Abstract
BACKGROUND
Hemolymphangioma of the jejunum is rare and lacks clinical specificity, and can manifest as gastrointestinal bleeding, abdominal pain, and intestinal obstruction. Computed tomography, magnetic resonance imaging, and other examinations show certain characteristics of the disease, but lack accuracy. Although capsule endoscopy and enteroscopy make up for this deficiency, the diagnosis also still requires pathology.
CASE SUMMARY
A male patient was admitted to the hospital due to abdominal distension and abdominal pain, but a specific diagnosis by computed tomography examination was not obtained. Partial resection of the small intestine was performed by robotic surgery, and postoperative pathological biopsy confirmed the diagnosis of hemolymphangioma. No recurrence in the follow-up examination was observed.
CONCLUSION
Robotic surgery is an effective way to treat hemolymphangioma through minimally invasive techniques under the concept of rapid rehabilitation.
Keywords: Hemolymphangioma, Enteroscopy, Robotic surgery, Rehabilitation, Case report
Core Tip: Endoscopy and computed tomography are often used for the diagnosis of hemolymphangioma of the jejunum. Laparotomy is a traditional treatment for this tumor. Our study was the first to introduce robotic surgical techniques, bringing new possibilities for the treatment of this tumor. This procedure can reduce surgical trauma and pain and accelerate recovery. In addition, robotic surgery can also improve the accuracy of the procedure. The presented patient recovered quickly and had no serious complications. Our results indicate that robotic surgery for jejunal angiolangioma is feasible, and provides better treatment options for the patients.
INTRODUCTION
Hemolymphangioma occurs in the transition area between the lymphatic system and the vascular system of the human body. The lesion site forms a cystic tumor composed of lymphatic vessels and blood vessels, commonly seen in the skin, soft tissues, and internal organs. The etiology of this disease is unknown, but is related to innate factors. In clinical presentation, the symptoms of hemolymphangioma vary according to the site and extent of the lesion. Some patients may have local swelling, pain, and compression, while others may not have obvious symptoms[1]. For those presenting with obvious symptoms, surgical resection is a commonly used treatment. Diagnosis of this tumor is based on the patient's clinical manifestations and imaging examination findings. Imaging examinations, including ultrasound, computed tomography (CT), magnetic resonance imaging, etc can show the size, location, relationship with the surrounding tissues, and whether there is metastasis[2]. The diseased tissue is obtained by surgical resection or needle biopsy for histological examination to determine the nature and type of the lesion. Surgical resection is a common treatment for hemolymphangioma.
In our case, robotic surgery was applied for the first time. Under the concept of Enhanced Recovery after Surgery, we achieved a good combination of minimally invasive and radical resection.
CASE PRESENTATION
Chief complaints
A 47-year-old man visited another hospital due to abdominal pain for 2 d, and abdominal CT suggested a small intestine-occupying lesion. The patient was admitted to our hospital for further examination on November 23, 2022. The abdominal pain occurred without obvious predisposing factors, and was not attributed to diet. Symptoms such as abdominal distension and fever were absent. The patient was healthy prior to admission.
History of present illness
A 47-year-old man visited another hospital due to abdominal pain for 2 d, and abdominal CT suggested a small intestine-occupying lesion. The patient was admitted to our hospital for further examination on November 23, 2022. The abdominal pain occurred without obvious predisposing factors, and was not attributed to diet. Symptoms, such as abdominal distension and fever were absent. The patient was healthy prior to admission.
Physical examination
Mild tenderness in the upper abdomen, without rebound tenderness or muscular tension was revealed on physical examination. In addition, the liver and spleen were not palpable.
Laboratory examinations
Routine blood parameters, biochemical functions, tumor markers, and blood gas findings were normal.
Imaging examinations
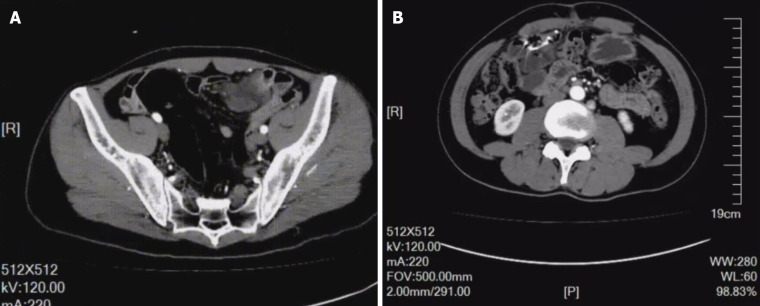
An enhanced CT scan showed significant localized annular enhancement in the left pelvic small bowel wall. The lumen was narrowed, and the outer wall of the small bowel was rough in texture. In addition, the adjacent mesentery was thickened with increased density, and slightly large lymph nodes were faintly visible inside the mesentery. No dilation of the proximal intestine was observed. Three-phase CT values were 52, 63, and 67 HU, respectively (Figure 1A; Video 1).
Figure 1.
Enhanced computed tomography and repeat enhanced computed tomography scan findings. A: Significant localized annular enhancement was seen in the left pelvic small bowel wall. The lumen was narrowed, and the outer wall of the small bowel was rough; B: The patient showed no tumor recurrence.
