Abstract
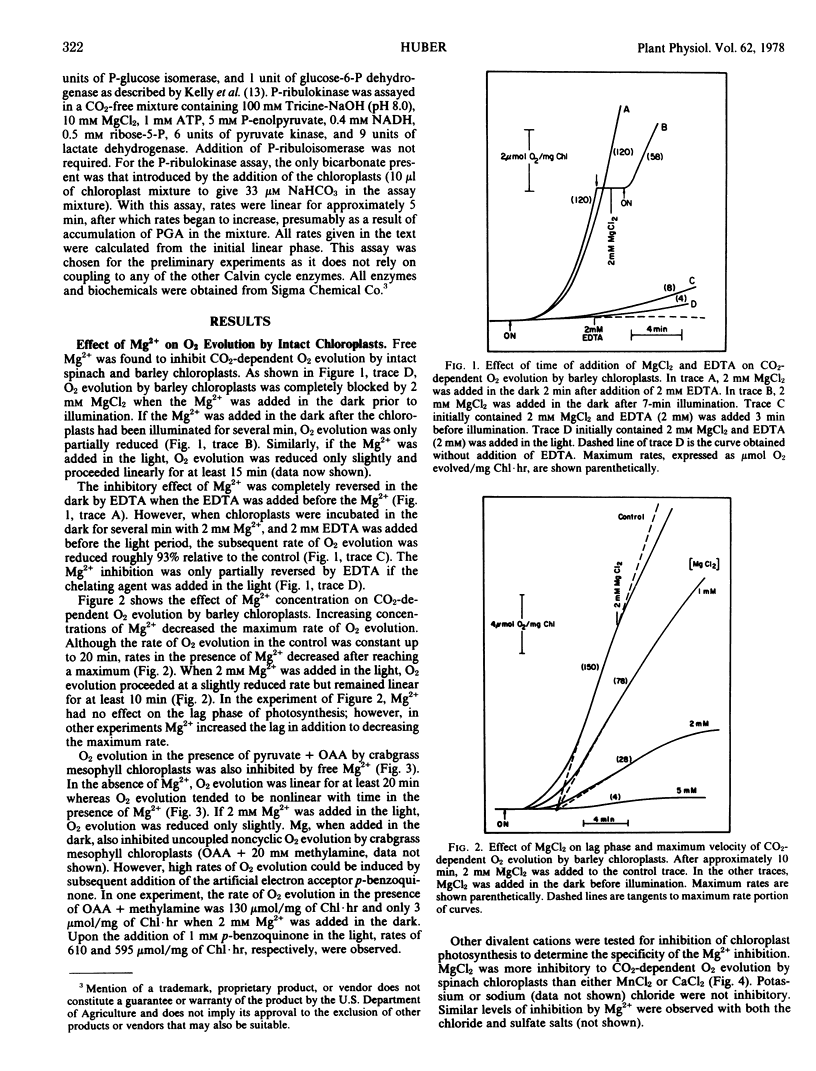
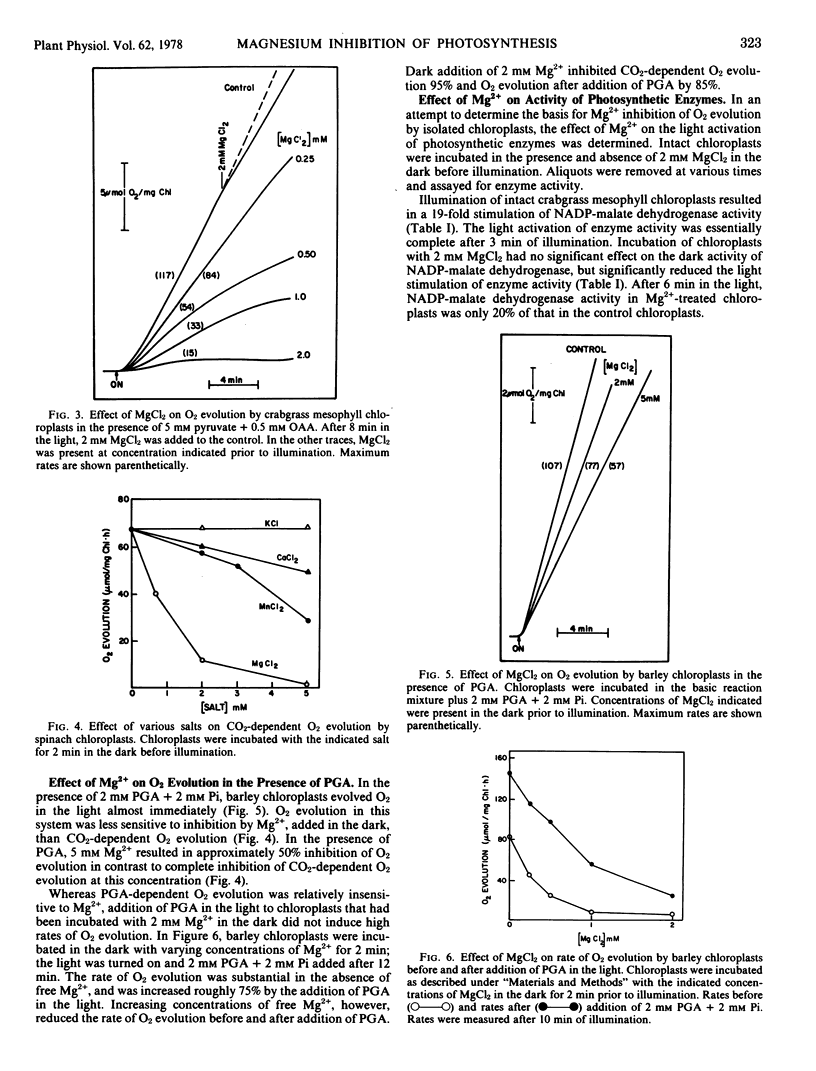
Magnesium was most inhibitory to photosynthetic reactions by intact chloroplasts when the magnesium was added in the dark before illumination. Two millimolar MgCl2, added in the dark, inhibited CO2-dependent O2 evolution by Hordeum vulgare L. and Spinacia oleracea L. (C3 plants) chloroplasts 70 to 100% and inhibited (pyruvate + oxaloacetate)-dependent O2 evolution by Digitaria sanguinalis L. (C4 plant) mesophyll chloroplasts from 80 to 100%. When Mg2+ was added in the light, O2 evolution was reduced only slightly. O2 evolution in the presence of phosphoglycerate was less sensitive to Mg2+ inhibition than was CO2-dependent O2 evolution.
