Abstract
Ulcerative colitis (UC) is a chronic recurrent inflammatory bowel disease. Despite ongoing advances in our understanding of UC, its pathogenesis is yet unelucidated, underscoring the urgent need for novel treatment strategies for patients with UC. Exosomes are nanoscale membrane particles that mediate intercellular communication by carrying various bioactive molecules, such as proteins, RNAs, DNA, and metabolites. The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is a cytosolic tripartite protein complex whose activation induces the maturation and secretion of proinflammatory cytokines interleukin-1β (IL-1β) and IL-18, triggering the inflammatory response to a pathogenic agent or injury. Growing evidence suggests that exosomes are new modulators of the NLRP3 inflammasome, with vital roles in the pathological process of UC. Here, recent evidence is reviewed on the role of exosomes and NLRP3 inflammasome in UC. First, the dual role of exosomes on NLRP3 inflammasome and the effect of NLRP3 inflammasome on exosome secretion are summarized. Finally, an outlook on the directions of exosome-NLRP3 inflammasome crosstalk research in the context of UC is proposed and areas of further research on this topic are highlighted.
Keywords: Ulcerative colitis, Exosomes, Inflammasome, Evidence, Therapeutics
Core Tip: Clarifying the regulatory circuits that control the abnormal immune state of the intestinal mucosa is essential for understanding ulcerative colitis (UC) pathogenesis and clinical management. The role of exosomes and NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes in UC has been continuously highlighted in recent years. In this review, the dual role of exosomes on NLRP3 inflammasome and the effect of NLRP3 inflammasome on exosome secretion are summarized. Furthermore, an outlook on the directions of exosome-NLRP3 inflammasome crosstalk research in the context of UC is proposed and areas of further research on this topic are highlighted.
INTRODUCTION
Ulcerative colitis (UC) is characterized by chronic, remitting, and recurrent mucosal inflammation[1]. Although its cause is not well understood, current evidence suggests innate and adaptive immunity play critical roles in its pathogenesis[2]. The events leading to UC involve disrupting the intestinal mucosal barrier, bringing the luminal microbial community and the mucosal immune system into direct contact[3]. Subsequently, innate immune cells, such as macrophages and dendritic cells, rapidly recognize microorganisms or their products entering the lamina propria from the intestinal lumen and transmit signals, awakening the innate defenses and the adaptive immune system[4]. A long-term feature of UC is inflammation maintained by various inflammatory mediators produced by activated immune cells, including proinflammatory cytokines and chemokines[5-8]. Another characteristic is enterocyte apoptosis sustained by several inflammatory cells, which prevents mucosal healing[2]. Considering these points, we can assume that clarifying the regulatory circuits that control the abnormal immune state of the intestinal mucosa is essential for understanding UC pathogenesis and clinical management.
The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome mediates the inflammatory cascade in vivo and is a critical regulator in inflammatory bowel disease development[9]. Its activation promotes pyroptosis and caspase-1-dependent secretion of interleukin-1β (IL-1β) and IL-18, leading to a sustained inflammatory response in the intestinal mucosa[10]. Since these two proinflammatory cytokines are present in released exosomes, one possible pathway for their unconventional secretion may occur through endosome release[11-14]. Exosomes are nanoscale membrane-derived particles that mediate intercellular communication by carrying many bioactive molecules, including proteins, RNAs, DNA, and metabolites[15,16]. They also carry out numerous functions, such as releasing cytokines and inhibiting or promoting inflammasome activation, depending on the transported molecules[17,18]. Increasing evidence suggests that crosstalk between exosomes and inflammasomes has a critical role in inflammatory diseases[19]. Therefore, systematically exploring this crosstalk in UC should have beneficial implications for the prevention and treatment.
NLRP3 INFLAMMASOME
Composition and distribution of NLRP3 inflammasome
Inflammasomes are cytosolic multiprotein complexes that initiate inflammatory cascade responses by identifying damage-associated molecular patterns (DAMPs), cellular distress signals of the host, pathogen-associated molecular patterns (PAMPs), and conserved components of infectious agents[20]. T and B lymphocytes, macrophages, antigen-presenting cells, and granulocytes all express the NLRP3 inflammasome[21]. It represents the most classical inflammasome subtype consisting of the NLRP3 receptor, apoptosis-associated speck-like protein (ASC) adapter, and caspase-1 effector proteins[22]. The NLRP3 receptor protein is composed of 3 domains: a C-terminal leucine-rich repeat domain, an N-terminal pyrin domain (PYD), and a central nucleotide-binding and oligomerization domain[23]. The ASC adapter contains several domains: 2 transactivation structural domains, the pyrin structural domain linked to the upstream NLRP3 receptor, and the caspase recruitment domain (CARD) connected to the downstream caspase-1[24,25].
Activation of NLRP3 inflammasome
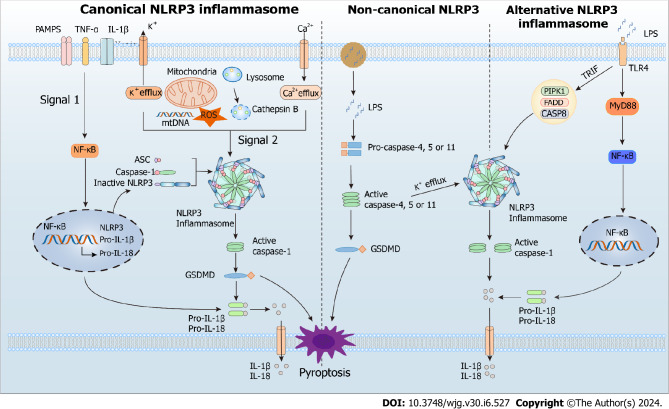
The innate immune system senses exogenous (PAMPs) or endogenous (DAMPs) danger signals by recognizing them with various pattern recognition receptors, such as Toll-like receptors and NOD-like receptors. During its involvement in the inflammatory response, NLRP3 inflammasome provides a molecular model that can be stimulated by many DAMPs (aluminum adjuvants, ATP, uric acid crystals, and β-amyloid peptides) and PAMPs (microbial toxins, viral RNA, and bacterial surface components). Currently, canonical, non-canonical, and alternate routes can all activate the NLRP3 inflammasome[26] (Figure 1).
Figure 1.
Canonical, non-canonical, and alternative modes of NOD-like receptor family pyrin domain containing 3 activation. NLRP3: NOD-like receptor family pyrin domain containing 3; PAMPs: Pathogen-associated molecular patterns; TNF-α: Tumor necrosis factor-alpha; IL-1β: Interleukin-1β; ROS: Reactive oxygen species; GSDMD: Gasdermin D; LPS: Lipopolysaccharide; ASC: Apoptosis-associated speck-like protein; IL-18: Interleukin-18.
Canonical NLRP3 inflammasome activation
In most cells, canonical NLRP3 inflammasome activation involves priming and activation steps. The priming step is initiated by a signal from the ligand bound to the pattern recognition receptor and promotes transcription of pro-IL-18, pro-IL-1β, and NLRP3 via NF-κB-dependent pathway[27-30]. The activation step leads to NLRP3 assembly and is promoted by various DAMPs or PAMPs through multiple molecular and cellular events, such as lysosomal disruption, mitochondrial DNA production, mitochondrial dysfunction, reactive oxygen species (ROS) release, and ion flux (Ca2+ influx and K+/Cl− efflux). The activated NLRP3 inflammasome induces cleavage and activation of caspase-1 via CARD-CARD and PYD-PYD interactions[30]. Subsequently, the activated caspase-1 recruits and cleaves the proinflammatory cytokines pro-IL-18 and pro-IL-1β, allowing their maturation and release[30]. In addition, it cleaves the pyroptotic substrate gasdermin D (GSDMD), enabling its translocation to the cell membrane, where it forms pores and triggers inflammatory programmed cell death called pyroptosis[31].
Non-canonical NLRP3 inflammasome activation
Human caspases 4 and 5, as well as murine caspase 11, are needed for non-canonical NLRP3 inflammasome activation. In this pathway, these caspases recognize and are activated by cytosolic lipopolysaccharide (LPS) from endocytosed gram-negative bacteria or, more often, their outer membrane vesicles[32]. The activated caspases catabolize GSDMD, leading to pyrolysis and promoting the release of mature IL-18 and IL-1β[33,34]. In addition to LPS, another signal called 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) activates the non-canonical pathway. This molecule is abundant in membranes of mammalian cells and is oxidized by ROS released from damaged or dead cells. The oxidized PAPC binds caspase-11 and caspase-4, initiating activation or inhibition of the NLRP3 inflammasome depending on the cell type[35].
