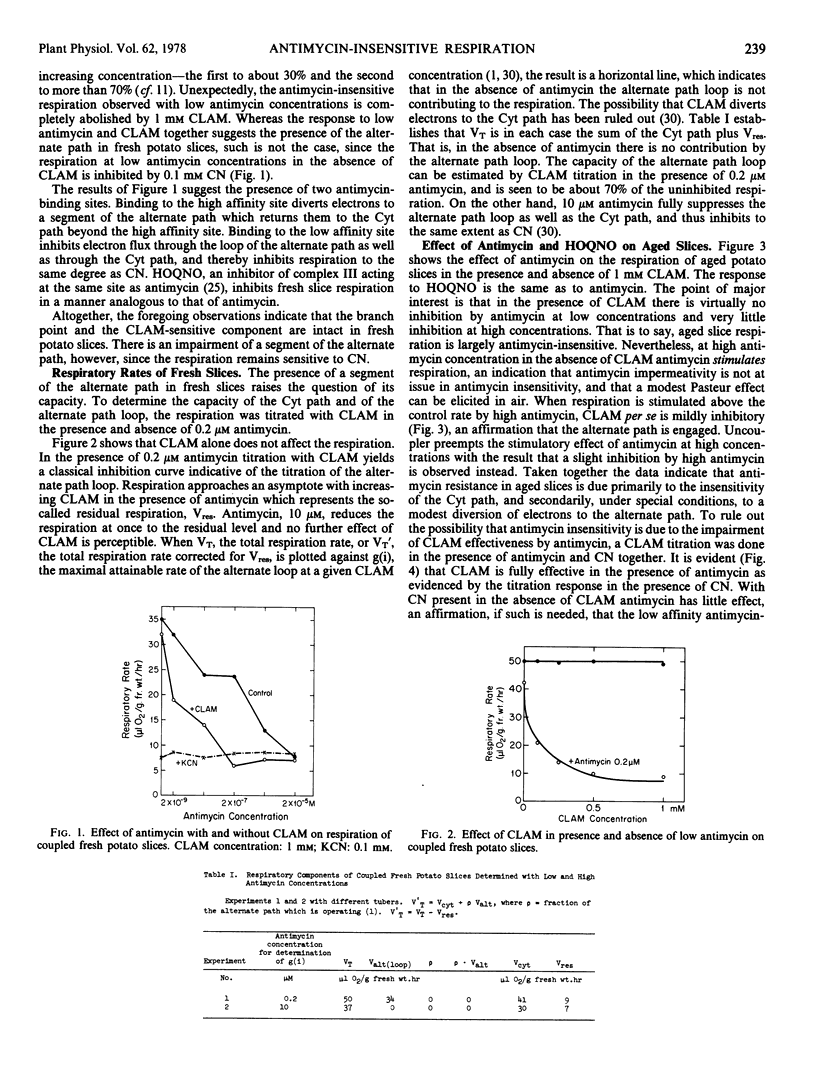
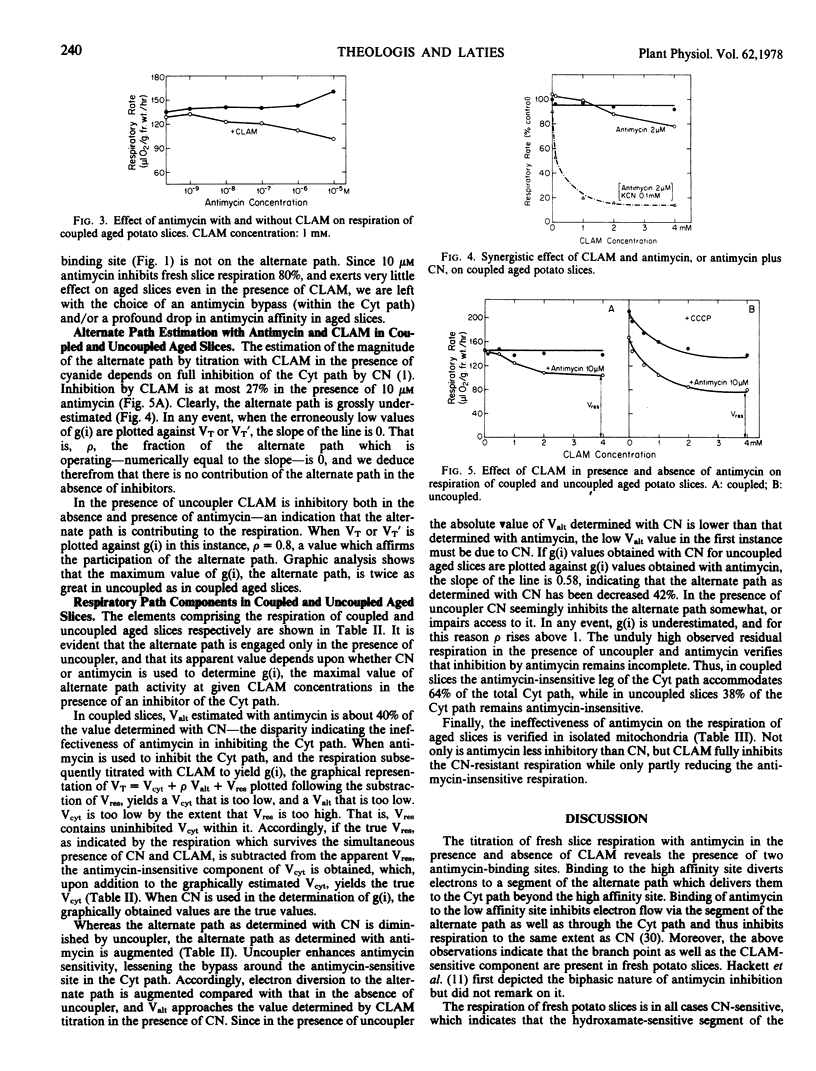
The effect of antimycin A on the respiration of fresh potato (Solanum tuberosum var. Russet Burbank) slices has been determined in the presence and absence of m-chlorobenzhydroxamic acid (CLAM). Two antimycin-binding sites are indicated. At low concentrations antimycin alone inhibits respiration only slightly. When CLAM and low antimycin are added together, respiration is sharply inhibited, as in response to cyanide. High antimycin alone is as inhibitory as cyanide. The branch point to the alternate path is intact in fresh slices, as is the hydroxamate-sensitive component. The full alternate path is inoperative, however, as indicated by the sensitivity to cyanide. The data suggest an alternate path loop which bypasses the high affinity antimycin site and returns electrons to the cytochrome path. Antimycin at high concentrations prevents articulation of the loop with the cytochrome path.
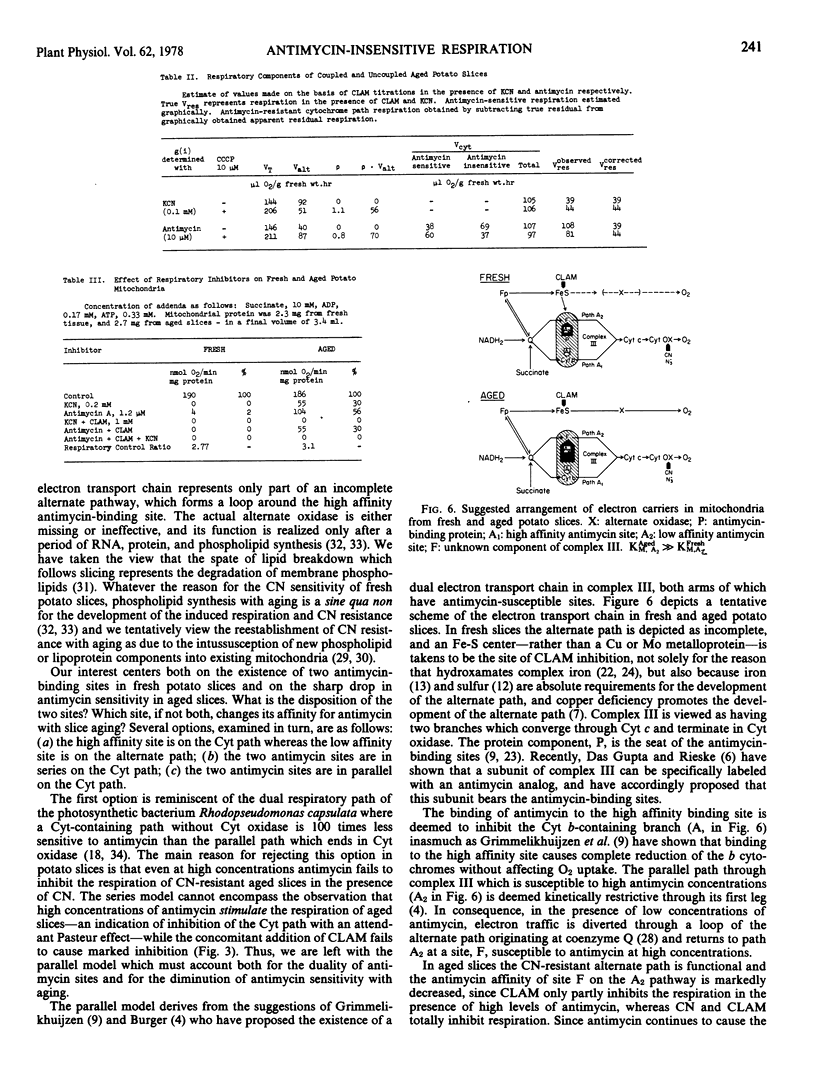
The respiration of aged slices is not only markedly resistant to antimycin at high concentrations, but quite insensitive to CLAM in the presence of antimycin. A model is proposed which involves parallel paths within complex III of the cytochrome path, with one path bearing the high affinity, and the other the low affinity antimycin site. With slice aging the antimycin affinity of the latter site is even further reduced, providing a relatively antimycin-insensitive bypass to both the high affinity antimycin-sensitive cytochrome path, and the CLAM-sensitive alternate path. The alternate path loop in fresh slices is presumed to feed into the low affinity antimycin-sensitive arm of the cytochrome path.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Bonner W. D., Jr, Slater E. C. Effect of antimycin of the potato mitochondrial cytochrome b system. Biochim Biophys Acta. 1970 Dec 8;223(2):349–353. doi: 10.1016/0005-2728(70)90191-x. [DOI] [PubMed] [Google Scholar]