FINAL DIAGNOSIS
Pathological examination revealed a jejunal hemolymphangioma, and immunohistochemical staining was positive for both D2-40 and CD32.
TREATMENT
Robot-assisted laparoscopic partial small bowel resection and lymph node dissection were performed under general anesthesia. During laparoscopic exploration, no significant ascites was found within the abdominal cavity, while the greater omentum was adhered to the right abdomen. Following release of the adhesion, the small bowel wall, approximately 250 cm away from Treitz ligament and 70 cm away from the ileocecum, was found to be thickened with a hard texture and a diameter of approximately 3 cm. There was no serosal invasion or obvious adhesion to surrounding tissues. Multiple enlarged lymph nodes were found within the small intestinal mesentery. Further exploration showed no significant organic changes or metastases in the liver, gallbladder, spleen, other small intestinal areas, colon and abdominopelvic cavity. The lines of resection were set 10 cm away from each end of the intestinal mass. The small intestinal mesentery was exposed by dissection, and the thicker vessel was clamped using a HemoLock clip. After resection, side-to-side anastomosis was performed. The stump was carefully treated to stop bleeding, and the seromuscular layer was embedded to reduce tension. The two horns of the stump were treated with half purse-string sutures to close the mesentery.
OUTCOME AND FOLLOW-UP
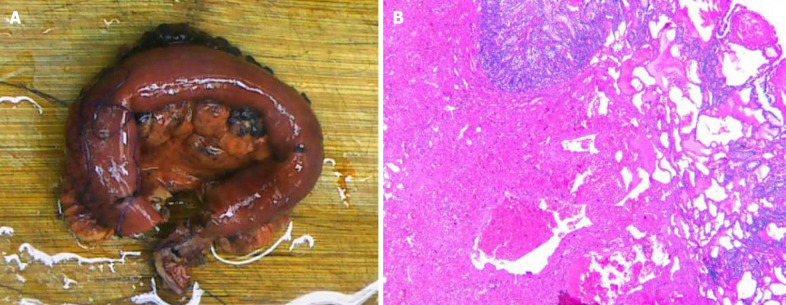
The patient underwent repeat abdominal enhanced CT which showed unobstructed anastomotic healing and no signs of intra-abdominal tumor recurrence 3 mo after surgery (Figure 1B; Video 2). The resected segments of the small intestine and mesentery were sent for pathological examination. Gross pathological evaluation revealed a 15-cm long lesion (perimeter: 4-5 cm) with a rough mucosal bulge (1.5 cm × 1.5 cm) 7 cm away from one cut end. The corresponding small intestinal serosal layer formed a 6 cm × 4 cm × 4 cm mass, which had a grey-red soft cut surface with outflow of dark red liquid. Hematoxylin and eosin staining showed cystic-like dilated blood and lymph vessels of varying sizes. The blood vessels were dilated into blood sinuses filled with red blood cells, while the lymph vessels containing lymphocytes were filled with eosinophilic proteins. These blood and lymph vessels showed a dopant distribution (Figure 2). Immunohistochemistry showed cluster of differentiation 34 (CD34) (focal +), CD31 (+), D2-40 (+), and Ki67 (dispersed +). Pathology suggested partial small intestinal hemolymphangioma invading the full-thickness bowel wall and mesentery. Both cut ends of the small bowel developed submucosal vascular proliferation accompanied by vascular dilation and congestion, and one peri-intestinal lymph node showed reactive hyperplasia. The patient had an excellent postoperative outcome without complications.
Figure 2.
Histopathology results. A: Pathological tissue removed from the patient; B: Immunohistochemical staining of pathological tissues.
DISCUSSION
Hematolymphangioma is a benign tumor originating from the mesenchymal-embryonic tissues, and is a type of low-flow vascular malformation instead of a true neoplasm[1]. Primary hematolymphangioma is the result of developmental abnormalities of the vasculature, embryonic angioplasty, and occlusion of veins and lymphatic capillaries. It is more common in children and adolescents (especially in females) and can occur at systemic sites mainly loose connective tissue. There have been a few reports of hematolymphangioma in the small intestine, spleen, esophagus and other organs[3].
Hematolymphangioma has varying clinical presentations, sizes and locations. The symptoms vary due to complications such as mass enlargement or bleeding, infection, perforation, torsion and rupture. In general, hematolymphangioma is rare in clinical practice, which may be due to the low incidence and lack of clinical presentations. In the current case, abdominal pain was the main symptom, and may have been a result of the space-occupying tumor. Gastrointestinal hematolymphangioma is diagnosed based on CT and endoscopic findings. CT is a very useful radiologic tool for diagnosis[2]. On CT images, hematolymphangioma presents with dilation of veins and lymphatic capillaries although with normal stromal tissue and vasculature. Malformed and dilated venous vessels usually present with thrombosis, potentially leading to dystrophic necrosis and calcium deposition. It is worth noting that the vessels of varying sizes within the hematolymphangioma may lead to enhancement with different characteristics on imaging. In the tumor rich in blood vessels, significant and persistent enhancement can be observed. Double balloon enteroscopy allows concurrent biopsy and endoscopy[4], and more importantly, it shows clearer pathological changes. In this patient, endoscopic biopsy was not selected due to the high risk of bleeding during biopsy.
