Abstract
The cytosolic and proplastid isoenzymes of 6-phosphogluconate dehydrogenase were purified from the developing endosperm of the castor bean (Ricinis communis L.). No differences in physical or kinetic properties were found for the purified isoenzymes. Each was composed of two identical 55,000 subunits. They had identical pH optima of 7.8 to 8.0 and similar MgCl2 stimulation for the oxidative decarboxylation of 6-phosphogluconate. The Km values for 6-phosphogluconate were 12 and 9.6 micromolar and for NADP+ were 4.1 and 5.4 micromolar for the cytosolic and proplastid isoenzymes, respectively. Therefore, the synthesis of two distinct 6-phosphogluconate dehydrogenase isoenzymes does not appear to have any kinetic significance for the developing seed. However, changes in the proplastid contribution toward carbohydrate metabolism occur in the developing seed and may necessitate independent gene expression to allow for a unique and flexible subcellular distribution of isoenzymes during development.
Full text
PDF


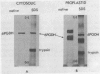
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Levin D. A. Chloroplast aldolase is controlled by a nuclear gene. Plant Physiol. 1970 Dec;46(6):819–820. doi: 10.1104/pp.46.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts S. A., Mayer R. J. Purification and properties of 6-phosphogluconate dehydrogenase from rabbit mammary gland. Biochem J. 1975 Nov;151(2):263–270. doi: 10.1042/bj1510263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Luca V., Dennis D. T. Isoenzyme of pyruvate kinase in proplastids from developing castor bean endosperm. Plant Physiol. 1978 Jun;61(6):1037–1039. doi: 10.1104/pp.61.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D. T., Green T. R. Soluble and particulate glycolysis in developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 2;64(3):970–975. doi: 10.1016/0006-291x(75)90142-4. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast phosphofructokinase: I. Proof of phosphofructokinase activity in chloroplasts. Plant Physiol. 1977 Aug;60(2):290–294. doi: 10.1104/pp.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard L. H. Gradient sievorptive chromatography. A focusing system for the separation of cellular components. Biochemistry. 1973 Sep 11;12(19):3627–3632. doi: 10.1021/bi00743a009. [DOI] [PubMed] [Google Scholar]
- PARR C. W. Inhibition of phosphoglucose isomerase. Nature. 1956 Dec 22;178(4547):1401–1401. doi: 10.1038/1781401a0. [DOI] [PubMed] [Google Scholar]
- Reid E. E., Lyttle C. R., Canvin D. T., Dennis D. T. Pyruvate dehydrogenase complex activity in proplastids and mitochondria of developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 6;62(1):42–47. doi: 10.1016/s0006-291x(75)80402-5. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A. Two isoenzymes of glucosephosphate isomerase from spinach leaves and their intracellular compartmentation. Eur J Biochem. 1974 Jun 1;45(1):77–82. doi: 10.1111/j.1432-1033.1974.tb03531.x. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Tetour M., Herbert M. Development and intracellular distribution of enzymes of the oxidative pentose phosphate cycle in radish cotyledons. Plant Physiol. 1975 Dec;56(6):836–840. doi: 10.1104/pp.56.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Dennis D. T. Isoenzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinis communis L. Plant Physiol. 1978 Jun;61(6):871–877. doi: 10.1104/pp.61.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews M. L., Kanji M. I., Carper W. R. 6-Phosphogluconate dehydrogenase. Purification and kinetics. J Biol Chem. 1976 Nov 25;251(22):7127–7131. [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Role of Aldolase in Photosynthesis. II Demonstration of Aldolase Types in Photosynthetic Organisms. Plant Physiol. 1968 May;43(5):793–798. doi: 10.1104/pp.43.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]