- Burger G., Lang B., Bandlow W., Kaudewitz F. Studies on the mechanism of electron transport in the bc1-segment of the respiratory chain in yeast. II. The binding of antimycin to mitochondrial particles and the function of two different binding sites. Biochim Biophys Acta. 1975 Aug 11;396(2):187–201. doi: 10.1016/0005-2728(75)90033-x. [DOI] [PubMed] [Google Scholar]
- Butow R. A., Zeydel M. The isolation of an antimycin-resistant mutant of orulopsis utilis. J Biol Chem. 1968 May 25;243(10):2545–2549. [PubMed] [Google Scholar]
- Das Gupta U., Rieske J. S. Identification of a protein component of the antimycin-binding site of the respiratory chain by photoaffinity labeling. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1247–1254. doi: 10.1016/0006-291x(73)91121-2. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Garland P. B. An antimycin A- and cyanide-resistant variant of Candida utilis arising during copper-limited growth. Biochem J. 1973 Aug;134(4):1051–1061. doi: 10.1042/bj1341051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. Observations on the antimycin A inhibition of biological oxidations. I. Stoichiometry and pH effects. Biochim Biophys Acta. 1962 Jul 2;60:236–248. doi: 10.1016/0006-3002(62)90399-2. [DOI] [PubMed] [Google Scholar]
- Grimmelikhuijzen C. J., Marres C. A., Slater E. C. Antimycin-insensitive mutants of Candida utilis II. The effects of antimycin on Cytochrome b. Biochim Biophys Acta. 1975 Mar 20;376(3):533–548. doi: 10.1016/0005-2728(75)90173-5. [DOI] [PubMed] [Google Scholar]