Alternative NLRP3 inflammasome activation
Alternative activation of the NLRP3 inflammasome possesses cell- and species-specific characteristics[36]. For example, the TLR4-TRIF-RIPK1-FADD-CASP8 axis activates an alternative inflammasome upstream of NLRP3 in porcine and human monocytes, but this activation response is absent in murine monocytes[34,36]. Interestingly, the alternative activation lacks typical features for canonical and non-canonical activation, such as ASC speckle formation, K+ efflux, or pyroptosis induction[34].
The role of NLRP3 inflammasome in UC
Susceptibility to UC significantly increases with single nucleotide polymorphisms rs10925019 and rs10754558 in the coding region of the NLRP3 gene[37,38]. Similarly, predisposition to inflammatory bowel disease correlates with polymorphisms affecting receptors downstream of NLRP3, including interleukin 1 receptor-like 1 and 2, interleukin 1 receptor type 1 and 2, and interleukin 18 receptor 1[39]. Disease activity of UC is associated with increased levels of inflammasome activation markers NLRP3, caspase-1, and ASC[40,41]. A similar effect is also observed in mice with colitis, where the upregulated markers positively correlate with disease severity and pathological damage[42,43]. Conversely, mice with colitis lacking NLRP3 or caspase-1 show significantly less severe pathology compared with wild-type mice with colitis[44,45]. Furthermore, NLRP3 promotes intestinal mucosal inflammation in vitro[46]. These findings demonstrate that NLRP3 inflammasome activity participates in UC pathogenesis and suggest that treating the disease may rely on regulating the NLRP3 inflammasome activation or its downstream cytokine effectors.
A small-molecule inhibitor of the NLRP3 inflammasome called MCC950 significantly reduces the secretion of IL-18 and IL-1β in mice, attenuating the inflammatory cascade response evoked by NLRP3 inflammasome activation[47]. Carboxyamidotriazole, wogonoside, or oroxylin A are other small-molecule compounds that also alleviate experimental colitis but with a mechanism that inhibits the NLRP3 inflammasome activation[48-50]. Although pharmacological inhibition of inflammasome overactivation benefits animals with UC, therapies targeting inflammasomes remain limited. Recent evidence suggests that dietary compounds or medicinal herbs reduce colonic inflammation in mice and, in some cases, even in patients with UC by targeting different inflammasome modulators to inactivate inflammasomes in the colon[51]. Thus, strategies for treating UC may involve using bioactive substances purified from food or traditional medicines to regulate inflammasome activity.
EXOSOMES
Biogenesis, biology, function, and regulation of exosomes
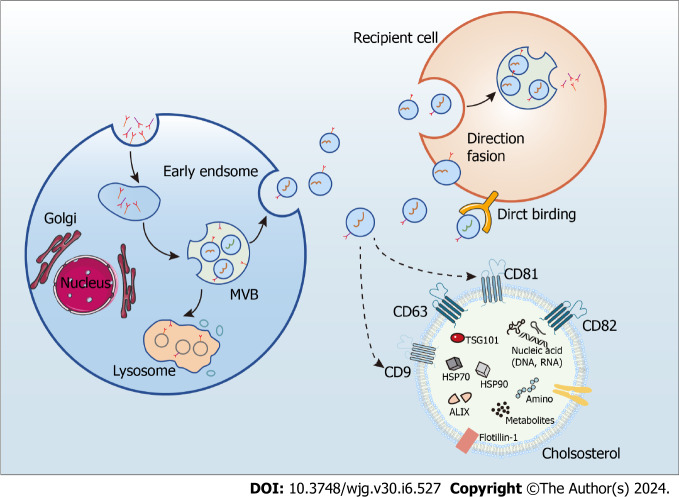
Exosomes are endosome-derived extracellular vesicles commonly found in body fluids, including sweat, blood, and urine, and characterized by a phospholipid bilayer, small vesicle morphology, and a diameter from 30 to 150 nm[15,16]. They mediate intercellular communication by carrying numerous biologically active molecules, such as DNA, RNAs, proteins, and metabolites, and their bioactive molecular composition depends on the cell type releasing them[15,16]. Notably, exosomes contain two classes of proteins: conserved and specific. While the make-up of specific proteins is determined by the cell type releasing the exosome and is subject to change from varying physiological conditions acting on the cell, that of the conserved proteins is constant, rendering them exosome markers. Noteworthy examples are programmed cell death 6 interacting protein, tumor susceptibility gene 10, members of the heat shock protein family HSP60, HSP70, and HSP90, and antigens CD9, CD63, CD81, and CD82[15,16]. Exosome biogenesis requires uptake, secretion, cargo sorting, and formation, achieved through the classical or direct pathways[52]. Whereas most cells utilize the classical, or exocytic, pathway of exosome biogenesis, T cells employ a direct pathway as a quick mechanism that generates exosomes directly from the plasma membrane[53] (Figure 2).
Figure 2.
The processes of exosome biogenesis and release. MVB: Multivesicular bodies.
Since released exosomes contain crucial molecules for transferring information between cells, they are implicated in the cancer microenvironment[54] and the pathogenesis of various illnesses, including autoimmune[55], cardiac[56], neurological[57], and liver disorders[58]. Furthermore, because exosomes collected from sick populations have different RNA profiles than exosomes collected from healthy ones[59-61], they are potential diagnostic and therapeutic biomarkers for many diseases[62,63].
Exosomes and UC
Exosomes are thought to play an immunomodulatory function owing to their involvement in immune synapse formation and antigen presentation[64,65]. Because UC is an immune disease, and the saliva of patients with UC contains large amounts of exosomal proteins, the role of exosomes in UC is unquestionable[66-68]. Indeed, animal experiments confirm that exosomal proteins are associated with proteasomal activity and inflammatory response, suggesting that some, such as saliva-derived exosomal proteasome 20S subunit alpha 7, can be used as an ideal biomarker for UC diagnosis[68]. Other potential UC biomarkers are exosome micro RNAs, with enhanced levels in individuals with UC. For instance, elevated levels of gut-derived miR-29b in the plasma of individuals with UC not only help diagnose the disease but also an impaired cardiac function via miR-29b-mediated extraintestinal inhibition of vital proteins, such as brain-derived neurotrophic factor[69]. Similarly, small GTPases that regulate exosome secretion also have increased levels in UC, such as RAB27A, member RAS oncogene family and RAB27B, member RAS oncogene family. The number of RAB27A- and RAB27B-positive immune cells in the intestinal mucosa of individuals with active UC is significantly higher than that of healthy patients, indicating that exosome-mediated immune regulation is involved in the pathological process of UC[70].
Currently, the role of various sources of exosomes in UC is being widely explored (Table 1)[71-105]. Mesenchymal stem cell (MSC) therapy is a cutting-edge one for treating various diseases, due to the strong immunomodulating and immunosuppressive properties of MSCs, and stem cell-derived exosomes may have a beneficial effect on UC, according to newly available evidence[71-91]. The ameliorative effects of MSC-derived exosomes on UC are regulated in multiple ways, including inhibition of inflammatory responses, regulation of immune cell homeostasis, improvement of intestinal flora structure, and inhibition of oxidative stress, ultimately leading to repair of intestinal mucosal damage and restoration of intestinal barrier function. Similarly, dendritic cell-derived exosomes were also found to have a reparative effect on intestinal injury in UC by inhibiting pathways associated with inflammation[92-94]. In addition, it was found that encapsulating triptolide with DC cell-derived exosomes could not only reduce the toxicity of the drug, but also accurately deliver the drug to the therapeutic target to induce immunosuppression in UC mice, providing a new perspective for immunosuppressive treatment of UC[95]. However, macrophage-derived exosomes do not always provide a benefit to UC. Some exosomal molecules, such as miR-590-3p produced by M2 macrophages, reduce mucosal damage and promote epithelial cell repair in mice with colitis[96]. However, others, such as exosome miR-21a-5p produced by M1 macrophages, exacerbate UC by inhibiting E-cadherin and activating type 2 innate lymphoid cells[97]. Furthermore, limited evidence suggests that gut-derived and serum-derived exosomes are beneficial in UC[98,100], whereas visceral adipose-derived exosomes aggravate UC[99]. Surprisingly, emerging evidence has recently suggested that human or bovine milk-derived exosomes express a favorable benefit in animals with colitis by decreasing oxidative stress and inflammation, indicating a new route for the development of therapeutic approaches for UC[103-105].
Table 1.
