Abstract
Heightened lactate production in cancer cells has been linked to various cellular mechanisms such as angiogenesis, hypoxia, macrophage polarisation and T‐cell dysfunction. The lactate‐induced lactylation of histone lysine residues is noteworthy, as it functions as an epigenetic modification that directly augments gene transcription from chromatin. This epigenetic modification originating from lactate effectively fosters a reliance on transcription, thereby expediting tumour progression and development. Herein, this review explores the correlation between histone lactylation and cancer characteristics, revealing histone lactylation as an innovative epigenetic process that enhances the vulnerability of cells to malignancy. Moreover, it is imperative to acknowledge the paramount importance of acknowledging innovative therapeutic methodologies for proficiently managing cancer by precisely targeting lactate signalling. This comprehensive review illuminates a crucial yet inadequately investigated aspect of histone lactylation, providing valuable insights into its clinical ramifications and prospective therapeutic interventions centred on lactylation.
Keywords: cancer, histone lactylation, metabolic reprogramming
The lactate‐induced lactylation of histone lysine residues bridges the metabolic reprogramming and epigenetic rewiring.
Histone lactylation fuels oncogene overexpression, expediting tumour progression and development.
Targeting lactate signalling exhibits with therapeutic efficacy in diversified cancers.

1. INTRODUCTION
In the past, lactate was primarily regarded as an outcome of energy metabolism; nevertheless, its distinctive biological significance has been progressively comprehended subsequent to the identification of the Warburg effect. 1 As a result of increased glycolytic levels, lactate plays various roles in promoting cancer, such as being used as a source of energy metabolism, participating in signal transduction pathways, regulating the tumour microenvironment (TME) and immune cells, and regulating specific enzyme modifications. 2 , 3 Consequently, lactate is involved in a wide range of biological aspects related to tumour regulation, including energy transport and growth. 4 , 5 , 6
Multiple studies conducted over several decades have consistently demonstrated that changes in patterns of gene expression are primarily attributed to the process of chromatin remodelling and posttranslational modifications (PTMs), commonly referred to as epigenetic alterations. 7 , 8 , 9 The alterations, which have the ability to be passed down and undone, have a significant impact on the specific control of cellular characteristics. 10 , 11 As a result, a variety of chromatin modifications, including methylation, acetylation, phosphorylation, ubiquitination, glycosylation and glutarylation, collectively contribute to distinct configurations that impact the efficacy of transcriptional machinery. 12 , 13 , 14 Significantly, the disturbance of epigenetic alterations and metabolic pathways is frequently linked to various illnesses, and the interaction between metabolism and chromatin presents numerous prospects for therapeutic interventions. 15 , 16
Recognising the interaction between metabolic remodelling and dynamic histone tuning is of utmost importance as it is currently in its early stages. The purpose of this review is to combine current information on the influence of lactate and histone modification on tumour and related gene regulation. Furthermore, we have clarified the scientific significance of future studies on histone alteration and recognised the obstacles that must be tackled, thus opening up new possibilities in the realm of cancer management through epigenetic/metabolic therapies.
In this review, we introduce a newly discovered epigenetic modification called histone lactylation modification, which is a dynamic and reversible epigenetic modification dependent on lactate. 17 , 18 , 19 Examining histone lactylation provides a thorough analysis of the latest advancements in researching this alteration in cancer, particularly emphasising its effects on reshaping tumour characteristics, regulating gene activity, promoting glycolysis in cancer stem cells and impacting the tumour's immune microenvironment (Figure 1). 20 , 21 , 22
FIGURE 1.

The correlation between histone lactylation and the interconnection of epigenetic remodelling and metabolic reprogramming during the progression of cancerous development. Metabolic disorders are visually represented in red, while epigenetic rewiring is depicted in green.
1.1. Warburg effect
For the aerobic respiration, pyruvate derived from glucose is transported into the mitochondria and undergoes conversion to acetyl‐CoA. Afterward, it proceeds into the tricarboxylic acid (TCA) cycle, which aids in oxidative phosphorylation (OXPHOS). 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Through this process, a grand sum of 36 adenosine triphosphate molecules are generated for each glucose molecule. 31 , 32 , 33 Instead of entering the TCA cycle, the pyruvate molecules are converted to lactate through the catalytic activity of cytosolic lactate dehydrogenases (LDHs). 34
In 1956, Otto Heinrich Warburg made the seminal observation that substantial lactate production occurs from glucose, particularly in tumour cells, a phenomenon now referred to as the Warburg effect. 19 , 35 The Warburg effect is distinguished by elevated glycolysis and diminished OXPHOS, conferring specific advantages to cancer cells. 36 , 37 , 38 , 39 In addition, glycolysis generates intermediate compounds that aid in the synthesis of nucleotides, proteins and fats, regardless of changes in oxygen concentrations. 40 Furthermore, the sustenance of glycolysis relies on maintaining the ratio of nicotinamide adenine dinucleotide (oxidative) (NAD+) to nicotinamide adenine dinucleotide (reductive) (NADH), which is achieved by converting pyruvate to lactate and then regenerating NAD+. 41 , 42 , 43 In conclusion, improved glycolysis promotes increased lactate generation, resulting in the acidification of the cellular surroundings.