- Grimmelikhuijzen C. J., Slater E. C. Antimycin-insensitive mutants of Candida utilis. I. Isolation and characterization of mutant 28. Biochim Biophys Acta. 1973 Apr 27;305(1):67–79. doi: 10.1016/0005-2728(73)90232-6. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W., Griffiths S. K., Niederpruem D. J. Studies on Development of Cyanide-resistant Respiration in Potato Tuber Slices. Plant Physiol. 1960 Jan;35(1):8–19. doi: 10.1104/pp.35.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Bonner W. D., Jr, Nyns E. J. Involvement of iron in the biogenesis of the cyanide-insensitive respiration in the yeast Saccharomycopsis lipolytica. Biochim Biophys Acta. 1977 Apr 11;460(1):94–100. doi: 10.1016/0005-2728(77)90155-4. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. The interaction of the radicals of ubiquinone in mitochondrial electron transport. FEBS Lett. 1976 Jun 15;65(3):278–280. doi: 10.1016/0014-5793(76)80128-7. [DOI] [PubMed] [Google Scholar]
- La Monica R. F., Marrs B. L. The branched respiratory system of photosynthetically grown Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976 Mar 12;423(3):431–439. doi: 10.1016/0005-2728(76)90198-5. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B., Burger G., Bandlow W. Activity of reduced ubiquinone: cytochrome c oxidoreductase with various ubiquinol-isoprenologues as substrate and corresponding inhibitory effect of antimycin in yeast. Biochim Biophys Acta. 1974 Oct 18;368(1):71–85. doi: 10.1016/0005-2728(74)90098-x. [DOI] [PubMed] [Google Scholar]
- Lang B., Burger G., Wolf K., Bandlow W., Kaudewitz F. Studies on the mechanism of electron trasport in the bc1-segment of the respiratory chain in yeast. III. Isolation and characterization of an antimycin resistant mutant ANT 8 in Schizosaccharomyces pombe. Mol Gen Genet. 