Robotic surgery has the following advantages over traditional laparoscopic surgery[5]. (1) Higher accuracy: the robotic arm of the surgical system can more accurately replicate the doctor's surgical movements, and can filter out human tremors or errors, thus providing higher accuracy than laparoscopic surgery; (2) less invasive: robotic surgery allows for smaller incisions and less tissue trauma, resulting in less post-operative pain and faster recovery times for patients; (3) better visualization: the robotic surgical system provides a high-definition, three-dimensional view of the surgical site, giving the surgeon a more detailed and accurate view of the surgery than laparoscopic surgery; (4) more dexterous: the robotic surgical system allows for more precise and intricate movements than laparoscopic surgery, enabling the surgeon to perform complex surgical procedures with greater ease; and (5) faster recovery times: due to the smaller incisions and less tissue trauma with robotic surgery, patients experience faster post-operative recovery times, allowing for earlier hospital discharge and a shorter overall recovery period[6]. Overall, robotic surgery is superior to conventional laparoscopic surgery in many ways, especially when performing complex procedures.
Generally, en bloc resection can provide the best results with a lower rate of recurrence; however, careful follow-up is required. Moreover, the rate of recurrence varies with the complexity, anatomical location and adequacy of resection. According to the literature, 10%-27% of lesions undergoing en bloc resection recur, whereas the rate could be up to 50%-100% in lesions undergoing partial resection. Compared to surgery, non-surgical treatments including cryotherapy, laser therapy, radiotherapy and localized injection of sclerosing agents are inferior[7].
CONCLUSION
Here, we report a male patient with a hematolymphangioma in the small intestine. Hematolymphangioma lacks typical clinical symptoms, and specific imaging examinations such as CT and magnetic resonance imaging are useful for confirming the diagnosis and selecting a suitable treatment regimen. In addition, endoscopy also facilitates accurate preoperative diagnosis and surgical strategy planning for hematolymphangioma. Although hematolymphangioma is extremely rare in clinical practice, especially cases involving the small intestine, it should also be considered in the context of recurrent and unexplained gastrointestinal symptoms. When the disease has been diagnosed or there is a space-occupying effect accompanied by clinical symptoms, it is necessary to undergo surgical resection as soon as possible, and robotic surgery has the advantages of less trauma and faster postoperative recovery, and is an important choice for the treatment of hematolymphangioma.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 13, 2023
First decision: November 1, 2023
Article in press: January 22, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demirli Atici S, Turkey; Kou G, China S-Editor: Qu XL L-Editor: Filipodia P-Editor: Qu XL
Contributor Information
Tian-Ning Li, Department of Clinical Lab, Tianjin First Central Hospital, Tianjin 300000, China.
Yan-Hong Liu, Nankai University, Tianjin Union Medical Center, Tianjin 300000, China.
Jia Zhao, Nankai University, Tianjin Union Medical Center, Tianjin 300000, China.
Hong Mu, Tianjin Medical University, Tianjin First Center Hospital, Tianjin 300000, China. tjmuhong2022@163.com.
Lei Cao, Nankai University, Tianjin Union Medical Center, Tianjin 300000, China.
References
- 1.Xiao NJ, Ning SB, Li T, Li BR, Sun T. Small intestinal hemolymphangioma treated with enteroscopic injection sclerotherapy: A case report and review of literature. World J Gastroenterol. 2020;26:1540–1545. doi: 10.3748/wjg.v26.i13.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan X, Dong Y, Yuan T, Yan Y, Tong D. Two cases of hemolymphangioma in the thoracic spinal canal and spinal epidural space on MRI: The first report in the literature. Medicine (Baltimore) 2017;96:e9524. doi: 10.1097/MD.0000000000009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosmidis I, Vlachou M, Koutroufinis A, Filiopoulos K. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res. 2010;5:56. doi: 10.1186/1749-799X-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Li Y, Yan K, Wu W, Yin S, Yang W, Xing B, Li X, Zhang X. Application of contrast-enhanced ultrasound in the diagnosis of solid pancreatic lesions--a comparison of conventional ultrasound and contrast-enhanced CT. Eur J Radiol. 2013;82:1385–1390. doi: 10.1016/j.ejrad.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Ye SP, Zhu WQ, Huang ZX, Liu DN, Wen XQ, Li TY. Role of minimally invasive techniques in gastrointestinal surgery: Current status and future perspectives. World J Gastrointest Surg. 2021;13:941–952. doi: 10.4240/wjgs.v13.i9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim KT. Current surgical management of duodenal gastrointestinal stromal tumors. World J Gastrointest Surg. 2021;13:1166–1179. doi: 10.4240/wjgs.v13.i10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi K, Umeda Y, Yoshida R, Fuji T, Yasui K, Yagi T, Fujiwara T. Innovative suture technique for robotic hepaticojejunostomy: double-layer interrupted sutures. Langenbecks Arch Surg. 2023;408:284. doi: 10.1007/s00423-023-03020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]