To support their growth, survival, proliferation and long‐term maintenance, cancer cells undergo this metabolic reprogramming. 44 , 45 An important feature of this altered metabolic condition is the increased absorption of glucose and subsequent transformation of glucose into lactate, leading to the acidic environment of the TME. 46 , 47 , 48 , 49 Furthermore, hypoxia plays a crucial role in promoting glycolysis and inhibiting OXPHOS, which is frequently observed in cancerous tissues. These aforementioned observations emphasise the role of lactate signalling in the development of tumours. 31 , 50
1.2. Lactate production and elimination
During the process of Warburg effect, a substantial quantity of lactate accumulates as a consequence of this metabolic reprogramming phenomenon. 51 , 52 , 53 Importantly, the buildup of lactate in the human organism presents a higher danger in contrast to the buildup of alternative molecular energy sources, since elevated levels of lactate in the bloodstream can lead to lactic acidosis. 42 , 54 The enzymatic activity of pyruvate dehydrogenase (PDH) enables the conversion of lactate to pyruvate, resulting in the elimination process. 55 , 56 , 57 , 58 Moreover, the buildup of lactate has the potential to trigger gluconeogenesis in cells of the liver and skeletal muscle. During energy expenditure, lactate is converted into glucose and subsequently released into the bloodstream to facilitate further glucose consumption. 59 , 60 , 61
Furthermore, alongside glycolysis, the process of glutamine catabolism serves as an additional approach for cancer cells to produce lactate. 61 The c‐Myc protein controls this metabolic pathway, enabling the movement of glutamine undergoes enzymatic conversion into glutamate through the action of the enzyme glutaminase. 62 , 63 , 64 Afterwards, the transformation of glutamate into α‐ketoglutarate takes place via the enzymatic activity of glutamate dehydrogenase or a cluster of transaminases. 65 , 66 During this process, carbon obtained from glutamine is converted into oxaloacetate, which is then changed into malate and leaves the mitochondria to be further converted into NADPH and pyruvate. 67 , 68
1.3. Lactate sensing, shuttling and signalling
Importantly, the transportation and detection of lactate rely on the utilisation of monocarboxylate transporter (MCT) and sodium MCT families, along with G protein‐coupled receptors (GPRs) called GPR81 and GPR132. 69 In cancer cells, glutamine serves as a vital carbon skeleton for the production of lactate and acts as an additional pathway for generating lactate. Out of the 14 recognised MCTs, MCT1–4 are present in different body tissues and play a role in the catalytic coupling of protons and the two‐way movement of monocarboxylic acid. 70
Under physiological conditions, the combined functioning of MCT1–4 facilitates the transfer of lactate between cells that undergo glycolysis and those that undergo oxidation, playing a crucial role in maintaining lactate balance in various tissues. 71 The MCT1 with strong affinity helps maintain balance of lactate by facilitating the movement of lactate across the cell membrane in response to the transmembrane lactate gradient. 72 Tumour cells and other cells with elevated levels of lactate within rely on MCT4, which has a low affinity for transporting lactate. The transportation process starts by attaching unbound protons to MCT, then proceeds with the attachment of lactate, which experiences a structural alteration inside the carrier and is subsequently released on the opposite side of the membrane. 30 , 73 Lactate is released prior to the release of protons. Of particular significance, increased levels of MCT1, MCT2 and MCT4 are strongly linked to the development of cancer, which is correlated with an adverse prognosis. 74 , 75 , 76 , 77 The transport of lactate, enabled by MCTs, creates internal links, and has a vital function in the collaborative metabolism of glycolytic cancer cells and oxidative cancer cells, ultimately aiding in the onset and advancement of tumours. 78
Classical metabolites have the ability to initiate direct signalling via GPRs. 79 Upon the binding of an agonist, the G protein‐coupled receptor (GPCR) alpha subunit undergoes a GDP–GTP exchange. Secondary messengers such as cyclic adenosine monophosphate (cAMP) and Ca2+ play a role in the following signalling events, and their variation depends on the specific alpha subunit type. 80 , 81 Notably, lactate is capable of signalling through GPR81 and GPR132, both of which are sensitive to protons. 82
GPR81 is detected in different tumours obtained from patients and cancer cell lines. In vivo studies have demonstrated a correlation between levels of GPR81 and tumour growth and metastasis in pancreatic cancer, with a significant decrease observed upon silencing of GPR81. 83 Crucially, lactate has been shown to actively promote the formation of new blood vessels by activating the phosphoinositide 3‐kinase/Akt (PI3K/Akt)–cAMP–CREB (cAMP response element‐binding protein) pathway, resulting in the production of pro‐angiogenic amphiregulin in breast cancer. 84 , 85 Moreover, lactate has been found to impede the efficiency of anti‐cancer immune response by communicating through GPR81 in dendritic cells that invade tumours, thereby compromising their capacity to effectively showcase antigens to T cells by reducing the expression of major histocompatibility complex class II (MHC‐II) molecules. 86 Furthermore, GPR132 is detected in different body tissues, including the respiratory system, digestive system and immune cells, specifically macrophages. 87 It is worth mentioning that the stimulation of GPR132 through lactate signalling in macrophages associated with tumours has been discovered to enhance a pro‐tumoural M2 phenotype, which is identified as alternatively activated and anti‐inflammatory, in models of breast cancer and Lewis lung carcinoma. 88 , 89 , 90 The expression of GPR132 exhibits a positive correlation with the existence of M2 macrophages and the incidence of metastasis. 91 (Figure 2)
FIGURE 2.
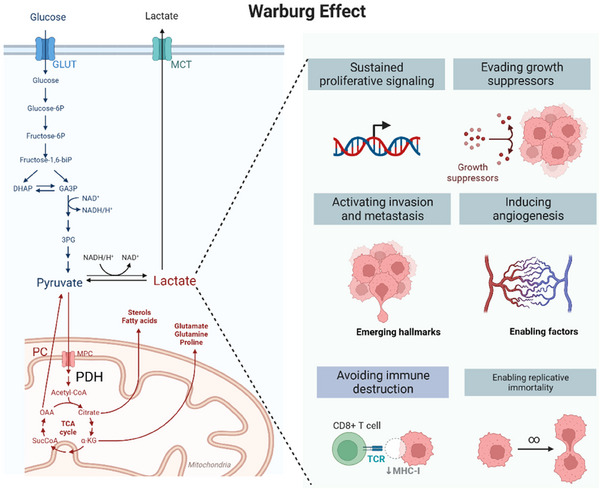
The illustration of the Warburg effect elicits an elevation in lactate levels, which is associated with multiple cancer‐related traits inclding enhanced signalling for cell proliferation, evasion of growth inhibitory factors, activation of invasion and metastasis, heightened capacity for angiogenesis, evasion of immune system destruction and facilitation of replicative immortality.
2. THE ROLE OF HISTONE LACTYLATION
While the significance of lactate as a catalyst for oncogenic signalling has been established, its role in transcriptional regulation remains uncertain. Notably, substantial evidence supports the crucial function of lactate in aiding the alteration of histone lysine residues. This alteration, such as other modifications of histones, plays a role in the regulation of transcriptional activation. 19 Thus far, the importance of histone lactylation in facilitating metabolic rewiring, altering the immunological environment, controlling cell destiny and impacting cancer stemness and senescence has been discovered (Figure 3 and Table 1). 92 , 93 , 94
FIGURE 3.

Oncogenic functions of histone lactylation in metabolic reprogramming, immunological remodelling, cell senescence and stemness maintenance.
TABLE 1.