Sources of exosomes and their roles in ulcerative colitis
|
Exosomes source
|
Pivotal molecules
|
Role of the exosomes
|
Conclusion
|
Ref.
|
| Stem cell | miR-378a-5p | Inhibiting pyroptosis through NLRP3/caspase-1 signaling | Beneficial | [71] |
| Stem cell | miR-539-5p | Inhibiting pyroptosis through NLRP3/caspase-1 signaling | Beneficial | [72] |
| Stem cell | miRNA | Suppressing pyroptosis | Beneficial | [73] |
| Stem cell | miR-203a-3p.2 | Suppressing macrophage pyroptosis induced by caspase11/4 | Beneficial | [74] |
| Stem cell | NA | Regulating the Treg population | Beneficial | [75] |
| Stem cell | NA | Modulating the gut metagenomics-metabolomics-farnesoid X receptor axis | Beneficial | [76] |
| Stem cell | NA | Polarizing M2b macrophages | Beneficial | [77] |
| Stem cell | miR-146a | Inhibiting SUMO1 expression and its binding to β-catenin | Beneficial | [78] |
| Stem cell | miR-216a-5p | Inducing macrophage M2 polarization by regulating the HMGB1/TLR4/NF-κB signaling pathway | Beneficial | [79] |
| Stem cell | NA | Regulating the Th17/Treg balance | Beneficial | [80] |
| Stem cell | NA | Repairing intestinal barrier via TSG-6 | Beneficial | [81] |
| Stem cell | miR-125a, miR-125b | Repressing Th17 cell differentiation | Beneficial | [82] |
| Stem cell | NA | Limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α | Beneficial | [83] |
| Stem cell | miR-181a | Improving gut microbiota composition, barrier function, and inflammatory status | Beneficial | [84] |
| Stem cell | NA | Suppressing inflammation | Beneficial | [85] |
| Stem cell | NA | Modulating Th1/Th17 and Treg cell responses | Beneficial | [86] |
| Stem cell | NA | Attenuating inflammation, oxidative stress and apoptosis | Beneficial | [87] |
| Stem cell | NA | Stimulating epithelial repair and decreasing epithelial apoptosis | Beneficial | [88] |
| Stem cell | NA | Modulating the expression of IL-7 in macrophages | Beneficial | [89] |
| Stem cell | NA | Downregulating intestine ferroptosis | Beneficial | [90] |
| Melatonin and stem cell | NA | Suppressing inflammation, oxidative stress, apoptosis, and fibrosis | Beneficial | [91] |
| Dendritic Cell | miR-146a | Targeting Traf6, IRAK-1, and NLRP3 in macrophages | Beneficial | [92] |
| Dendritic cell | NA | Preventing colon damage | Beneficial | [93] |
| Dendritic cell | NA | Downregulating the expression of IL-2, IFN-γ and TNF-α | Beneficial | [94] |
| Dendritic cell | NA | Carrying drug to dendritic cell | Beneficial | [95] |
| M2 macrophage | miR-590-3p | Suppressing LATS1 and activating the YAP/β-catenin signaling | Beneficial | [96] |
| M1 macrophage | MiR-21a-5p | Decreasing E-cadherin and subsequent ILC2 activation | Unfavorable | [97] |
| Intestinal | NA | Promoting wound healing | Beneficial | [98] |
| Visceral adipose tissue | miR-155 | Promoting macrophage M1 polarization | Unfavorable | [99] |
| Serum | NA | Inhibiting MCP-1 and MIP-1α expression via NLRP12-Notch signaling pathway | Beneficial | [100] |
| Serum | Proteins | Implicating macrophage activation | NA | [101] |
| Helicobacter pylori | NA | Aggravating intestinal epithelium barrier dysfunction by facilitating Claudin-2 expression | Unfavorable | [102] |
| Milk | NA | Suppressing inflammation | Beneficial | [103] |
| Cow and human milk | miRNA-320, 375, and Let-7 | Downregulating DNA methyltransferase 1 (DNMT1) and DNMT3 | Beneficial | [104] |
| Bovine colostrum | NA | Suppressing inflammation and oxidative stress | Beneficial | [105] |
NA: Not available.
EXOSOMES AND NLRP3 INFLAMMASOME CROSSTALK
Effects of exosomes on NLRP3 inflammasome
According to recent evidence, cells utilize exosome secretion to regulate NLRP3 inflammasome activation, suppressing inflammation and promoting damage repair (Table 2)[71-73,106-147]. Since most findings originate from research on various stem cell-derived exosomes, knowledge of how they regulate the NLPR3 inflammasome activation in differentiated cells remains limited. Nonetheless, the available evidence indicates that exosomes suppress the NLRP3 inflammasome mainly by regulating the pathways upstream of NLRP3, especially TLR-related ones and those related to oxidative stress. For example, exosome release lowers ROS production, reducing ROS levels available for the NLRP3 inflammasome activation[107,112,126]. In addition, exosomes help protect mitochondria from damage induced by oxidative stress states, possibly by exosome-carried mitochondrial proteins[112]. Abundant findings also suggest that exosomes regulate the activation of NLRP3 inflammasome by directly binding to NLRP3[71,121,130,137].
Table 2.
Sources of exosomes and their roles in NOD-like receptor family pyrin domain containing 3 inflammasome regulation
|
Exosomes source
|
Pivotal molecules
|
Role of the exosomes
|
Ref.
|
| Stem cell | miR-378a-5p | Inhibiting NLRP3 inflammasome activation | [71] |
| Stem cell | miR-539-5p | Inhibiting NLRP3 inflammasome activation | [72] |
| Stem cell | NA | Inhibiting NLRP3 inflammasome activation | [73] |
| Stem cell | miR-17 | Inhibiting NLRP3 inflammasome activation by targeting TXNIP | [106] |
| Stem cell | NA | Inhibiting NLRP3 inflammasome activation by down-regulating ROS levels | [107] |
| Stem cell | NA | Inhibiting TLR4-NLRP3-mediated pyroptosis | [108] |
| Plasma | NA | Inhibiting pyroptosis through the TLR4/NF-κB pathway | [109] |
| Stem cell | NA | Inhibiting NLRP3 inflammasome-mediated pyroptosis by promoting AMPK-dependent autophagic flux | [110] |
| Stem cell | circHIPK3 | Inhibiting pyroptosis by down-regulating miR-421 to increase FOXO3A expression | [111] |
| Stem cell | miRNA Let-7 | Inhibiting NLRP3 inflammasome activation by down-regulating ROS levels | [112] |
| Stem cell | miR-188-3p | Targeting NLRP3 | [113] |
| Stem cell | NA | Inhibiting the tumor suppressor Rb1-mediated NLRP3 inflammasome | [114] |
| Stem cell | NA | Inhibiting pyroptosis through the TLR4 pathway | [115] |
| Cancer cells | miR-21 | Repressing PTEN and BRCC3 to facilitate NLRP3 phosphorylation | [116] |
| Stem cell | circ_003564 | Attenuating inflammasome-related pyroptosis | [117] |
| Stem cell | miR-100-5p | Inhibiting the FOXO3A/NLRP3 pathway | [118] |
| Stem cell | miR-17-5p | Suppressing TXNIP-NLRP3 inflammasome | [119] |
| Pericyte | circEhmt1 | Upregulating NFIA levels to suppress NLRP3-mediated inflammasome formation | [120] |
| B cells | miR-BART15 | Targeting the miR-223 binding site in the NLRP3 3′-untranslated region | [121] |
| Stem cell | NA | Suppressing NLRP3 inflammasome activation | [122] |
| Stem cell | NA | Suppressing NLRP3 inflammasome activation | [123] |
| Stem cell | NA | Suppressing NLRP3 inflammasome activation | [124] |
| Stem cell | NA | Regulating pyroptosis via the miR-146a-5p-TRAF6 axis | [125] |
| M2 macrophage | NA | Suppressing the ROS/NLRP3 pathway | [126] |
| Stem cell | NA | Attenuating inflammasome-related pyroptosis | [127] |
| Cancer cells | NA | Suppressing NLRP3 inflammasome activation | [128] |
| Stem cell | miR-23b | Attenuating inflammasome-related pyroptosis | [129] |
| Stem cell | miR-223-3p | Targeting NLRP3 | [130] |
| Stem cell | NA | Suppressing NLRP3 inflammasome activation | [131] |
| Stem cell | NA | Modulating miR-126 via targeting HMGB1 | [132] |
| Plasma | NA | Promoting the autophagic degradation of NLRP3 | [133] |
| Stem cell | miR-223 | Downregulating NLRP3 expression | [134] |
| Dendritic cell | NA | Downregulating NLRP3 expression | [135] |
| M2 macrophage | microRNA-148a | Inhibiting the TLR4/NF-κB/NLRP3 pathway | [136] |
| Salivary | miR-223-3p | Attenuating inflammasome-related pyroptosis | [137] |
| Neutrophils | miR-30d-5p | Upregulating NLRP3 expression through the NF-κB pathway | [138] |
| Cancer cells | TRIM59 | Inducing the ubiquitination of ABHD5 to activate the NLRP3 inflammasome activation | [139] |
| Epithelium cells | NA | Upregulating the NLRP3 inflammasome | [140] |
| Serum | NA | Activating the NLRP3 inflammasome | [141] |
| Plasma | NA | Triggering NLRP3-dependent pyroptosis | [142] |
| Plasma | NA | Triggering NLRP3 inflammasome | [143] |
| Plasma | NA | Activating the NLRP3 inflammasome | [144] |
| Serum | NA | Inhibiting the NF-κB/NLRP3 pathway | [145] |
| Plasma | miRNA-223 | Inhibiting NLRP3 | [146] |
| Renal tissues | NA | Suppressing NLRP3 activation | [147] |
NA: Not available.
