Abstract
BACKGROUND
Early screening and accurate staging of diabetic retinopathy (DR) can reduce blindness risk in type 2 diabetes patients. DR’s complex pathogenesis involves many factors, making ophthalmologist screening alone insufficient for prevention and treatment. Often, endocrinologists are the first to see diabetic patients and thus should screen for retinopathy for early intervention.
AIM
To explore the efficacy of non-mydriatic fundus photography (NMFP)-enhanced telemedicine in assessing DR and its various stages.
METHODS
This retrospective study incorporated findings from an analysis of 93 diabetic patients, examining both NMFP-assisted telemedicine and fundus fluorescein angiography (FFA). It focused on assessing the concordance in DR detection between these two methodologies. Additionally, receiver operating characteristic (ROC) curves were generated to determine the optimal sensitivity and specificity of NMFP-assisted telemedicine, using FFA outcomes as the standard benchmark.
RESULTS
In the context of DR diagnosis and staging, the kappa coefficients for NMFP-assisted telemedicine and FFA were recorded at 0.775 and 0.689 respectively, indicating substantial intermethod agreement. Moreover, the NMFP-assisted telemedicine’s predictive accuracy for positive FFA outcomes, as denoted by the area under the ROC curve, was remarkably high at 0.955, within a confidence interval of 0.914 to 0.995 and a statistically significant P-value of less than 0.001. This predictive model exhibited a specificity of 100%, a sensitivity of 90.9%, and a Youden index of 0.909.
CONCLUSION
NMFP-assisted telemedicine represents a pragmatic, objective, and precise modality for fundus examination, particularly applicable in the context of endocrinology inpatient care and primary healthcare settings for diabetic patients. Its implementation in these scenarios is of paramount significance, enhancing the clinical accuracy in the diagnosis and therapeutic management of DR. This methodology not only streamlines patient evaluation but also contributes substantially to the optimization of clinical outcomes in DR management.
Keywords: Diabetes, Diabetic retinopathy, Non-mydriatic fundus photography-assisted telemedicine, Fundus fluorescein angiography
Core Tip: There is a high consistency between non-mydriatic fundus photography (NMFP)-assisted telemedicine and fundus fluorescein angiography (FFA) techniques. The area under the curve of NMFP-assisted telemedicine results for predicting a positive result from FFA was 0.955. The specificity, sensitivity, and a Youden index of NMFP-assisted telemedicine were 100%, 90.9% and 0.909, respectively. The NMFP-assisted telemedicine has a great significant value in the clinical diagnosis and treatment of diabetic retinopathy.
INTRODUCTION
Diabetic retinopathy (DR), a leading ocular pathology causing visual impairment, demonstrates an increased incidence and rate of blindness that correlate directly with the duration of diabetes and patient age[1,2]. The current global prevalence of DR among diabetic individuals is estimated at 34.6%, with a significant 10.2% progressing to severe visual impairment[3]. In China, approximately 87% of diabetic patients are treated in primary healthcare facilities, where ophthalmological resources are critically limited, and alarmingly, around 70% of these individuals do not receive standardized ophthalmic examinations[4]. Endocrinology departments typically serve as the initial consultation point for diabetic patients. However, there is a noted emphasis on metabolic markers such as glycemia and lipidemia, while fundus complications are often underprioritized. This leads to delayed referrals to ophthalmology services, usually at advanced stages of vision loss or blindness. At this juncture, the visual impairment induced by DR is largely irreversible, even with prompt and aggressive therapeutic interventions, significantly impacting patient quality of life and imposing substantial socio-economic burdens. Early and accurate diagnosis, followed by timely therapeutic intervention, is pivotal in arresting or mitigating the progression of DR[5]. DR can be divided into non-proliferative and proliferative types based on its clinical stage. Early screening for DR has become a priority in blindness prevention. Clinically, DR is stratified into non-proliferative and proliferative phases. Therefore, the development of an efficient and straightforward screening protocol for DR, especially tailored for endocrinologists and primary care practitioners, is imperative[6,7]. Fundus fluorescein angiography (FFA) is regarded as the gold standard for DR diagnosis. However, its invasive nature makes it unsuitable for mass DR screening. This study aims to evaluate the effectiveness of non-mydriatic fundus photography (NMFP)-assisted telemedicine in assessing DR and its various stages by assessing the concordance between NMFP-assisted telemedicine and FFA in diagnosing and staging DR.