The role of histone lactylation.
| Downstream targets | Cells | Function | Reference |
|---|---|---|---|
| Bmp5, Trpc5, Kit | PASMC | Promotes PASMC proliferation and vascular remodelling in hypoxic pulmonary hypertension | 96 |
| YTHDF2 | OCM1, CRMM1 | Drives ocular melanoma development | 94 |
| – | Saccharomyces cerevisiae | Attenuates dextran sulphate sodium‐induced colitis via suppressing macrophage pyroptosis and modulating the intestinal microbiota | 102 |
| METTL3 | Tumour infiltrating myeloid cells | Strengthens immunosuppressive functions of myeloid cells | 130 |
| PD‐L1 | HL‐60, NB4 | Suppresses T‐cell activation in acute myeloid leukaemia | 21 |
| Lrg1, Vegfa, IL10 | Monocyte | Promotes early remote activation of the reparative transcriptional response in monocytes post‐myocardial infarction | 105 |
| Rela, NFκB1 | Microglia | Upregulates senescence‐associated secretory phenotype and accelerates the pathogenesis of Alzheimer's disease | 100 |
| Oct4, Sall4, Mycn | iPSC | Facilitates cellular reprogramming | 114 |
Abbreviations: CRMM1, Conjunctival recurrent malignant melanoma 1; IL10, interleukin 10; METTL3, Methyltransferase Like 3; NFκB1, Nuclear Factor of Kappa Light Polypeptide Gene Enhancer in B Cells 1; OCM1, Ocular chroidal melanoma 1; PASMC, pulmonary artery smooth muscle cell; PD‐L1, programmed death‐ligand 1; YTHDF2, YTH N6‐methyladenosine RNA‐binding protein 2.
2.1. Histone lactylation and metabolic reprogramming
The regulation of lactylation by lactate has recently been established as a novel factor influencing the epigenetic landscape. 18 , 26 This discovery not only paves the way for comprehensive investigations into lactate metabolism but also provides crucial points of interest for future studies on functionality and mechanisms. Enhanced glycolysis and the accumulation of lactate are commonly observed in various types of cancer. 47 , 73 , 85 This metabolic shift leads to the intracellular production of lactate, which has been found to drive a recently discovered PTM known as lysine lactylation on core histones. A recent investigation performed worldwide lactylome profiling on a group of hepatitis B virus‐related hepatocellular carcinoma (HCC) and nearby liver tissues that were collected in advance. 95
By conducting an integrative analysis of lactylome and proteome, a grand total of 9275 Kla sites were discovered, out of which 9256 sites were found on proteins other than histones. The results suggest that Kla is a widespread alteration that goes beyond histone proteins and the regulation of transcription. Notably, Kla demonstrates a predilection for influencing enzymes involved in diverse metabolic pathways, including the TCA cycle, as well as carbohydrate, amino acid, fatty acid and nucleotide metabolism. 95 Moreover, in the framework of the pulmonary hypertension (PH) model, Chen et al. observed an augmentation of histone lactylation on hypoxia inducible factor 1 alpha (HIF‐1α) targets, namely Bmp5, Trpc5 and Kit, which subsequently stimulates the proliferation of pulmonary artery smooth muscle cells (PASMCs). Furthermore, the use of a LDH inhibitor as a pharmacological treatment led to a decrease in histone lactylation, which ultimately enhanced the proliferation of PASMC and vascular remodelling in hypoxic PH rats. 96
Furthermore, recent research indicates that metabolic adaptation is a crucial characteristic and requirement for the transition of macrophage phenotype. 89 , 97 Pyruvate kinase M2 (PKM2) is a fundamental molecular factor in the metabolic adaptations of pro‐inflammatory macrophages. 98 , 99 The regulation of PKM2 is primarily influenced by PTMs. Specifically, lactylation of PKM2 hinders its transformation from a tetramer to a dimer, thereby enhancing its pyruvate kinase activity and decreasing its nuclear distribution. 100 In summary, these findings emphasise the significance of lactylation as a key initiator of metabolic adjustments in diversified pathological events.
In addition, the involvement of histone lactylation has been suggested in the control of diverse forms of chemical alterations. For instance, Yu et al. noticed a connection between increased amounts of histone Kla and an adverse outlook in individuals with ocular melanoma. The strong positive correlation between ocular melanoma and intracellular histone Kla was further confirmed through the subsequent inhibition of histone Kla in ocular melanoma cells. Melanoma development is caused by the increase in YTHDF2 expression, which is achieved by enhancing histone Kla at the promoter region. YTHDF2, an m6A reader, plays a crucial part in enhancing the development of ocular melanoma. 94 While certain observations endorse the concept that histone Kla is crucial for the proliferation of tumours, alternative studies argue that histone Kla lacks the ability to convert healthy cells into cancerous ones. This suggests that histone Kla plays a role in facilitating the growth of developing tumours rather than initiating tumourigenesis initially. 15 , 20
2.2. Histone lactylation and immunological remodelling
Lactic acid, a substance produced during metabolism by both the host and intestinal microbiota, has been recognised as a crucial signalling molecule in the immune system. 69 , 101 Notably, Sun et al. successfully engineered Saccharomyces cerevisiae to produce lactic acid from glucose instead of ethanol, resulting in a high production yield. The application of this genetically modified strain of S. cerevisiae has been shown to effectively alleviate dextran sulfate sodium (DSS)‐induced colitis in mice through the inhibition of macrophage pyroptosis and modulation of the intestinal microbiota. This approach holds great potential as a safe and promising therapeutic strategy for the treatment of ulcerative colitis. Howbeit, additional research is needed to determine whether this engineered S. cerevisiae strain is also involved in tumourigenesis, particularly in the context of autoimmune diseases. 102
The rapid advancements in genomics and immunology have led to the emergence of immunotherapy as a groundbreaking therapeutic approach for tumour treatment. Immune checkpoint inhibitors, in particular, have demonstrated remarkable efficacy in various tumour types. 19 , 40 Histone lactylation was initially discovered in the macrophages present in the TME, which aligns with the observed high glucose uptake in this specific subpopulation. 5 Furthermore, the increased histone lactylation originating from tumours contributes to the polarisation of M2 tissue‐associated macrophages, thereby promoting immunological evasion during the progression of cancer. 100 , 103 , 104
