Abstract
The respiration of fresh slices of preclimacteric avocado (Persea americana Mill. var. Hass) and banana (Musa cavendishii var. Valery) fruits is stimulated by cyanide and antimycin. The respiration is sensitive to m-chlorobenzhydroxamic acid in the presence of cyanide but much less so in the presence of antimycin. In the absence of cyanide the contribution of the cyanide-resistant pathway to the coupled preclimacteric respiration is zero. In uncoupled slices, by contrast, the alternate path is engaged and utilized fully in avocado, and extensively in banana. Midclimacteric and peak climacteric slices are also cyanide-resistant and, in the presence of cyanide, sensitive to m-chlorobenzhydroxamic acid. In the absence of uncoupler there is no contribution by the alternate path in either tissue. In uncoupled midclimacteric avocado slices the alternate path is fully engaged. Midclimacteric banana slices, however, do not respond to uncouplers, and the alternate path is not engaged. Avocado and banana slices at the climacteric peak neither respond to uncouplers nor utilize the alternate path in the presence or absence of uncoupler.
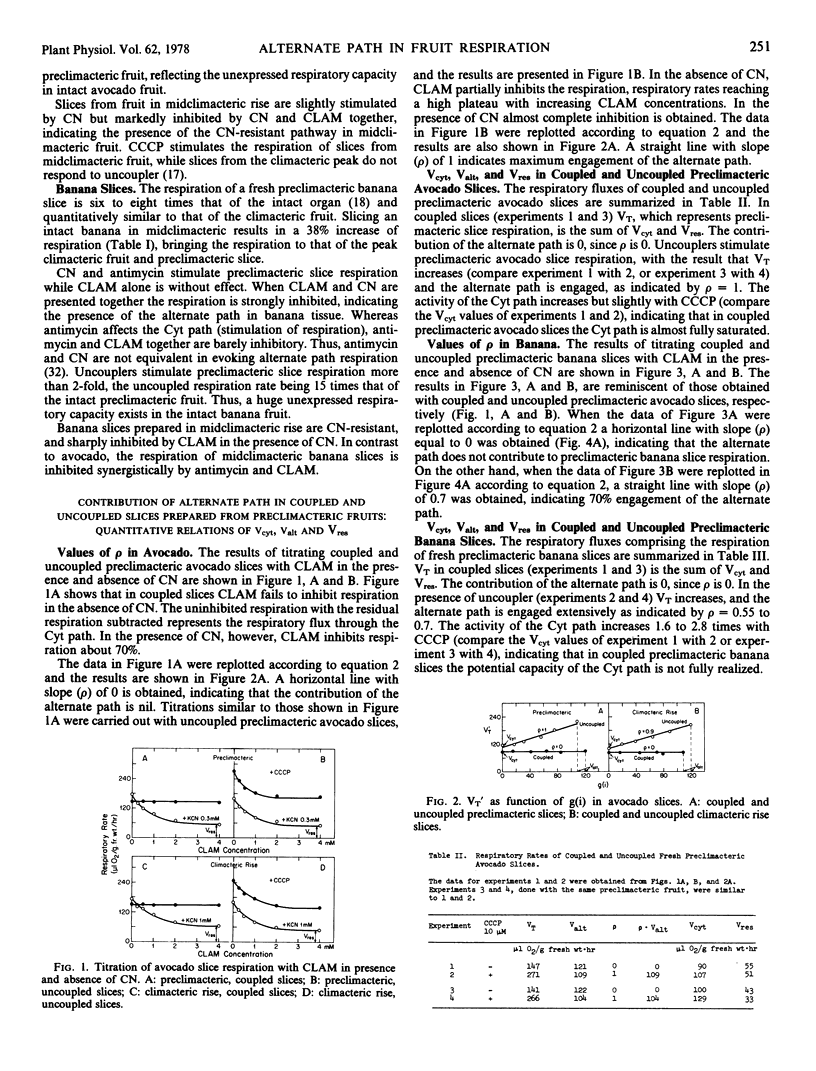
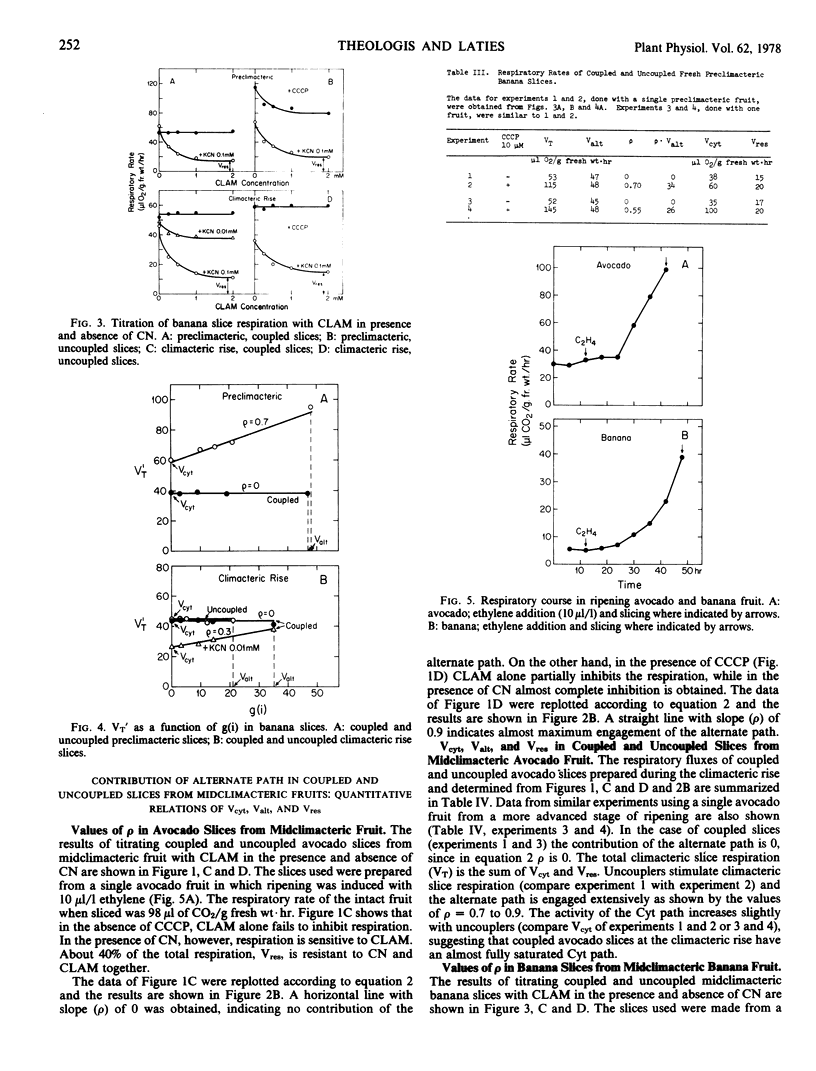
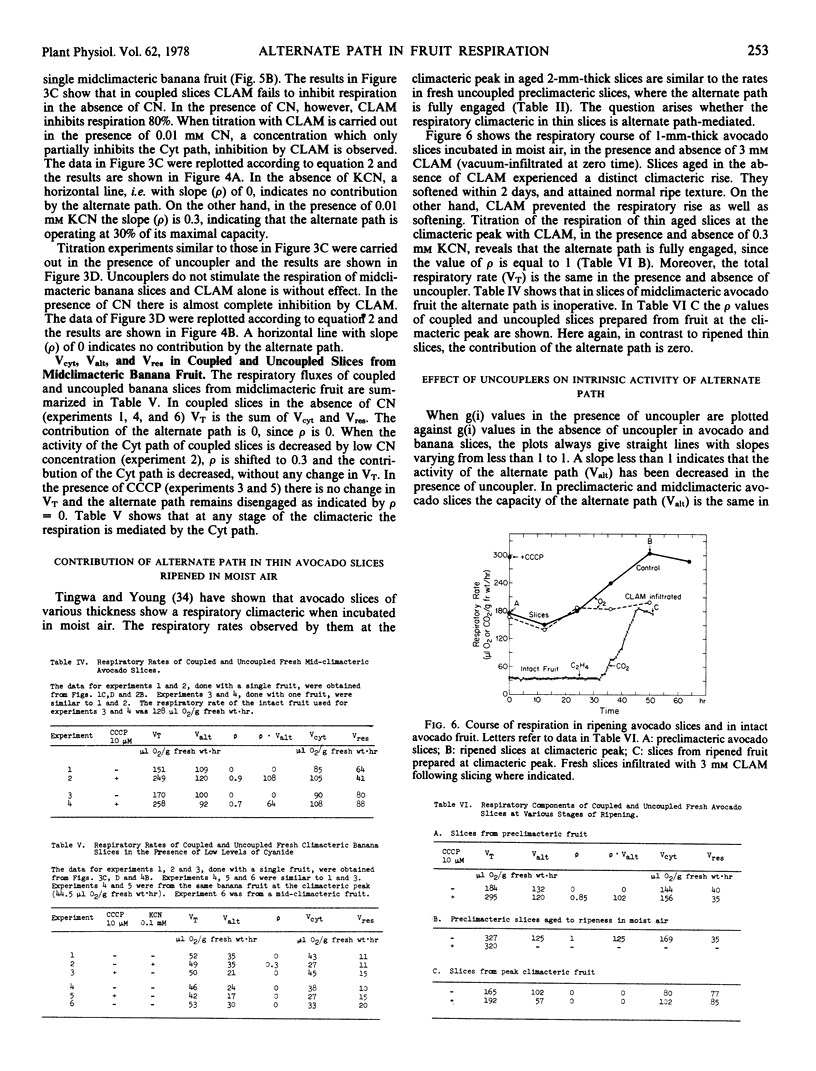
The maximal capacities of the cytochrome and alternate paths, Vcyt and Valt, respectively, have been estimated in slices from preclimacteric and climacteric avocado fruit and found to remain unchanged. The total respiratory capacity in preclimacteric and climacteric slices exceeds the respiratory rise which attends fruit ripening. In banana Valt decreases slightly with ripening.