MATERIALS AND METHODS
Subjects
Clinical data from diabetic patients diagnosed at the First Affiliated Hospital of University of Science and Technology of China between June 2019 and June 2021 were subject to a retrospective analysis. The study cohort consisted of 93 individuals (42 females and 51 males), each diagnosed through NMFP-assisted telemedicine and FFA. These patients were categorized based on the International Federation of Ophthalmological Societies’ 2002 guidelines for DR screening and staging: Stage I involved no evident retinopathy, only minor hemorrhages in the posterior pole; stage II featured scattered punctate hyperfluorescent spots with capillary hemangiomas; stage III mirrored stage II in symptoms; stage IV presented with fundus or vitreous hemorrhage due to neovascularization; stage V included fundus neovascularization accompanied by fibrous proliferation; and stage VI was characterized by both neovascularization and fibrous proliferation in the fundus, along with tractional retinal detachment. Stages I to III were classified under non-proliferative DR, while stages IV to VI fell under the proliferative DR category. Cases with no abnormal fundus findings were designated as no DR (NDR). This study adhered to the World Medical Association’ Declaration of Helsinki and received approval from the ethics committee of the First Affiliated Hospital of University of Science and Technology of China.
Methods
FFA: Patients initially underwent an allergy test following mydriasis. Those who exhibited no allergic reaction proceeded to receive a rapid intravenous injection of sodium fluorescein. Subsequently, their fundus was imaged using the Heidelberg confocal laser angiography system (Global Vision, Germany), a sophisticated apparatus designed for detailed fundus examination.
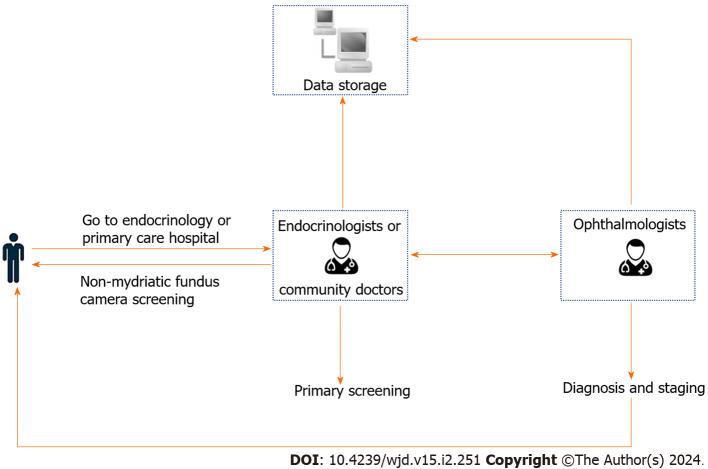
NMFP-assisted telemedicine: A skilled endocrinology technician, in a controlled darkroom setting, utilized the Optomed Aurora® handheld non-mydriatic fundus camera to capture detailed images of the patients’ fundus. Following a brief acclimatization period of five minutes in the examination room, two high-resolution color images were taken of each fundus’ posterior pole. These images focused on the macula and optic disc, covering a 45° field of view. To ensure optimal image quality, patients were given a five-minute rest period after capturing the image of one eye, followed by imaging of the other eye post-pupil recovery. Subsequently, these fundus images, along with patient data, were uploaded to a specialized diagnostic platform. Here, an ophthalmologist employed the platform’s advanced software for comprehensive analysis, diagnosis, and staging of the fundus condition (Figures 1 and 2).
Figure 1.
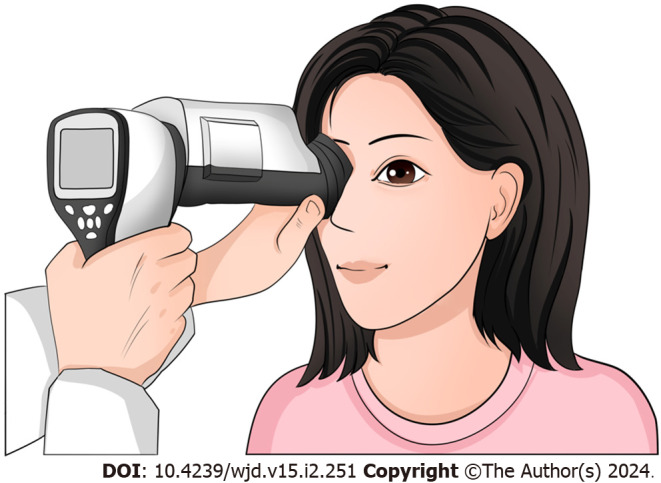
Operation of the non-mydriatic portable fundus camera when performing fundus examination.