Recently, histone lactylation is also involved in the response towards immunotherapy. For instance, the promotion of increased lactate accumulation facilitated the nuclear translocation of E3BP, a component of the PDH complex. It is worth noting that E3BP demonstrated the capacity to interact with lactylated histone, leading to significant histone lactylation and ultimately resulting in the upregulation of programmed death‐ligand 1 (PD‐L1) transcription. This finding emphasises the significance of nuclear translocation of metabolic enzymes in connecting histone lactylation and PD‐L1‐mediated T‐cell exhaustion. 21 Furthermore, the timely activation of reparative signals is necessary for the resolution of inflammation and the initiation of cardiac repair following myocardial infarction. The lactylation of histones facilitates the early activation of the reparative transcriptional response in monocytes, which is crucial for maintaining immune balance and initiating timely cardiac repair after myocardial infarction. 105
In addition to its essential role in histone lactylation, non‐histone lactylation has been established as a significant mediator in immunological remodelling. A noteworthy illustration of this phenomenon is observed in hepatocyte HSPA12A, which functions as a novel regulator to protect livers against ischemia–reperfusion (I/R) injury. The inhibition of glycolysis‐mediated HMGB1 lactylation and its subsequent secretion from hepatocytes achieves a protective mechanism, which effectively suppresses macrophage chemotaxis and inflammatory activation. 106
2.3. Histone lactylation and cell senescence
Cellular ageing plays a crucial role in the progression of age and is closely linked to various age‐related ailments, such as Alzheimer Disease (AD), retinopathies and tumours. 50 Comparatively higher concentrations of lactic acid were detected in aged mice and AD mouse models' senescent microglia and hippocampus tissues, when compared to their corresponding counterparts. Moreover, the levels of H3K18 lactylation and Pan‐Kla were notably increased in both senescent microglia and hippocampus tissues of naturally aged mice as well as AD modelling mice. The results suggest that focusing on this pathway may be a viable approach to slow down the ageing process and delay the onset of AD by reducing the effects of the senescence‐related secretory phenotype. 100 , 107
Hair follicle stem cells (HFSCs) undergo rapid activation and division during a new hair cycle. The quiescence of HFSCs is regulated by various intrinsic and extrinsic mechanisms as individuals age. HFSCs employ glycolytic metabolism and exhibit higher lactate production compared to other epidermal cells. Moreover, lactate generation plays a crucial role in the activation of HFSCs, as evidenced by the prevention of their activation upon deletion of LDH. While this study primarily focuses on the involvement of lactate in HFSC activation, further investigation is needed to explore the role of lactylation in successive studies. 108
Furthermore, it is important to note that the microenvironment within cancerous tissues exhibits immunosuppressive and pro‐tumourigenic characteristics, often characterised by elevated levels of senescence‐associated secretory phenotype factors across various cancer types. 109 In contrast, tissues impacted by chronic inflammatory diseases exhibit a pro‐inflammatory milieu that impedes the resolution mechanisms. 87 Despite the divergent immunological conditions, the metabolic profiles within the tissue microenvironments of cancer and inflammatory diseases are analogous: both are characterised by hypoxia, heightened lactate levels and other metabolic by‐products, as well as diminished nutrient levels. 110 , 111 While several studies have suggested the substantial involvement of ageing in tumourigenesis, the precise role of histone lactylation remains enigmatic and necessitates further investigation.
2.4. Histone lactylation and stemness maintenance
The process of somatic cell reprogramming offers valuable insights into the fundamental mechanisms underlying cell fate determination, particularly in relation to the regulation of stemness. 112 , 113 In a study conducted by Li et al., it was demonstrated that Glis1 directly interacts with chromatin, resulting in the activation of glycolytic genes while repressing somatic genes, ultimately leading to an upregulation of glycolysis. Furthermore, this increased glycolytic activity promotes higher levels of cellular acetyl‐CoA and lactate, which in turn enhance acetylation and lactylation at gene loci associated with pluripotency. This process facilitates the opening of these loci, thereby facilitating cellular reprogramming. 114
Moreover, lactate exhibits the potential to augment the stemness of CD8+ T cells and fortify the immune response directed towards tumours. Examination employing single cell transcriptomics reveals an elevated ratio of CD8+ T cells expressing the stem‐like T‐cell factor 1 (TCF‐1) marker within intra‐tumoural CD3+ cells. This characteristic has been corroborated via in vitro lactate treatment of T cells. The effects of lactate are mediated by the inhibition of histone deacetylase activity, resulting in increased acetylation at the H3K27 site of the Tcf7 super enhancer locus. This, in turn, leads to the upregulation of Tcf7 gene expression. Conversely, the modulation of abnormal lactate levels through the inhibition of LDH also promotes the stemness of CD8+ T cells and initiates immune responses against tumours. Collectively, these findings suggest that lactate may play a dual role in regulating the stemness of T cells. 115
3. CANCER THERAPY TARGETING HISTONE LACTYLATION
During cancerous initiation and progression, lactate has been traditionally regarded as a by‐product of aerobic glycolysis in metabolism. 108 Nevertheless, an increasing number of studies have indicated that lactate has the ability to control the advancement of cancer through various mechanisms including but not limited to cell cycle control, immune system suppression and energy metabolism. The recent discovery of lactylation has attracted considerable attention and has become a prominent topic of conversation in the realm of cancer studies. While several studies have demonstrated the successful therapeutic efficacy of targeting lactate metabolism in various types of cancer (Figure 4 and Table 2), the specific modulation of histone lactylation remains unresolved.
FIGURE 4.

Examples of targeting lactate metabolism in cancer treatment, including neuroblastoma, lung cancer, hepatocellular carcinoma, etc.
TABLE 2.