We have so far learned that stem cell-derived exosomes repress the NLRP3 inflammasome activation but will see that those from other cell types, including cancer, epithelial, immune, and endothelial cells, appear to promote it (Table 2). For instance, exosomal miR-30d-5p released by polymorphonuclear neutrophils induces macrophage pyroptosis and M1 macrophage polarization via the NF-κB pathway, promoting sepsis-associated acute lung injury[138]. Similarly, tumor-derived exosomal tripartite motif containing 59 protein induces proteasomal degradation of abhydrolase domain containing 5 lipolytic co-activator in macrophages. Consequently, this event reprograms macrophages into cells with tumor-promoting function and activates the NLRP3 inflammasome, mediating the IL-1β release and stimulating lung cancer progression[139]. When exposed to photooxidative blue light, retinal pigment epithelium-derived exosomes exacerbate potentially harmful oxidative responses by activating the NLRP3 inflammasome[140]. In hepatic ischemia-reperfusion injury, serum exosome levels rise significantly, freely crossing the blood-brain barrier due to their small size and stimulating pyroptosis of hippocampal and cortical tissues[141]. By triggering NLRP3-dependent pyroptosis in alveolar macrophages, plasma-derived exosomes help cause lung damage brought on by pancreatitis[142]. Exosomes in patients with COVID-19 increase inflammasome activity in distant endothelial cells, enhancing immunopathogenesis of the disease[143]. In addition, plasma-derived exosomes induce pyroptosis in intestinal epithelial cells via NLRP3 inflammasome activation in individuals with intestinal Behçet’s syndrome[144].
In summary, the above evidence suggests that exosomes play a dual role in NLRP3-mediated inflammatory response by attenuating or enhancing the inflammasome activity. The differences in how exosomes affect the inflammasome activity may depend on the cell type producing the exosomes and the specific circumstances of their release. Importantly, modulating the NLRP3 inflammasome activity by targeting exosomes is emerging as a promising strategy to combat inflammatory diseases[145-147].
Effects of NLRP3 inflammasome on exosomes
Some NLRP3 inflammasome activators also stimulate extracellular vesicle secretion, suggesting inflammasome activation enhances extracellular vesicle secretion[148]. After exposure to ATP, macrophages secrete exosomes carrying the major histocompatibility complex class II proteins[149]. Moreover, macrophages isolated from mice lacking the genes encoding the ASC adapter or NLRP3 cannot release these exosomes after exposure to ATP, indicating exosome release requires components of the NLRP3 complex[149]. Similarly, inflammasome activation increases exosome secretion caused by a viral infection or exposure to LPS/ATP[149]. We have seen previously that the release of mature IL-1β largely depends on the NLRP3 inflammasome activation. When synovial fibroblasts are treated with exogenous IL-1β, they show a significant increase in exosome secretion compared with the untreated control cells, implying IL-1β stimulates exosome release[150]. Although a few recent studies demonstrate that exosome secretion is induced by NLRP3 inflammasome activation, evidence supporting this claim is insufficient and requires additional confirmation[19].
Exosome-inflammasome crosstalk in UC
In inflammatory states, such as UC, MSCs have immunomodulating and homeostatic effects and may repair intestinal damage[151]. Increasing evidence indicates that MSCs maintain immunosuppressive signals through paracrine mediators instead of cell-to-cell contact and that paracrine processes predominantly mediate the therapeutic role of MSC-derived exosomes[71,152]. Although we know little about how MSC-derived exosomes suppress colonic inflammation, recent evidence suggests that crosstalk between exosomes and NLRP3 inflammasome constitutes the mechanism[71-73,92]. Thus, the roles of exosome-NLRP3 inflammasome crosstalk in inflammatory diseases are gaining much attention[19].
In mice with colitis, exosomes from human umbilical cord MSCs carrying miR-378a-5p significantly alleviate colonic inflammation and promote mucosal repair[71]. Mechanically, these exosomes inhibit the NLRP3 inflammasome activation, preventing caspase-1 cleavage and the IL-18 and IL-1β secretion and decreasing pyroptosis[71]. Similarly, exosomes from bone marrow MSCs containing miR-539-5p alleviate colitis by directly targeting the NLRP3-caspase-1 pathway to inhibit pyroptosis[72]. Moreover, hair follicle-derived MSCs inhibited pyroptosis by releasing exosomes in a paracrine manner, which ultimately exerted an alleviating effect in mice with colitis[73]. Other examples involving exosomes with small RNA cargo are dendritic cells-derived exosomes transporting miR-146a which exert a therapeutic effect by directly targeting the NLRP3-caspase-1 pathway to inhibit intestinal inflammation in mice with colitis[92] and human umbilical cord MSC-derived exosomes transferring miR-203a-3p.2 that reduce pyroptosis of macrophages caused by caspase-1 or -4[74].
Given these points, we can conclude that crosstalk between exosomes and the NLRP3 inflammasome holds promise for developing novel treatment strategies (Figure 3). Despite the scarcity of available evidence, the connection between MSC-derived exosomes with anti-inflammatory activity and the NLRP3 inflammasome offers a fresh viewpoint on using this system as a therapy for UC in the clinical setting.
Figure 3.
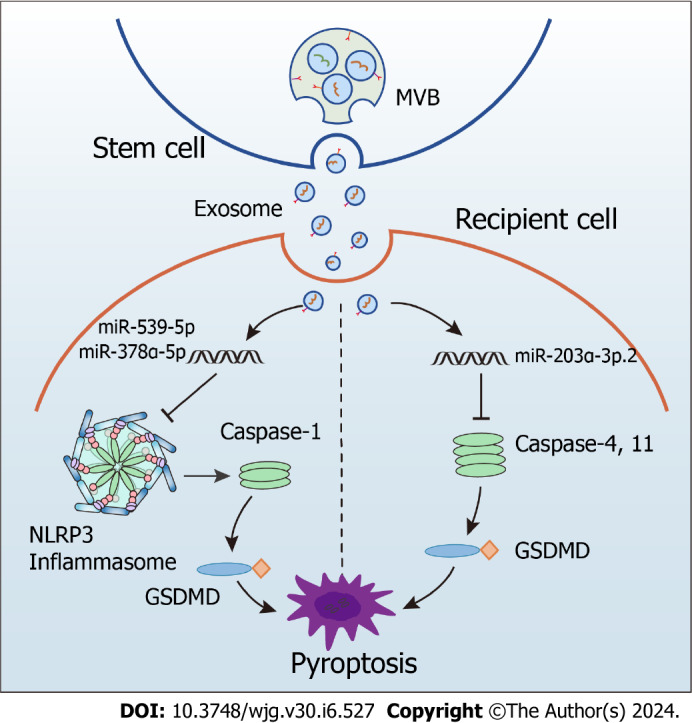
Inhibition of NOD-like receptor family pyrin domain containing 3 inflammasome activation by stem cell-derived exosomes in ulcerative colitis. NLRP3: NOD-like receptor family pyrin domain containing 3; MVB: Multivesicular bodies; GSDMD: Gasdermin D.
CONCLUSION
Since exosomes and the NLRP3 inflammasome play vital roles in UC, they are explored as potential new targets for preventing and treating the disease, attracting considerable attention. Importantly, crosstalk between exosomes and the NLRP3 inflammasome and its emerging therapeutic benefit is gaining increasing interest in biomedicine.
Exosomes are upstream components of the NLRP3 inflammasome pathway and attenuate or enhance the NLRP3 inflammasome activation. Based on the available data, MSC-derived exosomes repress the NLRP3 inflammasome activation in receptor cells, alleviating the inflammatory response. Therefore, these exosomes are therapeutically valuable and in stark contrast to most of those derived from non-stem cells that promote the NLRP3 inflammasome activation and exacerbate tissue inflammation. Potent effectors of the crosstalk are micro RNAs that repress the NLRP3 inflammasome activation and prevent pyroptotic cell death or promote the opposite effect, depending on the cell type releasing the exosomes and the external factors triggering exosome release. However, this contrasting effect of exosomes on the NLRP3 inflammasome and the factors that decide on its direction is supported by limited evidence. Similarly, evidence is lacking about the regulatory role of the NLRP3 inflammasome activation in exosome release. Thus, although crosstalk between exosomes and the NLRP3 inflammasome undoubtedly has a central role in UC research, further studies are necessary to elucidate it.