Figure 2.
Application of non-mydriatic fundus photography-assisted telemedicine.
Observational indicators
The study rigorously assessed the alignment between NMFP-assisted telemedicine and FFA in diagnosing and staging DR. Furthermore, it scrutinized the diagnostic efficacy of NMFP-assisted telemedicine in terms of sensitivity and specificity for DR, utilizing FFA results as the standard reference.
Statistical analysis
Statistical analysis was executed utilizing SPSS 23.0 software. The Kappa (κ) test was applied to evaluate the congruence between NMFP-assisted telemedicine and FFA test results, with κ values interpreted as follows: > 0.75 denoting exceptional concordance, 0.61-0.75 suggesting significant consistency, 0.41-0.60 indicating moderate agreement, and < 0.40 reflecting limited consistency. To ascertain the diagnostic predictive efficacy of NMFP-assisted telemedicine, receiver operating characteristic (ROC) curves were employed. A P value of less than 0.05 was set as the threshold for statistical significance.
RESULTS
The consistency check between NMFP-assisted telemedicine and FFA in the screening of DR
Of 23 patients (24.7%) were diagnosed with NDR and 70 patients (75.3%) with DR using NMFP-assisted telemedicine, whereas, FFA detected NDR in 16 patients (17.2%) and DR in 77 patients (82.8%). κ test analysis suggested consistency DR screening results between NMFP-assisted telemedicine and FFA with a κ value of 0.775 (P < 0.001) (Table 1).
Table 1.
Kappa test analysis between non-mydriatic fundus photography assisted telemedicine and fundus fluorescein angiography in the screening of diabetic retinopathy
|
Fundus fluorescein angiography
|
Non-mydriatic fundus photography-assisted telemedicine
|
Total (n)
|
|
|
NDR
|
DR
|
||
| NDR | 16 | 0 | 16 |
| DR | 7 | 70 | 77 |
| Total (n) | 23 | 70 | 93 |
NDR: No diabetic retinopathy; DR: Diabetic retinopathy.
The consistency check between NMFP-assisted telemedicine and FFA in the staging and diagnosis of DR
The 70 positive cases, diagnosed using both techniques, were categorized into stages I–VI, with 52 cases showing identical staging results. Among the patients diagnosed using NMFP-assisted telemedicine, the distribution across DR stages I, II, III, IV, V, and VI was 20, 16, 17, 11, 4, and 2, respectively. In contrast, for those diagnosed using FFA, the numbers were 15, 18, 21, 10, 5, and 1, respectively. The proportion of stage I patients diagnosed using NMFP-assisted telemedicine was slightly higher than that using FFA, while the proportion of patients at DR stages II and III diagnosed using NMFP-assisted telemedicine was lower than that using FFA. The κ test analysis indicated concordance between the results of NMFP-assisted telemedicine and FFA in the diagnosis and staging of DR, with a κ value of 0.689 (P < 0.001) (Table 2).
Table 2.
Kappa test analysis between non-mydriatic fundus photography assisted telemedicine and fundus fluorescein angiography in the staging and diagnosis of diabetic retinopathy
|
Fundus fluorescein angiography
|
No-mydriatic fundus photography-assisted telemedicine
|
Total (n)
|
|||||
|
I
|
II
|
III
|
IV
|
V
|
VI
|
||
| I | 15 | 0 | 0 | 0 | 0 | 0 | 15 |
| II | 2 | 13 | 1 | 2 | 0 | 0 | 18 |
| III | 3 | 3 | 15 | 0 | 0 | 0 | 21 |
| IV | 0 | 0 | 1 | 8 | 1 | 0 | 10 |
| V | 0 | 0 | 0 | 1 | 2 | 2 | 5 |
| VI | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total (n) | 20 | 16 | 17 | 11 | 4 | 2 | 70 |
Diagnostic prediction effectiveness of NMFP-assisted telemedicine by ROC curve
ROC analysis was conducted using the FFA result as the dependent variable (DR assignment = 1, NDR assignment = 0) and the NMFP-assisted telemedicine result (DR assignment = 1, NDR assignment = 0) as the independent variable. The area under the curve for NMFP-assisted telemedicine in predicting a positive result from FFA was 0.955 (0.914, 0.995; P < 0.001), with a specificity of 100%, sensitivity of 90.9%, and a Youden index of 0.909 (Figure 3).
