Dear Editor,
Basal ganglia calcification is a common incidental finding in brain imaging, being reported in 20%–30% of the elderly.1 More than 50 clinical diagnoses have been reported to be associated with calcium accumulation in the basal ganglia.2 This condition was previously called familial idiopathic basal ganglia calcification, Fahr syndrome, Fahr’s disease, and striopallidodentate calcinosis, but primary familial brain calcification (PFBC) was coined to imply that there is a genetic component. Seven Mendelian genes (SLC20A2, PDGFRB, PDGFB, XPR1, MYORG, JAM2, and CMPK2) for PFBC have been discovered.3 Here we report the first Korean case of PFBC caused by an XPR1 mutation, who presented with early-onset cognitive decline with apathy and mild parkinsonism.
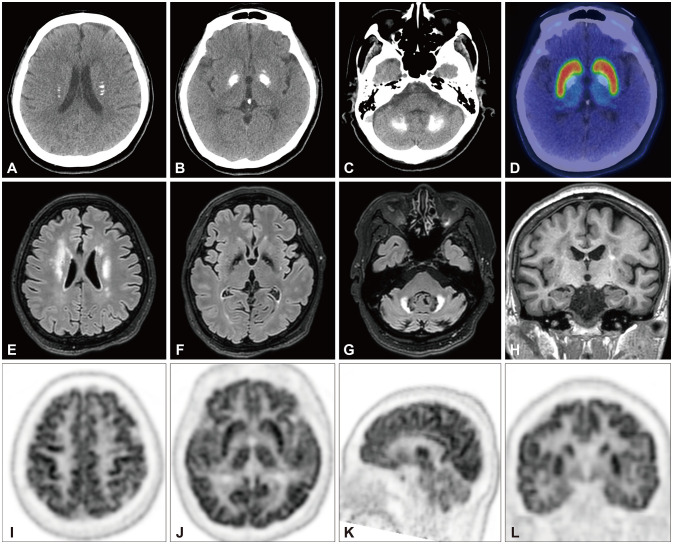
A 54-year-old female presented with a 3-year history of cognitive deficit. She complained of difficulty in pronouncing words, comprehending complex sentences, and mathematical calculations, which had progressed over the previous year. She exhibited apathy, depression, anxiety, sleep disturbance, and nocturia. She has been taking medications for hypertension, type-2 diabetes mellitus, and dyslipidemia. There was no family history of any neurological disorders. A neurological examination revealed a moderate degree of dysarthria, mild symmetric bradykinesia, and rigidity in both upper extremities. Her gait and postural stability were intact. She has scores of 10, 17, and 15 on the Unified Parkinson’s Disease Rating Scale part III score, Mini-Mental State Examination (MMSE), and Montreal Cognitive Assessment Test (MoCA), respectively. A comprehensive neuropsychological study using the Seoul Neuropsychological Screening Battery revealed mild cognitive impairment involving multiple cognitive domains including memory, attention, language, and visuospatial function. Her score on the Korean version of the Short Form of the Geriatric Depression Scale (SGDS-K) was 9, suggesting mild depression. Her score on the Withdrawal/Apathy/Lack of Vigor (WAV) subscale was 3/3. Laboratory studies revealed normal levels of serum calcium, inorganic phosphorous, 25-hydroxy vitamin D, osteocalcin, and parathyroid hormone. Low-dose methimazole was started since a thyroid function test indicated subclinical hypothyroidism. Other routine blood test results were normal. Computed tomography (Fig. 1A-C) and magnetic resonance imaging (MRI) (Fig. 1E-H) revealed dense calcifications in both corona radiata, the basal ganglia (especially the globus pallidus), and the dentate nucleus of the cerebellum. 18F-FP-CIT [(3-[18F]fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane] positron-emission tomography (PET) showed preserved dopamine transporter (DAT) binding (Fig. 1D). Fluorodeoxyglucose (FDG) PET showed diffusely decreased FDG uptake in the frontal and parietal lobes and the cerebellum, while showing relatively preserved uptake in the sensorimotor cortex and basal ganglia (Fig. 1I-L). Screening for mutations by whole-exome sequencing revealed the heterozygous missense mutation c.1871G>A (p.Arg624His, NM_004736.3) in XPR1 (OMIM 213600) (Supplementary Fig. 1 in the online-only Data Supplement). She was prescribed rivastigmine at 3 mg twice daily and 5 mg of escitalopram to control apathy and depression. Her general cognition as assessed using the MMSE and MoCA improved after 14 months, but this was not sustained to the 31-month follow-up. In contrast, SGDS-K and WAV scores showed sustained improvement up to 31 months (Supplementary Table 1 in the online-only Data Supplement).