Histone lactylation targeted drugs in cancer therapy.
| Targets | Drugs | Cells and species | Drug dose and exposure time | Reference |
|---|---|---|---|---|
| LDH | Oxamate | Nasopharyngeal carcinoma cells; human | 100 mmol/L; 72 h | 131 |
| PS™B | Colon cancer cells; human | 50 µmol/L; 24 h | 2 | |
| N‐hydroxyindole | Pancreatic carcinoma cells; human | 100 µmol/L; 48 h | 132 | |
| HK | Lonidamine | Melanoma and breast cancer cells; human | 300 µg/mL; 24 h | 126 |
| 2‐DG | Cervix adenocarcinoma cells; human | 10 mmol/L; 72 h | 133 | |
| WP‐1122 | Nude mice implanted with glioblastoma cells; human | 2.5 g/kg; 40 days | 134 | |
| GLUT | STF‐31 | Glioblastoma cells, oral squamous cell carcinoma cells, cervix adenocarcinoma cells, head neck cancer cells, colon carcinoma cells and osteosarcoma cells; human | 100 µmol/L; 24 h | 135 |
| WZB117 | Glioblastoma cells, oral squamous cell carcinoma cells, cervix adenocarcinoma cells, head neck cancer cells, colon carcinoma cells and osteosarcoma cells; human | 100 µmol/L; 24 h | 135 | |
| BAY‐876 | Triple‐negative breast cancer cells; human | 1 µmol/L; 5 days | 136 | |
| PFKFB3 | 3‐PO | Jurkat cells (T leukaemia cells); human | 10 µmol/L; 36 h | 137 |
| PFK‐158 | Small cell lung cancer cells; human | 20 µmol/L; 24 h | 138 | |
| AZ‐67 | Triple‐negative breast cancer cells; human | 15 nmol/L; 48 h | 139 | |
| PKM | Compound 3K | Ovarian cancer cells; human | 15 µmol/L; 48 h | 140 |
| Shikonin | Ovarian cancer cells; human | 1.5 µmol/L; 48 h | 141 | |
| Alkannin | Ovarian cancer cells; human | 20 µmol/L; 12 h | 142 | |
| SGLT | Phlorizin | Esophageal squamous cell carcinoma cells; human | 1.6 mmol/L; 72 h | 143 |
| Canagliflozin | Hepatocellular carcinoma cells; human | 15 µmol/L; 48 h | 144 | |
| Dapagliflozin | Colon cancer cells; human | .5 mmol/L; 35 min | 145 | |
| MCT | Simvastatin | Triple‐negative breast cancer cells; human | 20 µg/mL; 48 h | 146 |
| Quercetin | Colorectal cancer cells; human | 200 mmol/L; 24 h | 147 | |
| DIDS | Prostate cancer cells; human | 1 mmol/L; 48 h | 148 |
Abbreviations: HK, Hexokinase; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; PFKFB3, 6‐Phosphofructo‐2‐Kinase/Fructose‐2,6‐Bisphosphatase 3; PKM, pyruvate kinase M; SGLT, Sodium‐Glucose Cotransporter 1.
3.1. Targeting LDHs for cancer treatment
The LDH enzyme complex, composed of LDHA and LDHB subunits, has five different isoforms that are determined by the combination of LDHA and LDHB subunits. 47 The reduction of pyruvate to lactate is catalysed by LDHA, which occurs simultaneously with the regeneration of NAD+. On the other hand, LDHB is responsible for mediating the reverse reaction. Significantly, LDHA is highly expressed in various tumour tissues and is associated with adverse prognostic outcomes in individuals with cancer. 98
Preclinical trials have evaluated various LDHA inhibitors for their effectiveness. Oxamate, a compound similar to pyruvate and a competitive inhibitor of LDH, hinders the transformation of pyruvate into lactate, thereby affecting cellular glycolysis. 116 Studies have recorded that the application of oxamate to lymphoma cells hinders the advancement of acute lymphoblastic leukaemia cells Jurkat and DU528. 117 According to records, oxamate has been observed to hinder the growth of cells in nasopharyngeal and gastric cancer. 118 Gossypol, FX11 and quinoline 3‐sulphonamides are part of the group of LDHA inhibitors that compete with nicotinamide adenine dinucleotide. Several research studies have extensively recorded the effectiveness of these in inhibiting the proliferation of different types of cancer cells, such as those found in the colon, neuroblastoma, HCC, primary pancreatic cancer, melanoma and breast cancer. 118 Furthermore, N‐hydroxyindole‐based inhibitor of lactated dehydrogenase (NHI), galloflavin and [(R)‐3‐((2‐chlorophenyl)thio)‐4‐hydroxy‐6‐(4‐morpholinophenyl)‐6‐(thiophen‐3‐yl)‐5,6‐dihydropyridin‐2(1H)‐one] ((R)‐GNE‐140), functioning as LDHA inhibitors that compete with NADH, have exhibited the capability to impede the growth of cancerous cells, particularly those found in pancreatic and colorectal cancer.
Several specific compounds, such as 1‐(phenylseleno)‐4‐(trifluoromethyl) benzene (PSTMB), phthalimide and dibenzofuran derivatives, have shown the ability to function as LDHA inhibitors, effectively impeding the development and multiplication of cancer cells. 119 Particularly noteworthy are the novel LDH inhibitors, phthalimide and dibenzofuran derivatives, which display selective inhibition towards the LDHA isoenzyme. 120 Furthermore, recent studies have emphasised the inhibitory properties of small interfering RNAs (siRNAs) targeting LDHA. The investigation into the utilisation of siRNAs to specifically target LDHA with the intention of impeding the progression of cancer by inducing oxidative stress and cell death has demonstrated their potential as a therapeutic strategy for LDHA‐dependent malignancies. 121 Furthermore, the oncogenic driver Ewing's sarcoma breakpoint region 1‐friend leukemia virus integration 1 (EWS‐FLI1) in Ewing sarcoma has been observed to impede the proliferation of Ewing sarcoma cells by targeting the transcription of LDHA. 122 Consequently, these discoveries provide a solid basis for the advancement of LDHA inhibitors. Nevertheless, the development of more potent and selective compounds remains a significant obstacle that must be overcome to facilitate their clinical application.
3.2. Targeting MCTs for cancer treatment
MCTs are members of the solute carrier (SLC) transporter family, which encompasses 52 distinct membrane transporter families. Within this group, MCT1 and MCT4 play a pivotal role in the uptake and release of lactate, as well as the transportation of other monocarboxylates including pyruvate, β‐hydroxybutyrate and acetate. 69 , 123 The efficacy of lactate transport is contingent upon the intracellular and extracellular concentrations of lactate, pH levels and the concentrations of other MCT substrates. Multiple types of human cancer, including glioma, breast, colorectal, gastric, cervical and neuroblastoma, exhibit heightened expression of MCT1 and MCT4, which has been linked to unfavourable prognostic outcomes. 124 Inhibition of MCT4 prompts the buildup of intracellular lactate and consequent demise of hypoxic cancer cells. Experimental suppression of MCT4 has demonstrated its essential role in the migratory and invasive capabilities of lung cancer cells. 125 Consequently, directing therapeutic efforts towards MCTs holds promise as a viable strategy for cancer treatment.