Figure 3.
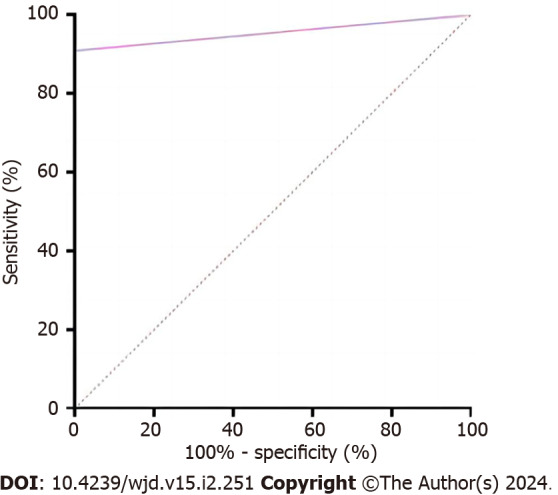
The receiver operating characteristic curve identified diagnostic prediction effectiveness of non-mydriatic fundus photography-assisted telemedicine (area under the curve 0.955 with 90.9% sensitivity and 100% specificity).
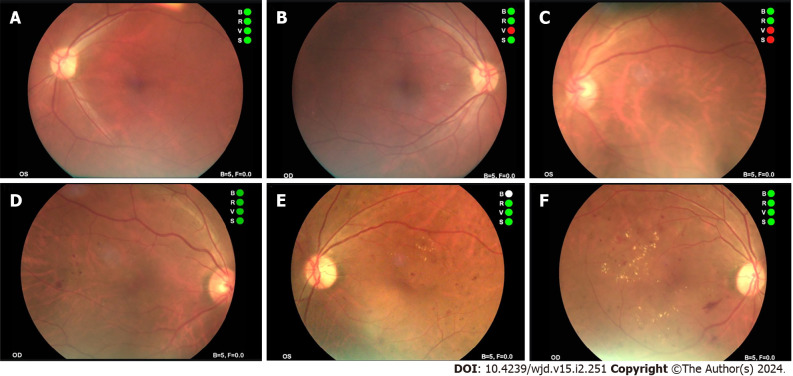
Typical fundus photos in different stages
Typical fundus photos in different stages examined by NMFP-assisted telemedicine were exhibited in Figure 4.
Figure 4.
Typical fundus photos in different stages of non-mydriatic fundus photography-assisted telemedicine. A and B: Photographs of normal fundus; C and D: Fundus photograph shows microhemangioma and retinal hemorrhage; E and F: Fundus photograph shows retinal hemorrhage and retinal hard exudates.
DISCUSSION
Traditional DR screening methods, including direct fundoscopy, indirect fundoscopy, and slit-lamp with preset lens methods, are known for their simplicity, speed, and patient cooperation[8,9]. However, these methods often exhibit poor accuracy and are not well-suited for mass DR screening. On the other hand, FFA, recognized as one of the most effective early DR detection methods, enables physicians to observe capillary non-perfusion patterns and disruptions in the blood-retinal barrier, providing insights into the source of macular edema-related leakage[10,11]. Consequently, FFA is regarded as the gold standard for DR diagnosis. Nevertheless, its invasive nature makes it unsuitable for pregnant women, patients with contrast allergies, or those with concurrent systemic illnesses, thereby limiting its applicability in mass DR screening.
NMFP, a technique that employs low-density light to capture high-resolution fundus images by enhancing camera sensitivity, has been utilized for fundus examinations since the 1980s[12]. Initially, Polaroid film and 35 mm transparencies were used, but technological advancements have significantly improved NMFP, resulting in high-quality, directly savable, and shareable images. This method offers several advantages, such as mydriasis-free operation, convenience, safety, and effectiveness. Numerous studies on DR screening have indicated that NMFP is a straightforward, objective, and cost-effective technique that enhances the efficiency of DR screening[13].