In conclusion, the therapeutic potential of exosomes has gained much attention since these vesicles transfer biologically active cargo between cells and could deliver drugs to treat diseases. However, because exosomes originating from different sources and exposed to specific intervention conditions have unique cargo composition and properties, selecting those most suitable for therapeutic use represents a challenge requiring substantial effort for clarification. Moreover, encapsulation and targeted delivery of drugs (e.g., biologics and small molecule drugs) through exosomes is a novel approach that both reduce drugs toxicity and improve efficacy. Therefore, large-scale prospective clinical trials exploring therapeutic efficacy and adverse events of exosomes in UC will be the focus of upcoming studies on the basis of sufficient basic research evidence.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 22, 2023
First decision: November 20, 2023
Article in press: January 9, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ertan A, United States; Hassan SA, United States S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Xin Li, School of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, Guizhou Province, China.
Li-Jiang Ji, Department of Anorectal Surgery, Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu 215500, Jiangsu Province, China.
Kai-Di Feng, College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
Hua Huang, Department of Anorectal Surgery, Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu 215500, Jiangsu Province, China.
Mei-Rou Liang, College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
Shi-Jin Cheng, College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
Xiu-Dong Meng, School of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, Guizhou Province, China. xiudong19@163.com.
References
- 1.Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357–1373. doi: 10.1016/j.mayocp.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Wang F, Tang X. Uncovering the shared molecule and mechanism between ulcerative colitis and atherosclerosis: an integrative genomic analysis. Front Immunol. 2023;14:1219457. doi: 10.3389/fimmu.2023.1219457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 4.Zou J, Liu C, Jiang S, Qian D, Duan J. Cross Talk between Gut Microbiota and Intestinal Mucosal Immunity in the Development of Ulcerative Colitis. Infect Immun. 2021;89:e0001421. doi: 10.1128/IAI.00014-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun T, Nguyen A, Gommerman JL. Dendritic Cell Subsets in Intestinal Immunity and Inflammation. J Immunol. 2020;204:1075–1083. doi: 10.4049/jimmunol.1900710. [DOI] [PubMed] [Google Scholar]
- 6.Ji L, Zhou Q, Huang J, Lu D. Macrophages in ulcerative colitis: A perspective from bibliometric and visual analysis. Heliyon. 2023;9:e20195. doi: 10.1016/j.heliyon.2023.e20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Zhang J, Ma J, Liu J, Wang F, Tang X. Inhibiting Ferroptosis: A Novel Approach for Ulcerative Colitis Therapeutics. Oxid Med Cell Longev. 2022;2022:9678625. doi: 10.1155/2022/9678625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan J, Pandey SP, Barnes BJ, Turner JR, Abraham C. T Cell-Intrinsic IRF5 Regulates T Cell Signaling, Migration, and Differentiation and Promotes Intestinal Inflammation. Cell Rep. 2020;31:107820. doi: 10.1016/j.celrep.2020.107820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Zhao Y, Ma Y, Wang Z, Rong L, Wang B, Zhang N. Biological functions of NLRP3 inflammasome: A therapeutic target in inflammatory bowel disease. Cytokine Growth Factor Rev. 2021;60:61–75. doi: 10.1016/j.cytogfr.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Chen LH, Yang H, Fang YC, Wang SW, Wang M, Yuan QT, Wu W, Zhang YM, Liu ZJ, Nan FJ, Xie X. GPR84 signaling promotes intestinal mucosal inflammation via enhancing NLRP3 inflammasome activation in macrophages. Acta Pharmacol Sin. 2022;43:2042–2054. doi: 10.1038/s41401-021-00825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol. 2013;87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Gee HY, Lee MG. Unconventional protein secretion - new insights into the pathogenesis and therapeutic targets of human diseases. J Cell Sci. 2018;131 doi: 10.1242/jcs.213686. [DOI] [PubMed] [Google Scholar]
- 14.Shi TT, Zhao RX, Xin Z, Hou ZJ, Wang H, Xie RR, Li DM, Yang JK. Tear-derived exosomal biomarkers of Graves' ophthalmopathy. Front Immunol. 2022;13:1088606. doi: 10.3389/fimmu.2022.1088606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paktinat S, Hashemi SM, Ghaffari Novin M, Mohammadi-Yeganeh S, Salehpour S, Karamian A, Nazarian H. Seminal exosomes induce interleukin-6 and interleukin-8 secretion by human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2019;235:71–76. doi: 10.1016/j.ejogrb.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Chen X, Tao J, Shi A, Zhang J, Yu P. Exosomes Regulate NLRP3 Inflammasome in Diseases. Front Cell Dev Biol. 2021;9:802509. doi: 10.3389/fcell.2021.802509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11:4436–4451. doi: 10.7150/thno.54004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 22.Seoane PI, Lee B, Hoyle C, Yu S, Lopez-Castejon G, Lowe M, Brough D. The NLRP3-inflammasome as a sensor of organelle dysfunction. J Cell Biol. 2020;219 doi: 10.1083/jcb.202006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci. 2017;130:3955–3963. doi: 10.1242/jcs.207365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Wang D, Liu M, Yu H, Chen Q, Wu Y, Bao R, Zhang Y, Wang T. The development from hyperuricemia to gout: key mechanisms and natural products for treatment. Acupunct Herbal Med. 2022;2:25–32. [Google Scholar]
- 26.Wang L, Hauenstein AV. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889. doi: 10.1016/j.mam.2020.100889. [DOI] [PubMed] [Google Scholar]
- 27.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA, Pasare C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing Y, Yao X, Li H, Xue G, Guo Q, Yang G, An L, Zhang Y, Meng G. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling-Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J Immunol. 2017;199:1561–1566. doi: 10.4049/jimmunol.1700175. [DOI] [PubMed] [Google Scholar]
- 30.Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141–1160. doi: 10.1038/s41423-021-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matikainen S, Nyman TA, Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204:3063–3069. doi: 10.4049/jimmunol.2000373. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–2127. doi: 10.1038/s41423-021-00740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, Kagan JC. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Hanaei S, Sadr M, Rezaei A, Shahkarami S, Ebrahimi Daryani N, Bidoki AZ, Rezaei N. Association of NLRP3 single nucleotide polymorphisms with ulcerative colitis: A case-control study. Clin Res Hepatol Gastroenterol. 2018;42:269–275. doi: 10.1016/j.clinre.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J, Liu J. NLRP3 gene is associated with ulcerative colitis (UC), but not Crohn's disease (CD), in Chinese Han population. Inflamm Res. 2014;63:979–985. doi: 10.1007/s00011-014-0774-9. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Oh J, Xue M, Huh WJ, Wang J, Gonzalez-Hernandez JA, Rice TA, Martin AL, Song D, Crawford JM, Herzon SB, Palm NW. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science. 2022;378:eabm3233. doi: 10.1126/science.abm3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranson N, Veldhuis M, Mitchell B, Fanning S, Cook AL, Kunde D, Eri R. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int J Mol Sci. 2018;20 doi: 10.3390/ijms20010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Zhang J, Wang Y, Huang J, Yang X, Ma J, Liu Z, Wang F, Tang X. Modified Gegen Qinlian decoction ameliorates DSS-induced chronic colitis in mice by restoring the intestinal mucus barrier and inhibiting the activation of γδT17 cells. Phytomedicine. 2023;111:154660. doi: 10.1016/j.phymed.2023.154660. [DOI] [PubMed] [Google Scholar]
- 42.Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, Liu W, Si J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol Spectr. 2021;9:e0073021. doi: 10.1128/Spectrum.00730-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng B, Huang Y, Chen S, Xu R, Xu L, Qiu J, Shi F, Liu S, Zha Q, Ouyang D, He X. Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis. Cell Mol Immunol. 2022;19:925–943. doi: 10.1038/s41423-022-00891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 45.Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors. Dig Dis. 2012;30 Suppl 1:82–90. doi: 10.1159/000341681. [DOI] [PubMed] [Google Scholar]