Numerous MCT blockers have exhibited effectiveness in preclinical experiments. Various malignancies, such as cervical cancer, breast cancer, colorectal cancer, prostate cancer, pharyngeal squamous cell carcinoma and other types of cancer, have been effectively treated using classical inhibitors of MCT1/MCT4, such as quercetin, phloretin and α‐cyano4‐hydroxycinnamate. 126 Additionally, lonidamine, DIDS, simvastatin and other MCT inhibitors have exhibited therapeutic potential for a range of cancer types. 126 , 127 Additionally, the chaperone protein CD147, which is common to both MCT1 and MCT4, has been observed to promote the migration, invasion and metastasis of cancer cells. Therefore, the targeting of CD147 offers a promising and innovative strategy for suppressing the function of both transporters. 128 Notably, p‐chloromercuric besylate, an organomercury compound, and AC‐73, which selectively hinders CD147 dimerisation, are among the compounds that disrupt MCT binding to CD147. Nevertheless, the clinical utilisation of CD147 is constrained by its involvement as a co‐chaperone for other membrane proteins, thereby prompting concerns regarding safety. 129
4. CONCLUSIONS AND FUTURE DIRECTIONS
Lactate serves as both a terminus of glycolysis and a universal metabolic fuel for energy, as evidenced by the emerging concept of the ‘lactate shuttle’ facilitating its movement between cells and transmission of signals. 89 , 95 , 108 The prominence of glycolytic‐dependent metabolism in tumours and rapidly proliferating cells has positioned lactate as a crucial participant in the reprogramming of energy metabolism, allowing cells to efficiently acquire ample energy within a limited timeframe. 60 , 126 In addition, lactate has the potential to create advantageous circumstances for the development of tumours through its influence on the acidic microenvironment of the tumour, the recruitment of immune cells, and other mechanisms.
In addition to its fundamental role in accelerating tumourigenesis, histone lactylation also serves as a significant diagnostic marker in various cancers, such as ocular melanoma, 94 colorectal cancer 4 and HCC. 95 Notably, elevated levels of global lactylation (pan‐lactylation) are specifically upregulated in tumour tissues, indicating a higher likelihood of earlier recurrence and increased aggressiveness in ocular melanomas. 94 Furthermore, there is a significant correlation between elevated levels of pan‐lactylation and H3K18la and decreased overall survival rates in individuals diagnosed with colorectal cancer. 4 Additionally, it has been observed that HCC patients also exhibit heightened lactylation levels, resulting in the subsequent upregulation of adenylate kinase 2 lactylation. This molecular alteration further promotes the proliferation and metastasis of HCC. 95 Cumulatively, these findings substantiate the notion that aberrant histone lactylation is significantly elevated across diverse tumour classifications, thus exerting a pivotal influence on the malignant progression of cancer. Nonetheless, the absence of a comprehensive evaluation regarding the disparity in histone lactylation levels between metastatic sites and primary tumours necessitates further investigation, thereby warranting future exploratory endeavours.
Furthermore, the newly discovered occurrence of lactate‐induced lactylation enhances our comprehension of the pro‐tumourigenesis consequences of lactate generation, circulation and utilisation. 66 , 128 Analogous to other epigenetic alterations, lactylation possesses the capacity to alter histone proteins, thereby influencing the spatial organisation of chromatin, affecting DNA accessibility and governing the expression of related genes. Moreover, the degree of lactylation is intricately associated with the localised concentration of lactate, establishing a correlation between epigenetics and metabolic reprogramming. 18 , 19 , 109
Significantly, this field encounters numerous challenges. Primarily, histone lactylation exhibits shared ‘writers’ and ‘erasers’ with other types of histone modifications, rendering the specific modulation of histone lactylation notably difficult. For example, most studies have silenced EP300 to reduce histone lactylation level, which simultaneously reduced the global acetylation levels. Therefore, it is important to find a novel method to specifically modulate global lactate level. Additionally, therapeutic endeavours predominantly focus on multi‐functional LDHs and MCTs. Apart from regulating the lactate cascade, these enzymes also exert a crucial influence on other chemical reactions. Consequently, the development of a specific inhibitor targeting histone lactylation necessitates further investigation in our subsequent studies. In addition, the majority of studies substantiate the oncogenic function of histone lactylation in both the initiation and advancement of tumourigenesis. Nevertheless, it is imperative to thoroughly investigate whether histone lactylation may also exert an inhibitory influence on tumour progression, as most modifications exhibit a dualistic impact on carcinogenesis.
In light of the significant role of lactate signalling in diverse cellular processes, it is crucial to conduct thorough investigations into the underlying mechanisms of oncometabolic‐mediated epigenetic reprogramming in future research endeavours. Considering that histone lactylation is indicative of an open chromatin state, which is closely associated with super‐enhancer and 3D‐chromosome architecture, it is warranted to pursue further endeavours aimed at developing a more precise model for epigenetic modulation. By investigating the correlation between histone lactylation and cancer characteristics, we propose that histone lactylation plays a crucial role in predisposing cells to a malignant state, highlighting the significance of identifying innovative therapeutic approaches or dual‐targeting methods to combat lactylation for effective cancer management.
AUTHOR CONTRIBUTIONS
Meili Li and Peirong Lu planned the project. Yu Zhang and Hang Song collected relevant information and clinical samples. All authors analysed the data. Yu Zhang wrote the paper. All authors discussed the results and commented on the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGEMENTS
This study was supported by grants from the Suzhou Municipal Health Commission (no. KJXW2020077) and the Science and Technology Program of Suzhou (nos. SKJY2021015 and SYSD2020048).
Zhang Y, Song H, Li M, Lu P. Histone lactylation bridges metabolic reprogramming and epigenetic rewiring in driving carcinogenesis: Oncometabolite fuels oncogenic transcription. Clin Transl Med. 2024;14:e1614. 10.1002/ctm2.1614
Yu Zhang and Hang Song authors contributed equally to this manuscript.
Contributor Information
Meili Li, Email: limeili0319@163.com.
Peirong Lu, Email: lupeirong@suda.edu.cn.
DATA AVAILABILITY STATEMENT
All relevant data are available from the authors upon request.