Research by Piyasena et al[14] demonstrated that NMFP surpasses mydriatic examination in sensitivity for detecting fundus lesions, particularly smaller ones like tiny retinal hemorrhages, microaneurysms, and neovascularization in various fundus areas[15]. Yaslam et al[16] also suggested that NMFP is more patient-friendly and can be widely employed for fundus examinations in both type 1 and type 2 diabetes cases. Dunn et al[17] showed that NMFP exhibits higher sensitivity in DR screening compared to direct fundoscopy, enhancing diagnostic accuracy for fundus pathology. In the current study, no significant difference was observed in the concordance rate between NMFP (75.3%) and FFA (82.8%), underscoring the substantial utility of NMFP in DR screening. When using FFA results as the gold standard, NMFP-assisted telemedicine demonstrated high diagnostic sensitivity and specificity in DR screening.
NMFP encompasses single-field, double-field, and seven-field photography, with most options offering a 45° field of view. Equipment models primarily include handheld, desktop, TV-type, and stereo cameras[18-21]. The advantages of the handheld NMFP employed in this study include its compact size, portability, and versatility to operate in various body positions, enabling high-definition fundus photography. Technological advancements and the widespread use of the internet have transformed DR screening into an efficient mode, with NMFP playing a pivotal role in this screening method. NMFP-assisted telemedicine relies on dedicated information transfer software tailored for remote screening of DR patients. In addition to storing and transmitting images, the software can record patients’ medical history and physical examination results from each visit, streamlining data extraction, comparison, retrieval, query, and analysis for subsequent consultations. The advantages of NMFP-assisted telemedicine include: (1) Enhanced feasibility: Elderly diabetic patients with a lengthy disease course, post-cataract surgery individuals, and patients with angle-closure glaucoma often struggle with mydriasis. NMFP-assisted telemedicine significantly improves the screening feasibility for patients with small pupils[22]; (2) Patient engagement: NMFP-assisted telemedicine allows the storage of fundus images, enabling patients to visualize changes in their fundus. This facilitates timely and effective health education, empowers patients with essential knowledge about DR, and helps them comprehend disease progression. Consequently, it enhances patient compliance and shifts the focus from disease treatment to disease prevention; (3) Improved compliance: By avoiding the mydriasis procedure in fundus examination, NMFP-assisted telemedicine enhances patient compliance; (4) Accessibility: NMFP-assisted telemedicine is user-friendly, portable, and suitable for bedside data collection, making it particularly valuable for fundus screening in pediatric patients or those with limited mobility[23]; (5) Versatile use: This straightforward procedure makes fundus screening accessible to non-ophthalmologists, including endocrinologists and community physicians; (6) Telemedicine potential: Combining NMFP with telemedicine holds great promise in emphasizing the significance of ocular fundus examinations in endocrinology inpatients and primary care hospitals. It facilitates access to ophthalmic consultative services and supports clinical and epidemiologic research[16,24]. Digital fundus photography and network technology enable the acquisition of patient information, remote screening, consultation, and diagnosis, coupled with digital storage and information sharing; and (7) Cost-effectiveness: For endocrinology departments, NMFP testing is cost-effective with reusable equipment. For patients, the cost of NMFP testing is lower and within the range covered by medical insurance, making NMFP more suitable for large-scale screening in DR diagnosis. However, NMFP also has some potential risks. For instance, in telemedicine systems, patient medical information and images are transmitted over the internet, which could lead to data security and privacy issues. Moreover, remote diagnosis may lack direct face-to-face interaction with patients. Therefore, in practical applications, doctors must be vigilant in protecting patient privacy and strive to explain the diagnostic results to the patients as clearly as possible.