- 46.Ning L, Ye N, Ye B, Miao Z, Cao T, Lu W, Xu D, Tan C, Xu Y, Yan J. Qingre Xingyu recipe exerts inhibiting effects on ulcerative colitis development by inhibiting TNFα/NLRP3/Caspase-1/IL-1β pathway and macrophage M1 polarization. Cell Death Discov. 2023;9:84. doi: 10.1038/s41420-023-01361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8:8618. doi: 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du X, Chen W, Wang Y, Chen C, Guo L, Ju R, Li J, Zhang D, Zhu L, Ye C. Therapeutic efficacy of carboxyamidotriazole on 2,4,6-trinitrobenzene sulfonic acid-induced colitis model is associated with the inhibition of NLRP3 inflammasome and NF-κB activation. Int Immunopharmacol. 2017;45:16–25. doi: 10.1016/j.intimp.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Zhou W, Liu X, Zhang X, Tang J, Li Z, Wang Q, Hu R. Oroxylin A inhibits colitis by inactivating NLRP3 inflammasome. Oncotarget. 2017;8:58903–58917. doi: 10.18632/oncotarget.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, Zhao Y, Yao J, Zhao L, Wu Z, Wang Y, Pan D, Miao H, Guo Q, Lu N. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem Pharmacol. 2015;94:142–154. doi: 10.1016/j.bcp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Huang J, Zheng Y, Ma J, Lu M, Ma X, Wang F, Tang X. Exploration of the Potential Mechanisms of Wumei Pill for the Treatment of Ulcerative Colitis by Network Pharmacology. Gastroenterol Res Pract. 2021;2021:4227668. doi: 10.1155/2021/4227668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucci M, Mannavola F, Passarelli A, Stucci LS, Cives M, Silvestris F. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget. 2018;9:20826–20837. doi: 10.18632/oncotarget.24846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ventimiglia LN, Fernández-Martín L, Martínez-Alonso E, Antón OM, Guerra M, Martínez-Menárguez JA, Andrés G, Alonso MA. Cutting Edge: Regulation of Exosome Secretion by the Integral MAL Protein in T Cells. J Immunol. 2015;195:810–814. doi: 10.4049/jimmunol.1500891. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miao C, Wang X, Zhou W, Huang J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res. 2021;169:105680. doi: 10.1016/j.phrs.2021.105680. [DOI] [PubMed] [Google Scholar]
- 56.Barile L, Moccetti T, Marbán E, Vassalli G. Roles of exosomes in cardioprotection. Eur Heart J. 2017;38:1372–1379. doi: 10.1093/eurheartj/ehw304. [DOI] [PubMed] [Google Scholar]
- 57.Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, Zheng Y. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–8944. doi: 10.7150/thno.62330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, Xu X, Zuo Y, Zhao Y, Wei YQ, Wei XW, Peng Y. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18:74. doi: 10.1186/s12943-019-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soares Martins T, Trindade D, Vaz M, Campelo I, Almeida M, Trigo G, da Cruz E Silva OAB, Henriques AG. Diagnostic and therapeutic potential of exosomes in Alzheimer's disease. J Neurochem. 2021;156:162–181. doi: 10.1111/jnc.15112. [DOI] [PubMed] [Google Scholar]
- 61.Han Z, Peng X, Yang Y, Yi J, Zhao D, Bao Q, Long S, Yu SX, Xu XX, Liu B, Liu YJ, Shen Y, Qiao L. Integrated microfluidic-SERS for exosome biomarker profiling and osteosarcoma diagnosis. Biosens Bioelectron. 2022;217:114709. doi: 10.1016/j.bios.2022.114709. [DOI] [PubMed] [Google Scholar]
- 62.He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duya P, Chen Y, Bai L, Li Z, Li J, Chai R, Bian Y, Zhao S. Nature products of traditional Chinese medicine provide new ideas in γδT cell for tumor immunotherapy. Acupunct Herbal Med. 2022;2:78–83. [Google Scholar]
- 64.Li Q, Wang H, Peng H, Huyan T, Cacalano NA. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers (Basel) 2019;11 doi: 10.3390/cancers11101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colletti M, Galardi A, De Santis M, Guidelli GM, Di Giannatale A, Di Luigi L, Antinozzi C. Exosomes in Systemic Sclerosis: Messengers Between Immune, Vascular and Fibrotic Components? Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, Xu W, Mao F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. 2020;95:1287–1307. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front Immunol. 2019;10:1464. doi: 10.3389/fimmu.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng X, Chen F, Zhang Q, Liu Y, You P, Sun S, Lin J, Chen N. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell. 2017;8:686–695. doi: 10.1007/s13238-017-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lian H, Zhong XS, Xiao Y, Sun Z, Shen Y, Zhao K, Ma X, Li Y, Niu Q, Liu M, Powell DW, Liu C, Li Q. Exosomal miR-29b of Gut Origin in Patients With Ulcerative Colitis Suppresses Heart Brain-Derived Neurotrophic Factor. Front Mol Biosci. 2022;9:759689. doi: 10.3389/fmolb.2022.759689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu AT, Lu JT, Ran ZH, Zheng Q. Exosome in intestinal mucosal immunity. J Gastroenterol Hepatol. 2016;31:1694–1699. doi: 10.1111/jgh.13413. [DOI] [PubMed] [Google Scholar]
- 71.Cai X, Zhang ZY, Yuan JT, Ocansey DKW, Tu Q, Zhang X, Qian H, Xu WR, Qiu W, Mao F. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther. 2021;12:416. doi: 10.1186/s13287-021-02492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D, Xue H, Tan J, Liu P, Qiao C, Pang C, Zhang L. Bone marrow mesenchymal stem cells-derived exosomes containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1 signalling to alleviate inflammatory bowel disease. Inflamm Res. 2022;71:833–846. doi: 10.1007/s00011-022-01577-z. [DOI] [PubMed] [Google Scholar]
- 73.Chang Y, Zhang Y, Jiang Y, Zhao L, Lv C, Huang Q, Guan J, Jin S. From Hair to Colon: Hair Follicle-Derived MSCs Alleviate Pyroptosis in DSS-Induced Ulcerative Colitis by Releasing Exosomes in a Paracrine Manner. Oxid Med Cell Longev. 2022;2022:9097530. doi: 10.1155/2022/9097530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Y, Tang X, Fang A, Yan J, Kofi Wiredu Ocansey D, Zhang X, Mao F. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int Immunopharmacol. 2022;110:108925. doi: 10.1016/j.intimp.2022.108925. [DOI] [PubMed] [Google Scholar]
- 75.Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, Hashemi SM. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236:5906–5920. doi: 10.1002/jcp.30275. [DOI] [PubMed] [Google Scholar]
- 76.Ocansey DKW, Zhang Z, Xu X, Liu L, Amoah S, Chen X, Wang B, Zhang X, Mao F. Mesenchymal stem cell-derived exosome mitigates colitis via the modulation of the gut metagenomics-metabolomics-farnesoid X receptor axis. Biomater Sci. 2022;10:4822–4836. doi: 10.1039/d2bm00559j. [DOI] [PubMed] [Google Scholar]
- 77.Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X, Lan P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Pei B, Yan J, Xu X, Fang AN, Ocansey DKW, Zhang X, Qian H, Xu W, Mao F. hucMSC-Derived Exosomes Alleviate the Deterioration of Colitis via the miR-146a/SUMO1 Axis. Mol Pharm. 2022;19:484–493. doi: 10.1021/acs.molpharmaceut.1c00450. [DOI] [PubMed] [Google Scholar]
- 79.Qian W, Huang L, Xu Y, Lu W, Wen W, Guo Z, Zhu W, Li Y. Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm Bowel Dis. 2023;29:602–619. doi: 10.1093/ibd/izac225. [DOI] [PubMed] [Google Scholar]
- 80.Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Mirsanei Z, Hashemi SM. Regulation of the Th17/Treg balance by human umbilical cord mesenchymal stem cell-derived exosomes protects against acute experimental colitis. Exp Cell Res. 2022;419:113296. doi: 10.1016/j.yexcr.2022.113296. [DOI] [PubMed] [Google Scholar]
- 81.Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y, Zhang X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315. doi: 10.1186/s13287-021-02404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang R, Huang H, Cui S, Zhou Y, Zhang T. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020;11:603. doi: 10.1038/s41419-020-02788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu F, Wei C, Wu H, Shuai B, Yu T, Gao F, Yuan Y, Zuo D, Liu X, Zhang L, Fan H. Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int Immunopharmacol. 2022;113:109426. doi: 10.1016/j.intimp.2022.109426. [DOI] [PubMed] [Google Scholar]