REFERENCES
- 1. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alwahsh M, Knitsch R, Marchan R, et al. Metabolic profiling of thymic epithelial tumors hints to a strong Warburg effect, glutaminolysis and precarious redox homeostasis as potential therapeutic targets. Cancers (Basel). 2022;14(6):1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daverio Z, Balcerczyk A, Rautureau GJP, Panthu B. How Warburg‐associated lactic acidosis rewires cancer cell energy metabolism to resist glucose deprivation. Cancers (Basel). 2023;15(5):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li W, Zhou C, Yu L, et al. Tumor‐derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. 2023;20(1):114‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitamura F, Semba T, Yasuda‐Yoshihara N, et al. Cancer‐associated fibroblasts reuse cancer‐derived lactate to maintain a fibrotic and immunosuppressive microenvironment in pancreatic cancer. JCI Insight. 2023;8(20):e163022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dou X, Fu Q, Long Q, et al. PDK4‐dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat Metab. 2023;5(11):1887‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chai P, Jia R, Li Y, et al. Regulation of epigenetic homeostasis in uveal melanoma and retinoblastoma. Prog Retin Eye Res. 2022;89:101030. [DOI] [PubMed] [Google Scholar]
- 8. Chai P, Yu J, Wang X, Ge S, Jia R. BMP9 promotes cutaneous wound healing by activating Smad1/5 signaling pathways and cytoskeleton remodeling. Clin Transl Med. 2021;11(1):e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chai P, Lan P, Li S, et al. Mechanistic insight into allosteric activation of human pyruvate carboxylase by acetyl‐CoA. Mol Cell. 2022;82(21):4116‐4130.e4116. [DOI] [PubMed] [Google Scholar]
- 10. Wang T, Ye Z, Li Z, et al. Lactate‐induced protein lactylation: a bridge between epigenetics and metabolic reprogramming in cancer. Cell Prolif. 2023;56(10):e13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J, Xu W, Wu Y, et al. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene. 2023;42(45):3319‐3330. [DOI] [PubMed] [Google Scholar]
- 12. Guo W, Zhou B, Bie F, et al. Single‐cell RNA sequencing analysis reveals transcriptional heterogeneity of multiple primary lung cancer. Clin Transl Med. 2023;13(10):e1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong MCS, Huang J, Liang PS. Is the practice of colorectal cancer screening questionable after the NordICC trial was published? Clin Transl Med. 2023;13(10):e1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu ZM, Huo FC, Zhang J, Shan HJ, Pei DS. Crosstalk between m6A modification and alternative splicing during cancer progression. Clin Transl Med. 2023;13(10):e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Y, Ji Z, Gong Y, et al. Numb/Parkin‐directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023;42(2):112033. [DOI] [PubMed] [Google Scholar]
- 16. Morris O, Deng H, Tam C, Jasper H. Warburg‐like metabolic reprogramming in aging intestinal stem cells contributes to tissue hyperplasia. Cell Rep. 2020;33(8):108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Visan I. Histone lactylation. Nat Immunol. 2019;20(12):1558. [DOI] [PubMed] [Google Scholar]
- 18. Izzo LT, Wellen KE. Histone lactylation links metabolism and gene regulation. Nature. 2019;574(7779):492‐493. [DOI] [PubMed] [Google Scholar]
- 19. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun L, Zhang Y, Yang B, et al. Lactylation of METTL16 promotes cuproptosis via m(6)A‐modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14(1):6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang ZW, Zhang XN, Zhang L, et al. STAT5 promotes PD‐L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. 2023;8(1):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pandkar MR, Sinha S, Samaiya A, Shukla S. Oncometabolite lactate enhances breast cancer progression by orchestrating histone lactylation‐dependent c‐Myc expression. Transl Oncol. 2023;37:101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhat PJ, Darunte L, Kareenhalli V, Dandekar J, Kumar A. Can metabolic plasticity be a cause for cancer? Warburg–Waddington legacy revisited. Clin Epigenetics. 2011;2(2):113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siska PJ, Singer K, Evert K, Renner K, Kreutz M. The immunological Warburg effect: can a metabolic‐tumor‐stroma score (MeTS) guide cancer immunotherapy? Immunol Rev. 2020;295(1):187‐202. [DOI] [PubMed] [Google Scholar]
- 25. Lee CH, Gundem G, Lee W, et al. Persistent severe hyperlactatemia and metabolic derangement in lethal SDHB‐mutated metastatic kidney cancer: clinical challenges and examples of extreme Warburg effect. JCO Precis Oncol. 2017:1:PO.16.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothman DL, Shulman RG. Two transition states of the glycogen shunt and two steady states of gene expression support metabolic flexibility and the Warburg effect in cancer. Neoplasia. 2021;23(9):879‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez‐Cenizo L, Formentini L, Aldea M, et al. Up‐regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+‐ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J Biol Chem. 2010;285(33):25308‐25313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazzio EA, Boukli N, Rivera N, Soliman KF. Pericellular pH homeostasis is a primary function of the Warburg effect: inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci. 2012;103(3):422‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang SH, Li J, Dong FY, et al. Increased serotonin signaling contributes to the Warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;153(1):277‐291.e219. [DOI] [PubMed] [Google Scholar]
- 30. Wilde L, Roche M, Domingo‐Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu S, Han L, Hu X, et al. N6‐methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: implication in colorectal cancer. J Hematol Oncol. 2021;14(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cucielo MS, Cesario RC, Silveira HS, et al. Melatonin reverses the Warburg‐type metabolism and reduces mitochondrial membrane potential of ovarian cancer cells independent of MT1 receptor activation. Molecules. 2022;27(14):4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jenniskens JCA, Offermans K, Simons C, et al. Energy balance‐related factors and risk of colorectal cancer expressing different levels of proteins involved in the Warburg effect. Cancer Epidemiol Biomarkers Prev. 2022;31(3):633‐646. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Sun Z, Liang M, et al. An unrevealed molecular function of corannulene Buckybowl glycoconjugates in selective tumor annihilation by targeting the cancer‐specific Warburg effect. Adv Sci (Weinh). 2022;9(10):e2105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitroda SP, Wakim BT, Sood RF, et al. STAT1‐dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC Med. 2009;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asgari Y, Zabihinpour Z, Salehzadeh‐Yazdi A, Schreiber F, Masoudi‐Nejad A. Alterations in cancer cell metabolism: the Warburg effect and metabolic adaptation. Genomics. 2015;105(5‐6):275‐281. [DOI] [PubMed] [Google Scholar]