1975;137(4):353–363. doi: 10.1007/BF00703260. [DOI] [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C., Lee C. P., Berden J. A., Wegdam H. J. High-energy forms of cytochrome b. I. The effectof ATP and antimycin on cytochrome b in phosphorylating sub-mitochondrial particles. Biochim Biophys Acta. 1970 Dec 8;223(2):354–364. doi: 10.1016/0005-2728(70)90192-1. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of action of the respiratory inhibitor, antimycin. Biochim Biophys Acta. 1973 Dec 7;301(2):129–154. doi: 10.1016/0304-4173(73)90002-5. [DOI] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. 13. Redox state changes of cytochrome b 562 in mung bean seedling mitochondria treated with antimycin A. Biochim Biophys Acta. 1972 Apr 20;267(1):48–64. doi: 10.1016/0005-2728(72)90137-5. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson P. F., Moreland D. E. Cyanide-resistant Respiration of Sweet Potato Mitochondria. Plant Physiol. 1975 Feb;55(2):365–369. doi: 10.1104/pp.55.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Dependence of Wound-induced Respiration in Potato Slices on the Time-restricted Actinomycin-sensitive Biosynthesis of Phospholipid. Plant Physiol. 1977 Jul;60(1):5–10. doi: 10.1104/pp.60.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Inhibition of the Development of Induced Respiration and Cyanide-insensitive Respiration in Potato Tuber Slices by Cerulenin and Dimethylaminoethanol. Plant Physiol. 1977 Jul;60(1):11–16. doi: 10.1104/pp.60.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannoni D., Melandri B. A., Baccarini-Melandri A. Energy transduction in photosynthetic bacteria. X. Composition and function of the branched oxidase system in wild type and respiration deficient mutants of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976 Mar 12;423(3):413–430. doi: 10.1016/0005-2728(76)90197-3. [DOI] [PubMed] [Google Scholar]