The presence, severity, and staging of DR are pivotal factors in the screening of DR, particularly in primary care hospitals facing limitations in medical resources. These examination results play a critical role in determining whether patients should be referred to higher-level hospitals for further consultation and prompt treatment. This approach ensures the early detection of DR, timely intervention, and facilitates the implementation of a hospital-based hierarchical diagnosis and treatment system. A real-world, multicenter, and prospective study have demonstrated the efficacy of NMFP in both DR screening and grading[25]. In our study, the results obtained from NMFP-assisted telemedicine and FFA exhibited remarkable consistency across different DR stages. Besides screening for various fundus lesions, NMFP-assisted telemedicine also allows for DR staging. The proportion of patients in stage I diagnosed using NMFP-assisted telemedicine was slightly higher than those diagnosed using FFA. In contrast, for patients in stages II and III, NMFP-assisted telemedicine had a lower proportion of diagnoses compared to FFA. NMFP-assisted telemedicine can promptly identify early signs such as hard exudates and arterial aneurysms. However, FFA excels in providing a comprehensive characterization of retinal blood flow, including all aneurysms, identification of exudates and microhemorrhages, and observation of vascular permeability alterations. Consequently, NMFP-assisted telemedicine may occasionally yield false-negative results.
Limitations
This article still has some limitations and deficiencies. Firstly, the sample size is small, and secondly, it lacks a cross-sectional comparison with other DR screening methods, which will be a direction for future research.
CONCLUSION
In summary, early screening and accurate staging of DR can reduce the risk of blindness in patients with type 2 diabetes. The pathogenesis of DR is complex, with numerous factors affecting its onset and progression. Therefore, the prevention and treatment of DR cannot rely solely on screening by ophthalmologists. Because endocrinology is often the first department that diabetic patients visit, endocrinologists need to screen diabetic patients for retinopathy detection and early intervention. NMFP-assisted telemedicine can be comprehensively used to facilitate fundus examination among endocrinology inpatients and diabetic patients in primary hospitals. In addition, the captured images and basic information from NMFP-assisted telemedicine can be archived and uploaded to a software platform to help ophthalmologists diagnose and create medical reports, thereby enabling effective prevention, control, and management of diabetes and its fundus complications. Furthermore, the collected image data can be summarized, analyzed, stored digitally, and shared for big data analysis. Diabetic patients diagnosed using NMFP-assisted telemedicine can promptly observe their fundus lesions and gain essential knowledge about DR in a timely and effective manner, thereby improving their awareness of the disease and compliance with treatment and preventing the onset and progression of DR.
ARTICLE HIGHLIGHTS
Research background
Prompt detection and precise classification of diabetic retinopathy (DR) in individuals with type 2 diabetes can lessen the likelihood of blindness. Given DR’s intricate causes, relying solely on ophthalmologist examinations may not be enough for effective prevention and treatment. Since endocrinologists frequently encounter diabetic patients initially, they play a crucial role in early DR screening and intervention.
Research motivation
We endeavored to offer fresh perspectives on the screening approaches for DR.
Research objectives
This study investigates the effectiveness of telemedicine enhanced by non-mydriatic fundus photography (NMFP) in evaluating DR and its different stages.
Research methods
This study retrospectively analyzed 93 diabetic patients, comparing NMFP-assisted telemedicine with fundus fluorescein angiography (FFA) in detecting DR. It aimed to evaluate the agreement between these methods and used receiver operating characteristic (ROC) curves to assess the accuracy of NMFP against the FFA benchmark.
Research results
In diagnosing and staging DR, NMFP-assisted telemedicine and FFA showed substantial agreement with kappa coefficients of 0.775 and 0.689, respectively. NMFP’s predictive accuracy for positive FFA outcomes, indicated by a ROC curve area of 0.955 (confidence interval 0.914 to 0.995) and a P-value < 0.001, was high. The model demonstrated 100% specificity, 90.9% sensitivity, and a Youden index of 0.909.
Research conclusions
This study introduces NMFP-assisted telemedicine as a practical, accurate method for examining the fundus, especially suitable for endocrinology inpatient care and primary healthcare for diabetic patients. Its use in these settings is crucial for improving the accuracy of diagnosing and treating DR. This approach simplifies patient assessments and significantly improves clinical results in DR management. The new theories proposed by this study include the importance of integrating NMFP-assisted telemedicine into endocrinology and primary healthcare for enhanced DR management. The new method proposed is the application of NMFP-assisted telemedicine itself for fundus examination in diabetic patients.