Fig. 1. Brain computed tomography showed dense calcifications in both basal ganglia, cerebellum, and corona radiata (A-C). 18F-FP-CIT PET showed preserved DAT uptake (D). Fluid-attenuated inversion-recovery MRI showed nonspecific hyperintensities in bilateral frontal white matter and the cerebellum (E-G). T1-weighted MRI showed mild mesial temporal lobe atrophy (H). Brain FDG PET showed decreased FDG uptakes in the frontal and parietal lobes and the cerebellum, while those in the sensorimotor cortex and basal ganglia were relatively preserved (I-L). 18F-FP-CIT, (3-[18F]fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane; DAT, dopamine transporter; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron-emission tomography.
PFBC cases with a Mendelian gene mutation have been rarely reported in Koreans. XPR1 mutations are known to be inherited in an autosomal dominant manner, but they can also occur de novo.4 Reduced penetrance may also account for the lack of a family history in our patient.3
Parkinsonism was reported in 16.7%–80% of PFBC cases, with a prevalence of 28.6% in XPR1 mutation carriers.3 Presynaptic dopamine loss was observed in carriers of SLC20A2, MYROG, and XPR1 mutations.5,6,7 Our case showing neither presynaptic DAT loss nor striatal hypometabolism suggests that parkinsonism is not always related to dopaminergic dysfunction in XPR1-mutation carriers. Cognitive deficits are reported as the most frequent nonmotor symptom in PFBC, with apathy being frequently reported.8 Apathy is related to dysfunction of the systems that control voluntary actions in prefrontal-basal ganglia circuits: orbital-medial prefrontal cortex (PFC)-ventral basal ganglia or lateral PFC-dorsal caudate and dorsal pallidum.9 Cholinergic and serotonergic systems appear to be involved.10 Although our patient showed hypometabolism in structures associated with apathy, her symptoms continued to improve after a selective serotonin reuptake inhibitor treatment. This suggests that biochemical changes were responsible for her nonmotor symptoms.
This case report has some limitations. First, we were unable to obtain the patient’s pedigree or a detailed family history. Second, due to her multiple vascular risk factors and the presence of white-matter hyperintensities in brain MRI, her cognitive decline and apathy might have been attributable to vascular pathology.
Footnotes
Ethics Statement: This study was reviewed and approved by Yonsei University College of Medicine, Yongin Severance Hospital, Institutional Review Board (IRB No. 9-2023-0105). Informed consent was waived by IRB because medical records were used only for this study.
- Conceptualization: Yun Joong Kim.
- Data curation: Sojung Yoon.
- Formal analysis: Sojung Yoon.
- Methodology: Seok Jong Chung.
- Project administration: Sojung Yoon.
- Supervision: Seok Jong Chung, Yun Joong Kim.
- Validation: Seok Jong Chung.
- Visualization: Sojung Yoon.
- Writing—original draft: Sojung Yoon, Yun Joong Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This research was supported by a grant of the Korea Health Technology R&D Project through the Korean Healthy Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00265377).
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2023.0284.