- 84.Gu L, Ren F, Fang X, Yuan L, Liu G, Wang S. Exosomal MicroRNA-181a Derived From Mesenchymal Stem Cells Improves Gut Microbiota Composition, Barrier Function, and Inflammatory Status in an Experimental Colitis Model. Front Med (Lausanne) 2021;8:660614. doi: 10.3389/fmed.2021.660614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma ZJ, Wang YH, Li ZG, Wang Y, Li BY, Kang HY, Wu XY. Immunosuppressive Effect of Exosomes from Mesenchymal Stromal Cells in Defined Medium on Experimental Colitis. Int J Stem Cells. 2019;12:440–448. doi: 10.15283/ijsc18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, Xu H, Rui K, Yin K, Wang S. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Experimental Colitis via Modulating Th1/Th17 and Treg Cell Responses. Front Immunol. 2020;11:598322. doi: 10.3389/fimmu.2020.598322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnhoorn MC, Plug L, Jonge ESMM, Molenkamp D, Bos E, Schoonderwoerd MJA, Corver WE, van der Meulen-de Jong AE, Verspaget HW, Hawinkels LJAC. Mesenchymal Stromal Cell-Derived Exosomes Contribute to Epithelial Regeneration in Experimental Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2020;9:715–717.e8. doi: 10.1016/j.jcmgh.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X, Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y, Qin H, Sun C, Shao B, Li G, Qin Y, Kong D, Ren S, Wang H, Wang Z, Zhang J. Endometrial Regenerative Cell-Derived Exosomes Attenuate Experimental Colitis through Downregulation of Intestine Ferroptosis. Stem Cells Int. 2022;2022:3014123. doi: 10.1155/2022/3014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang CL, Chen CH, Chiang JY, Sun CK, Chen YL, Chen KH, Sung PH, Huang TH, Li YC, Chen HH, Yip HK. Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res. 2019;11:2706–2724. [PMC free article] [PubMed] [Google Scholar]
- 92.Bauer KM, Nelson MC, Tang WW, Chiaro TR, Brown DG, Ghazaryan A, Lee SH, Weis AM, Hill JH, Klag KA, Tran VB, Thompson JW, Ramstead AG, Monts JK, Marvin JE, Alexander M, Voth WP, Stephens WZ, Ward DM, Petrey AC, Round JL, O'Connell RM. CD11c+ myeloid cell exosomes reduce intestinal inflammation during colitis. JCI Insight. 2022;7 doi: 10.1172/jci.insight.159469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, Lin D, Liu J, Xie H, Sun X, Wu Z. Exosomes Derived from Dendritic Cells Treated with Schistosoma japonicum Soluble Egg Antigen Attenuate DSS-Induced Colitis. Front Pharmacol. 2017;8:651. doi: 10.3389/fphar.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang X, Meng S, Jiang H, Chen T, Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 2010;45:1168–1177. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 95.Rao Q, Ma G, Li M, Wu H, Zhang Y, Zhang C, Ma Z, Huang L. Targeted delivery of triptolide by dendritic cell-derived exosomes for colitis and rheumatoid arthritis therapy in murine models. Br J Pharmacol. 2023;180:330–346. doi: 10.1111/bph.15958. [DOI] [PubMed] [Google Scholar]
- 96.Deng F, Yan J, Lu J, Luo M, Xia P, Liu S, Wang X, Zhi F, Liu D. M2 Macrophage-Derived Exosomal miR-590-3p Attenuates DSS-Induced Mucosal Damage and Promotes Epithelial Repair via the LATS1/YAP/ β-Catenin Signalling Axis. J Crohns Colitis. 2021;15:665–677. doi: 10.1093/ecco-jcc/jjaa214. [DOI] [PubMed] [Google Scholar]
- 97.Lu J, Liu D, Tan Y, Deng F, Li R. M1 Macrophage exosomes MiR-21a-5p aggravates inflammatory bowel disease through decreasing E-cadherin and subsequent ILC2 activation. J Cell Mol Med. 2021;25:3041–3050. doi: 10.1111/jcmm.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang C, Zhang M, Sung J, Wang L, Jung Y, Merlin D. Autologous Exosome Transfer: A New Personalised Treatment Concept to Prevent Colitis in a Murine Model. J Crohns Colitis. 2020;14:841–855. doi: 10.1093/ecco-jcc/jjz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei M, Gao X, Liu L, Li Z, Wan Z, Dong Y, Chen X, Niu Y, Zhang J, Yang G. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano. 2020;14:5099–5110. doi: 10.1021/acsnano.0c01860. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, Huang J, Li H, Li P, Xu C. Serum exosomes derived from Hp-positive gastritis patients inhibit MCP-1 and MIP-1α expression via NLRP12-Notch signaling pathway in intestinal epithelial cells and improve DSS-induced colitis in mice. Int Immunopharmacol. 2020;88:107012. doi: 10.1016/j.intimp.2020.107012. [DOI] [PubMed] [Google Scholar]
- 101.Wong WY, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP, Tai WC. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 2016;16:1131–1145. doi: 10.1002/pmic.201500174. [DOI] [PubMed] [Google Scholar]
- 102.Guo Y, Xu C, Gong R, Hu T, Zhang X, Xie X, Chi J, Li H, Xia X, Liu X. Exosomal CagA from Helicobacter pylori aggravates intestinal epithelium barrier dysfunction in chronic colitis by facilitating Claudin-2 expression. Gut Pathog. 2022;14:13. doi: 10.1186/s13099-022-00486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stremmel W, Weiskirchen R, Melnik BC. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm Intest Dis. 2020;5:117–123. doi: 10.1159/000507626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reif S, Elbaum-Shiff Y, Koroukhov N, Shilo I, Musseri M, Golan-Gerstl R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients. 2020;12 doi: 10.3390/nu12092589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han G, Cho H, Kim H, Jang Y, Jang H, Kim DE, Kim ES, Kim EH, Hwang KY, Kim K, Yang Y, Kim SH. Bovine colostrum derived-exosomes prevent dextran sulfate sodium-induced intestinal colitis via suppression of inflammation and oxidative stress. Biomater Sci. 2022;10:2076–2087. doi: 10.1039/d1bm01797g. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D, Zheng M, Chen Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140–150. doi: 10.1016/j.ebiom.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L, Wang Y, Shi Y, Fang C, Mei S, Chen Q, Zhao J, Lin X, Fan S, Jin Y, Chen P. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1–15. doi: 10.1016/j.freeradbiomed.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 108.Tavakoli Dargani Z, Singla DK. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ Physiol. 2019;317:H460–H471. doi: 10.1152/ajpheart.00056.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang K, Ru J, Zhang H, Chen J, Lin X, Lin Z, Wen M, Huang L, Ni H, Zhuge Q, Yang S. Melatonin Enhances the Therapeutic Effect of Plasma Exosomes Against Cerebral Ischemia-Induced Pyroptosis Through the TLR4/NF-κB Pathway. Front Neurosci. 2020;14:848. doi: 10.3389/fnins.2020.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeng Q, Zhou Y, Liang D, He H, Liu X, Zhu R, Zhang M, Luo X, Wang Y, Huang G. Exosomes Secreted From Bone Marrow Mesenchymal Stem Cells Attenuate Oxygen-Glucose Deprivation/Reoxygenation-Induced Pyroptosis in PC12 Cells by Promoting AMPK-Dependent Autophagic Flux. Front Cell Neurosci. 2020;14:182. doi: 10.3389/fncel.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, Zhu B, Zhao R, Yu XY, Li Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728–6742. doi: 10.7150/thno.42259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun L, Zhu M, Feng W, Lin Y, Yin J, Jin J, Wang Y. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid Med Cell Longev. 2019;2019:4506303. doi: 10.1155/2019/4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson's disease. Mol Ther Nucleic Acids. 2021;23:1334–1344. doi: 10.1016/j.omtn.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y, Xie W, Liu B, Huang H, Luo W, Zhang Y, Pan X, Yu XY, Shen Z, Li Y. Stem cell-derived exosomes repair ischemic muscle injury by inhibiting the tumor suppressor Rb1-mediated NLRP3 inflammasome pathway. Signal Transduct Target Ther. 2021;6:121. doi: 10.1038/s41392-021-00520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singla DK, Johnson TA, Tavakoli Dargani Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells. 2019;8 doi: 10.3390/cells8101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng HY, Hsieh CH, Lin PH, Chen YT, Hsu DS, Tai SK, Chu PY, Yang MH. Snail-regulated exosomal microRNA-21 suppresses NLRP3 inflammasome activity to enhance cisplatin resistance. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y, Chen Y, Wang Z, Xu C, Qiao S, Liu T, Qi K, Tong D, Li C. Bone Marrow Mesenchymal Stem Cell Exosome Attenuates Inflammasome-Related Pyroptosis via Delivering circ_003564 to Improve the Recovery of Spinal Cord Injury. Mol Neurobiol. 2022;59:6771–6789. doi: 10.1007/s12035-022-03006-y. [DOI] [PubMed] [Google Scholar]