Research perspectives
Future research should focus on expanding the use of NMFP-assisted telemedicine in various healthcare settings for DR management, and conducting larger, long-term studies to evaluate its effectiveness. Additionally, exploring technological improvements and interdisciplinary collaborations can enhance the accuracy and impact of this approach in DR diagnosis and treatment.
Footnotes
Institutional review board statement: This study was conducted in accordance with the tenets of the World Medical Association’s Declaration of Helsinki and had been approved by the ethics committee of the First Affiliated Hospital of University of Science and Technology of China.
Informed consent statement: All the subjects signed informed consent.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 23, 2023
First decision: December 8, 2023
Article in press: January 12, 2024
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Motlagh B, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
Contributor Information
Wan Zhou, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China. zwan@ustc.edu.cn.
Xiao-Jing Yuan, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Jie Li, Department of Endocrinology, Anhui Provincial Hospital, Affiliated to Anhui Medical University, Hefei 230001, Anhui Province, China.
Wei Wang, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Hao-Qiang Zhang, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Yuan-Yuan Hu, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Shan-Dong Ye, Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Data sharing statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Chalke SD, Kale PP. Combinational Approaches Targeting Neurodegeneration, Oxidative Stress, and Inflammation in the Treatment of Diabetic Retinopathy. Curr Drug Targets. 2021;22:1810–1824. doi: 10.2174/1389450122666210319113136. [DOI] [PubMed] [Google Scholar]
- 2.Lamacchia O, Sorrentino MR, Picca G, Paradiso M, Maiellaro P, De Cosmo S. Cardio-ankle vascular index is associated with diabetic retinopathy in younger than 70 years patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;155:107793. doi: 10.1016/j.diabres.2019.107793. [DOI] [PubMed] [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha M, Choi SY, Kim M, Na JK, Park YH. Diabetic Nephropathy in Type 2 Diabetic Retinopathy Requiring Panretinal Photocoagulation. Korean J Ophthalmol. 2019;33:46–53. doi: 10.3341/kjo.2018.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018;63:601–608. doi: 10.1016/j.survophthal.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HV, Tan GS, Tapp RJ, Mital S, Ting DS, Wong HT, Tan CS, Laude A, Tai ES, Tan NC, Finkelstein EA, Wong TY, Lamoureux EL. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology. 2016;123:2571–2580. doi: 10.1016/j.ophtha.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Chen R, Lu Y, Dou X, Ye B, Cai Z, Pu Z, Mou L. Single-Field Non-Mydriatic Fundus Photography for Diabetic Retinopathy Screening: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2019;62:61–67. doi: 10.1159/000499106. [DOI] [PubMed] [Google Scholar]
- 8.Mujeeb S, Rodrigues GR, Nayak RR, Kamath AR, Kamath SJ, Kamath G. Urine protein: Urine creatinine ratio correlation with diabetic retinopathy. Indian J Ophthalmol. 2021;69:3359–3363. doi: 10.4103/ijo.IJO_1269_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvican L, Clowes J, Gillow T. Preservation of sight in diabetes: developing a national risk reduction programme. Diabet Med. 2000;17:627–634. doi: 10.1046/j.1464-5491.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Zuo Y, Wang N, Tong B. Fundus fluorescence Angiography in diagnosing diabetic retinopathy. Pak J Med Sci. 2017;33:1328–1332. doi: 10.12669/pjms.336.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Xie J, Zhang L, Cui Y, Zhang G, Wang J, Zhang A, Chen X, Huang T, Meng Q. Differential distribution of manifest lesions in diabetic retinopathy by fundus fluorescein angiography and fundus photography. BMC Ophthalmol. 2020;20:471. doi: 10.1186/s12886-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn HP, Teo KZ, Smyth JW, Weerasinghe LS, Costello J, Pampapathi P, Keay L, Green T, Vukasovic M, Bruce BB, Newman NJ, Biousse V, White AJ, McCluskey P, Fraser CL. Using non-mydriatic fundus photography to detect fundus pathology in Australian metropolitan emergency departments: A prospective prevalence and diagnostic accuracy study. Emerg Med Australas. 2021;33:302–309. doi: 10.1111/1742-6723.13619. [DOI] [PubMed] [Google Scholar]