The aging of thin preclimacteric avocado slices in moist air results in ripening with an accompanying climacteric rise. In this case the alternate path is fully engaged at the climacteric peak, and the respiration represents the total potential respiratory capacity present in preclimacteric tissue. The respiratory climacteric in intact avocado and banana fruits is cytochrome path-mediated, whereas the respiratory climacteric of ripened thin avocado slices comprises the alternate as well as the cytochrome path. The ripening of intact fruits is seemingly independent of the nature of the electron transport path.
Uncouplers are thought to stimulate glycolysis to the point where the glycolytic flux exceeds the oxidative capacity of the cytochrome path, with the result that the alternate path is engaged.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977 Mar;59(3):411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. K., Frenkel C. Upsurge in respiration and peroxide formation in potato tubers as influenced by ethylene, propylene, and cyanide. Plant Physiol. 1977 Mar;59(3):515–518. doi: 10.1104/pp.59.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Shirley P. S. Effect of cyanide on NADPH oxidation by granules from human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):445–454. [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Smith B. N., Epstein S., Laties G. G. The prevalence of carbon-13 in respiratory carbon dioxide as an indicator of the types of endogenous substrate. The change from lipid to carbohydrate during the respiratory rise in potato slices. J Gen Physiol. 1970 Jan;55(1):1–17. doi: 10.1085/jgp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. Controlling Influence of Thickness on Development & Type of Respiratory Activity in Potato Slices. Plant Physiol. 1962 Sep;37(5):679–690. doi: 10.1104/pp.37.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Biale J. B. Stimulations of oxygen uptake by electron transfer inhibitors. Plant Physiol. 1966 May;41(5):797–802. doi: 10.1104/pp.41.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Boveris A., Bonner W. D., Jr, Moore A. L. Hydrogen peroxide generation by the alternate oxidase of higher plants. Biochem Biophys Res Commun. 1976 Aug 9;71(3):695–703. doi: 10.1016/0006-291x(76)90887-1. [DOI] [PubMed] [Google Scholar]
- Richmond A., Biale J. B. Protein and nucleic acid metabolism in fruits. I. Studies of amino acid incorporation during the climacteric rise in respiration of the avocado. Plant Physiol. 1966 Oct;41(8):1247–1253. doi: 10.1104/pp.41.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Induction of ethylene of cyanide-resistant respiration. Biochem Biophys Res Commun. 1976 May 17;70(2):663–671. doi: 10.1016/0006-291x(76)91098-6. [DOI] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. The mechanism of ethylene and cyanide action in triggering the rise in respiration in potato tubers. Plant Physiol. 1975 Jan;55(1):73–78. doi: 10.1104/pp.55.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Antimycin-insensitive Cytochrome-mediated Respiration in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):238–242. doi: 10.1104/pp.62.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Cyanide-resistant Respiration in Fresh and Aged Sweet Potato Slices. Plant Physiol. 1978 Aug;62(2):243–248. doi: 10.1104/pp.62.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingwa P. O., Young R. E. The effect of tonicity and metabolic inhibitors on respiration and ripening of avocado fruit slices. Plant Physiol. 1974 Dec;54(6):907–910. doi: 10.1104/pp.54.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]