- 118.Liang C, Liu Y, Xu H, Huang J, Shen Y, Chen F, Luo M. Exosomes of Human Umbilical Cord MSCs Protect Against Hypoxia/Reoxygenation-Induced Pyroptosis of Cardiomyocytes via the miRNA-100-5p/FOXO3/NLRP3 Pathway. Front Bioeng Biotechnol. 2020;8:615850. doi: 10.3389/fbioe.2020.615850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu J, Jiang Y, Wu X, Wu Z, Qin J, Zhao Z, Li B, Xu Z, Lu X, Wang X, Liu X. Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome. Stem Cell Res Ther. 2022;13:349. doi: 10.1186/s13287-022-03037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ye L, Guo H, Wang Y, Peng Y, Zhang Y, Li S, Yang M, Wang L. Exosomal circEhmt1 Released from Hypoxia-Pretreated Pericytes Regulates High Glucose-Induced Microvascular Dysfunction via the NFIA/NLRP3 Pathway. Oxid Med Cell Longev. 2021;2021:8833098. doi: 10.1155/2021/8833098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Wang Q, Su H, Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J Biosci Bioeng. 2021;131:671–678. doi: 10.1016/j.jbiosc.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 123.Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma Y, Jiang J, Wang J, Zhong L, Chen M, Wang H, Hou Y, Zhu R, Zhang Q. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. 2019;508:735–741. doi: 10.1016/j.bbrc.2018.11.189. [DOI] [PubMed] [Google Scholar]
- 124.Kang X, Jiang L, Chen X, Wang X, Gu S, Wang J, Zhu Y, Xie X, Xiao H, Zhang J. Exosomes derived from hypoxic bone marrow mesenchymal stem cells rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis. Exp Cell Res. 2021;405:112635. doi: 10.1016/j.yexcr.2021.112635. [DOI] [PubMed] [Google Scholar]
- 125.Hua T, Yang M, Song H, Kong E, Deng M, Li Y, Li J, Liu Z, Fu H, Wang Y, Yuan H. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J Nanobiotechnology. 2022;20:324. doi: 10.1186/s12951-022-01522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu H, Qi L, Ren C, Yan S. M2 Macrophage-Derived Exosomes Regulate Myocardial Ischemia-Reperfusion And Pyroptosis Via ROS/NLRP3 Pathway. Heart Surg Forum. 2022;25:E698–E708. doi: 10.1532/hsf.4919. [DOI] [PubMed] [Google Scholar]
- 127.Dessouki FBA, Kukreja RC, Singla DK. Stem Cell-Derived Exosomes Ameliorate Doxorubicin-Induced Muscle Toxicity through Counteracting Pyroptosis. Pharmaceuticals (Basel) 2020;13 doi: 10.3390/ph13120450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bottino LZMF, Rodrigues-Junior DM, Farias IS, Branco LM, Iyer NG, de Albuquerque GE, Vettore AL, Bortoluci KR. Extracellular vesicles derived from head and neck squamous cells carcinoma inhibit NLRP3 inflammasomes. Curr Res Immunol. 2021;2:175–183. doi: 10.1016/j.crimmu.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu LT, Wang BY, Fan YH, He ZY, Zheng WX. Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage. Neural Regen Res. 2023;18:560–567. doi: 10.4103/1673-5374.346551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun Z, Gao Z, Wu J, Zheng X, Jing S, Wang W. MSC-Derived Extracellular Vesicles Activate Mitophagy to Alleviate Renal Ischemia/Reperfusion Injury via the miR-223-3p/NLRP3 Axis. Stem Cells Int. 2022;2022:6852661. doi: 10.1155/2022/6852661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang T, Li W, Peng A, Wang Q. Exosomes derived from heat shock preconditioned bone marrow mesenchymal stem cells alleviate cisplatin-induced ototoxicity in mice. J Biol Eng. 2022;16:24. doi: 10.1186/s13036-022-00304-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang W, Wang Y, Kong Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294–303. doi: 10.1167/iovs.18-25617. [DOI] [PubMed] [Google Scholar]
- 133.Qian J, Wang X, Su G, Shu X, Huang Z, Jiang H, Zhu Q. Platelet-rich plasma-derived exosomes attenuate intervertebral disc degeneration by promoting NLRP3 autophagic degradation in macrophages. Int Immunopharmacol. 2022;110:108962. doi: 10.1016/j.intimp.2022.108962. [DOI] [PubMed] [Google Scholar]
- 134.Huang Y, Lu D, Ma W, Liu J, Ning Q, Tang F, Li L. miR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages. Mol Immunol. 2022;143:68–76. doi: 10.1016/j.molimm.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Cui B, Sun J, Li SP, Zhou GP, Chen XJ, Sun LY, Wei L, Zhu ZJ. CD80(+) dendritic cell derived exosomes inhibit CD8(+) T cells through down-regulating NLRP3 expression after liver transplantation. Int Immunopharmacol. 2022;109:108787. doi: 10.1016/j.intimp.2022.108787. [DOI] [PubMed] [Google Scholar]
- 136.Dai Y, Wang S, Chang S, Ren D, Shali S, Li C, Yang H, Huang Z, Ge J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 137.Xia Y, Zhou K, Sun M, Shu R, Qian J, Xie Y. The miR-223-3p Regulates Pyroptosis Through NLRP3-Caspase 1-GSDMD Signal Axis in Periodontitis. Inflammation. 2021;44:2531–2542. doi: 10.1007/s10753-021-01522-y. [DOI] [PubMed] [Google Scholar]
- 138.Jiao Y, Zhang T, Zhang C, Ji H, Tong X, Xia R, Wang W, Ma Z, Shi X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit Care. 2021;25:356. doi: 10.1186/s13054-021-03775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, Zhao W, Geng B. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. 2020;39:176. doi: 10.1186/s13046-020-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang W, Ma Y, Zhang Y, Yang J, He G, Chen S. Photo-Oxidative Blue-Light Stimulation in Retinal Pigment Epithelium Cells Promotes Exosome Secretion and Increases the Activity of the NLRP3 Inflammasome. Curr Eye Res. 2019;44:67–75. doi: 10.1080/02713683.2018.1518458. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L, Liu H, Jia L, Lyu J, Sun Y, Yu H, Li H, Liu W, Weng Y, Yu W. Exosomes Mediate Hippocampal and Cortical Neuronal Injury Induced by Hepatic Ischemia-Reperfusion Injury through Activating Pyroptosis in Rats. Oxid Med Cell Longev. 2019;2019:3753485. doi: 10.1155/2019/3753485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu XB, Sun HY, Luo ZL, Cheng L, Duan XM, Ren JD. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165685. doi: 10.1016/j.bbadis.2020.165685. [DOI] [PubMed] [Google Scholar]
- 143.Sur S, Steele R, Isbell TS, Ray R, Ray RB. Circulatory Exosomes from COVID-19 Patients Trigger NLRP3 Inflammasome in Endothelial Cells. mBio. 2022;13:e0095122. doi: 10.1128/mbio.00951-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hou CC, Ma HF, Ye JF, Luo D, Bao HF, Guan JL. Plasma exosomes derived from patients with intestinal Behçet's syndrome induce intestinal epithelial cell pyroptosis. Clin Rheumatol. 2021;40:4143–4155. doi: 10.1007/s10067-021-05755-y. [DOI] [PubMed] [Google Scholar]
- 145.Li H, Lu R, Pang Y, Li J, Cao Y, Fu H, Fang G, Chen Q, Liu B, Wu J, Zhou Y, Zhou J. Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-κB/NLRP3 Pathway. Front Pharmacol. 2020;11:1080. doi: 10.3389/fphar.2020.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi X, Xie X, Sun Y, He H, Huang H, Liu Y, Wu H, Dai M. Paeonol inhibits NLRP3 mediated inflammation in rat endothelial cells by elevating hyperlipidemic rats plasma exosomal miRNA-223. Eur J Pharmacol. 2020;885:173473. doi: 10.1016/j.ejphar.2020.173473. [DOI] [PubMed] [Google Scholar]
- 147.Bai L, Li J, Li H, Song J, Zhou Y, Lu R, Liu B, Pang Y, Zhang P, Chen J, Liu X, Wu J, Liang C, Zhou J. Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem Pharmacol. 2019;169:113619. doi: 10.1016/j.bcp.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 148.Cypryk W, Nyman TA, Matikainen S. From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front Immunol. 2018;9:2188. doi: 10.3389/fimmu.2018.02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT, Weinman SA. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219 doi: 10.1083/jcb.201912074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, Ochi M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16:R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang J, Zhang L, Luo X, Ma X, Wang G, Yang Y, Yan Y, Qian H, Zhang X, Xu W, Mao F. Systematic Exposition of Mesenchymal Stem Cell for Inflammatory Bowel Disease and Its Associated Colorectal Cancer. Biomed Res Int. 2018;2018:9652817. doi: 10.1155/2018/9652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asami T, Ishii M, Fujii H, Namkoong H, Tasaka S, Matsushita K, Ishii K, Yagi K, Fujiwara H, Funatsu Y, Hasegawa N, Betsuyaku T. Modulation of murine macrophage TLR7/8-mediated cytokine expression by mesenchymal stem cell-conditioned medium. Mediators Inflamm. 2013;2013:264260. doi: 10.1155/2013/264260. [DOI] [PMC free article] [PubMed] [Google Scholar]