- 13.Phiri R, Keeffe JE, Harper CA, Taylor HR. Comparative study of the polaroid and digital non-mydriatic cameras in the detection of referrable diabetic retinopathy in Australia. Diabet Med. 2006;23:867–872. doi: 10.1111/j.1464-5491.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- 14.Piyasena MMPN, Yip JLY, MacLeod D, Kim M, Gudlavalleti VSM. Diagnostic test accuracy of diabetic retinopathy screening by physician graders using a hand-held non-mydriatic retinal camera at a tertiary level medical clinic. BMC Ophthalmol. 2019;19:89. doi: 10.1186/s12886-019-1092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedard C, Sherry Liu S, Patterson C, Gerstein H, Griffith L. Systematic review: Can non-mydriatic cameras accurately detect diabetic retinopathy? Diabetes Res Clin Pract. 2017;129:154–159. doi: 10.1016/j.diabres.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Yaslam M, Al Adel F, Al-Rubeaan K, AlSalem RK, Alageel MA, Alsalhi A, AlNageeb D, Youssef AM. Non-mydriatic fundus camera screening with diagnosis by telemedicine for diabetic retinopathy patients with type 1 and type 2 diabetes: a hospital-based cross-sectional study. Ann Saudi Med. 2019;39:328–336. doi: 10.5144/0256-4947.2019.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn HP, Browning SD, Thomson D, Yates WB, McCluskey P, Keay L, White AJ, Fraser CL. Impact on patient management of non-mydriatic fundus photography compared to direct ophthalmoscopy in a regional Australian emergency department. Emerg Med Australas. 2022;34:186–193. doi: 10.1111/1742-6723.13845. [DOI] [PubMed] [Google Scholar]
- 18.Bawankar P, Shanbhag N, K SS, Dhawan B, Palsule A, Kumar D, Chandel S, Sood S. Sensitivity and specificity of automated analysis of single-field non-mydriatic fundus photographs by Bosch DR Algorithm-Comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS One. 2017;12:e0189854. doi: 10.1371/journal.pone.0189854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neubauer AS, Rothschuh A, Ulbig MW, Blum M. Digital fundus image grading with the non-mydriatic Visucam(PRO NM) versus the FF450(plus) camera in diabetic retinopathy. Acta Ophthalmol. 2008;86:177–182. doi: 10.1111/j.1600-0420.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 20.Soto-Pedre E, Hernaez-Ortega MC. Screening coverage for diabetic retinopathy using a three-field digital non-mydriatic fundus camera. Prim Care Diabetes. 2008;2:141–146. doi: 10.1016/j.pcd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Gajiwala UR, Pachchigar S, Patel D, Mistry I, Oza Y, Kundaria D, B R S. Non-mydriatic fundus photography as an alternative to indirect ophthalmoscopy for screening of diabetic retinopathy in community settings: a comparative pilot study in rural and tribal India. BMJ Open. 2022;12:e058485. doi: 10.1136/bmjopen-2021-058485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubauer AS, Chryssafis C, Thiel M, Priglinger S, Welge-Lüssen U, Kampik A. [Screening for diabetic retinopathy and optic disc topography with the "retinal thickness analyzer" (RTA)] Ophthalmologe. 2005;102:251–258. doi: 10.1007/s00347-004-1098-x. [DOI] [PubMed] [Google Scholar]
- 23.Le Jeune C, Chebli F, Leon L, Anthoine E, Weber M, Péchereau A, Lebranchu P. Reliability and reproducibility of disc-foveal angle measurements by non-mydriatic fundus photography. PLoS One. 2018;13:e0191007. doi: 10.1371/journal.pone.0191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafiz F, Chalakkal RJ, Hong SC, Linde G, Hu R, O'Keeffe B, Boobin Y. A new approach to non-mydriatic portable fundus imaging. Expert Rev Med Devices. 2022;19:303–314. doi: 10.1080/17434440.2022.2070004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Shi J, Peng Y, Zhao Z, Zheng Q, Wang Z, Liu K, Jiao S, Qiu K, Zhou Z, Yan L, Zhao D, Jiang H, Dai Y, Su B, Gu P, Su H, Wan Q, Liu J, Hu L, Ke T, Chen L, Xu F, Dong Q, Terzopoulos D, Ning G, Xu X, Ding X, Wang W. Artificial intelligence-enabled screening for diabetic retinopathy: a real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.