The patient's serial cognitive scores
Sanger sequencing result of the proband showing a heterozygous missense mutation c.1871G>A (p.Arg624His, NM_004736.3) in the XPR1. This variant is considered likely pathogenic variants since it is in a well-established functional domain and a mutational hot spot, and it is not known as a benign variant (PM1, moderate evidence). Also, it is not included in control population databases such as Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium (PM2, moderate evidence). Lastly, multiple lines of in silico data support a deleterious effect on the gene or its product (PP3, supporting evidence). According to the American College of Medical Genetics and Genomics (ACMG) Standards and Guideline, this variant was concluded as likely pathogenic because it satisfied criteria (PM1, PM2, PP3) of the categories for classifying pathogenic variants.
References
- 1.Yamada M, Asano T, Okamoto K, Hayashi Y, Kanematsu M, Hoshi H, et al. High frequency of calcification in basal ganglia on brain computed tomography images in Japanese older adults. Geriatr Gerontol Int. 2013;13:706–710. doi: 10.1111/ggi.12004. [DOI] [PubMed] [Google Scholar]
- 2.Baba Y, Broderick DF, Uitti RJ, Hutton ML, Wszolek ZK. Heredofamilial brain calcinosis syndrome. Mayo Clin Proc. 2005;80:641–651. doi: 10.4065/80.5.641. [DOI] [PubMed] [Google Scholar]
- 3.Balck A, Schaake S, Kuhnke NS, Domingo A, Madoev H, Margolesky J, et al. Genotype–phenotype relations in primary familial brain calcification: systematic MDSGene review. Mov Disord. 2021;36:2468–2480. doi: 10.1002/mds.28753. [DOI] [PubMed] [Google Scholar]
- 4.López-Sánchez U, Nicolas G, Richard AC, Maltête D, Charif M, Ayrignac X, et al. Characterization of XPR1/SLC53A1 variants located outside of the SPX domain in patients with primary familial brain calcification. Sci Rep. 2019;9:6776. doi: 10.1038/s41598-019-43255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anheim M, López-Sánchez U, Giovannini D, Richard AC, Touhami J, N’Guyen L, et al. XPR1 mutations are a rare cause of primary familial brain calcification. J Neurol. 2016;263:1559–1564. doi: 10.1007/s00415-016-8166-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen SY, Lin WC, Chang YY, Lin TK, Lan MY. Brain hypoperfusion and nigrostriatal dopaminergic dysfunction in primary familial brain calcification caused by novel MYORG variants: case report. BMC Neurol. 2020;20:329. doi: 10.1186/s12883-020-01910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichikawa Y, Tanaka M, Kurita E, Nakajima M, Tanaka M, Oishi C, et al. Novel SLC20A2 variant in a Japanese patient with idiopathic basal ganglia calcification-1 (IBGC1) associated with dopa-responsive parkinsonism. Hum Genome Var. 2019;6:44. doi: 10.1038/s41439-019-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone MG, Della Rocca F. Neuropsychiatric manifestations of Fahr’s disease, diagnostic and therapeutic challenge: a case report and a literature review. Clin Neuropsychiatry. 2022;19:121–131. doi: 10.36131/cnfioritieditore20220206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol. 2006;253(suppl 7):vii54–vii61. doi: 10.1007/s00415-006-7012-5. [DOI] [PubMed] [Google Scholar]
- 10.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient's serial cognitive scores
Sanger sequencing result of the proband showing a heterozygous missense mutation c.1871G>A (p.Arg624His, NM_004736.3) in the XPR1. This variant is considered likely pathogenic variants since it is in a well-established functional domain and a mutational hot spot, and it is not known as a benign variant (PM1, moderate evidence). Also, it is not included in control population databases such as Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium (PM2, moderate evidence). Lastly, multiple lines of in silico data support a deleterious effect on the gene or its product (PP3, supporting evidence). According to the American College of Medical Genetics and Genomics (ACMG) Standards and Guideline, this variant was concluded as likely pathogenic because it satisfied criteria (PM1, PM2, PP3) of the categories for classifying pathogenic variants.
Data Availability Statement
All data generated or analyzed during the study are included in this published article (and its supplementary information files).