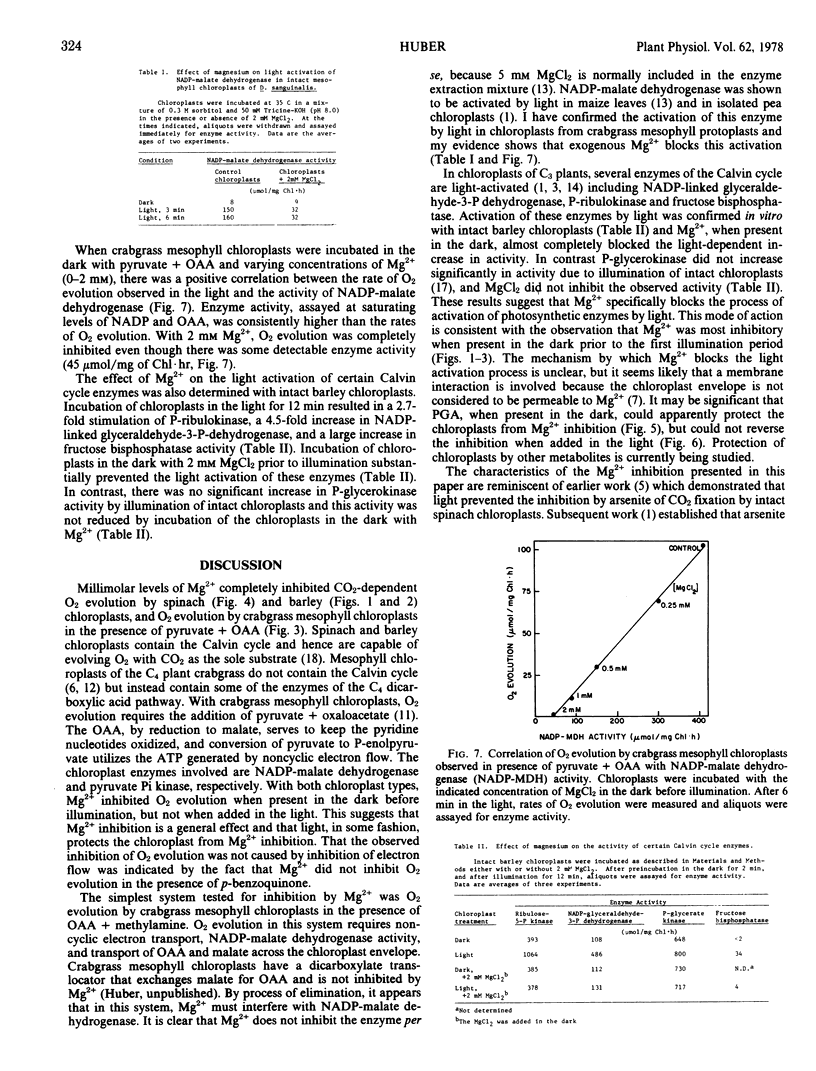
Magnesium prevented the light activation of several photosynthetic enzymes. Two millimolar Mg2+ blocked the light activation of NADP-malate dehydrogenase in D. sanguinalis mesophyll chloroplasts, and the light activation of phosphoribulokinase, NADP-linked glyceraldehyde-3-phosphate dehydrogenase, and fructose 1,6-diphosphatase in barley chloroplasts. The results suggest that Mg2+ inhibits chloroplast photosynthesis by preventing the light activation of certain enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Properties of phosphoribulokinase of whole chloroplasts. Plant Physiol. 1974 Feb;53(2):136–139. doi: 10.1104/pp.53.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G. L., Gander J. E. Uridine diphosphate glucose pyrophosphorylase from Sorghum vulgare. Purification and kinetic properties. J Biol Chem. 1972 Mar 10;247(5):1387–1397. [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Regulation of oxaloacetate, aspartate, and malate formation in mesophyll protoplast extracts of three types of c(4) plants. Plant Physiol. 1975 Aug;56(2):324–331. doi: 10.1104/pp.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Energy charge control of the Calvin cycle enzyme 3-phosphoglyceric acid kinase. Biochem Biophys Res Commun. 1973 Mar 5;51(1):139–143. doi: 10.1016/0006-291x(73)90519-6. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