- 38. Vadivalagan C, Krishnan A, Chen SJ, et al. The Warburg effect in osteoporosis: cellular signaling and epigenetic regulation of energy metabolic events to targeting the osteocalcin for phenotypic alteration. Cell Signal. 2022;100:110488. [DOI] [PubMed] [Google Scholar]
- 39. Chen F, Liao J, Wu P, et al. Oridonin inhibits the occurrence and development of colorectal cancer by reversing the Warburg effect via reducing PKM2 dimer formation and preventing its entry into the nucleus. Eur J Pharmacol. 2023;954:175856. [DOI] [PubMed] [Google Scholar]
- 40. Zhong X, He X, Wang Y, et al. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J Hematol Oncol. 2022;15(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyu Y, Zhang Y, Wang Y, et al. HIF‐1alpha regulated WTAP overexpression promoting the Warburg effect of ovarian cancer by m6A‐dependent manner. J Immunol Res. 2022;2022:6130806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerlinger M, Santos CR, Spencer‐Dene B, et al. Genome‐wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J Pathol. 2012;227(2):146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol. 2012;13(4):352‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin L, Huang H, Liao W, et al. MACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effect. Oncogene. 2015;34(21):2700‐2710. [DOI] [PubMed] [Google Scholar]
- 45. Hardonniere K, Saunier E, Lemarie A, et al. The environmental carcinogen benzo[a]pyrene induces a Warburg‐like metabolic reprogramming dependent on NHE1 and associated with cell survival. Sci Rep. 2016;6:30776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pavlides S, Tsirigos A, Vera I, et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer's disease, and “neuron‐glia metabolic coupling”. Aging (Albany NY). 2010;2(4):185‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Y, Ma H. circRNA PLOD2 promotes tumorigenesis and Warburg effect in colon cancer by the miR‐513a‐5p/SIX1/LDHA axis. Cell Cycle. 2022;21(23):2484‐2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu H, Liang W, Han M, et al. Mechanisms regulating wound healing: functional changes in biology mediated by lactate and histone lactylation. J Cell Physiol. 2023;238(10):2243‐2252. [DOI] [PubMed] [Google Scholar]
- 49. Huang Q, Li S, Chen X, et al. Association between serum lactate dehydrogenase and lymph node metastasis in cervical cancer. Oncol Lett. 2023;26(5):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chai P, Ni H, Zhang H, Fan X. The evolving functions of autophagy in ocular health: a double‐edged sword. Int J Biol Sci. 2016;12(11):1332‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guido C, Whitaker‐Menezes D, Capparelli C, et al. Metabolic reprogramming of cancer‐associated fibroblasts by TGF‐beta drives tumor growth: connecting TGF‐beta signaling with “Warburg‐like” cancer metabolism and L‐lactate production. Cell Cycle. 2012;11(16):3019‐3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pan L, Feng F, Wu J, et al. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270. [DOI] [PubMed] [Google Scholar]
- 53. Cao Z, Xu D, Harding J, et al. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Sci Transl Med. 2023;15(717):eadd2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rastogi S, Mishra SS, Arora MK, et al. Lactate acidosis and simultaneous recruitment of TGF‐beta leads to alter plasticity of hypoxic cancer cells in tumor microenvironment. Pharmacol Ther. 2023;250:108519. [DOI] [PubMed] [Google Scholar]
- 55. Peng J, Li W, Tan N, Lai X, Jiang W, Chen G. USP47 stabilizes BACH1 to promote the Warburg effect and non‐small cell lung cancer development via stimulating Hk2 and Gapdh transcription. Am J Cancer Res. 2022;12(1):91‐107. [PMC free article] [PubMed] [Google Scholar]
- 56. Chen IT, Aoki T, Huang YT, et al. White spot syndrome virus induces metabolic changes resembling the Warburg effect in shrimp hemocytes in the early stage of infection. J Virol. 2011;85(24):12919‐12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sgarbi G, Righetti R, Del Dotto V, et al. The pro‐oncogenic protein IF(1) does not contribute to the Warburg effect and is not regulated by PKA in cancer cells. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166879. [DOI] [PubMed] [Google Scholar]
- 58. Jaworska M, Szczudlo J, Pietrzyk A, et al. The Warburg effect: a score for many instruments in the concert of cancer and cancer niche cells. Pharmacol Rep. 2023;75(4):876‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frisardi V, Canovi S, Vaccaro S, Frazzi R. The significance of microenvironmental and circulating lactate in breast cancer. Int J Mol Sci. 2023;24(20):15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scott DA, Richardson AD, Filipp FV, et al. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J Biol Chem. 2011;286(49):42626‐42634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie F, Zhang H, Zhu K, et al. PRMT5 promotes ovarian cancer growth through enhancing Warburg effect by methylating ENO1. MedComm (2020). 2023;4(2):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kodama M, Nakayama KI. A second Warburg‐like effect in cancer metabolism: the metabolic shift of glutamine‐derived nitrogen: a shift in glutamine‐derived nitrogen metabolism from glutaminolysis to de novo nucleotide biosynthesis contributes to malignant evolution of cancer. Bioessays. 2020;42(12):e2000169. [DOI] [PubMed] [Google Scholar]
- 63. Galicia‐Vazquez G, Aloyz R. Metabolic rewiring beyond Warburg in chronic lymphocytic leukemia: how much do we actually know? Crit Rev Oncol Hematol. 2019;134:65‐70. [DOI] [PubMed] [Google Scholar]
- 64. Shlomi T, Benyamini T, Gottlieb E, Sharan R, Ruppin E. Genome‐scale metabolic modeling elucidates the role of proliferative adaptation in causing the Warburg effect. PLoS Comput Biol. 2011;7(3):e1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dai W, Shen J, Yan J, et al. Glutamine synthetase limits beta‐catenin‐mutated liver cancer growth by maintaining nitrogen homeostasis and suppressing mTORC1. J Clin Invest. 2022;132(24):e161408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Encarnacion‐Rosado J, Sohn ASW, Biancur DE, et al. Targeting pancreatic cancer metabolic dependencies through glutamine antagonism. Nat Cancer. 2023;5:85‐99. doi: 10.1038/s43018-023-00647-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Quek LE, van Geldermalsen M, Guan YF, et al. Glutamine addiction promotes glucose oxidation in triple‐negative breast cancer. Oncogene. 2022;41(34):4066‐4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Linder SJ, Bernasocchi T, Martinez‐Pastor B, et al. Inhibition of the proline metabolism rate‐limiting enzyme P5CS allows proliferation of glutamine‐restricted cancer cells. Nat Metab. 2023;5(12):2131‐2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blaszczak W, Williams H, Swietach P. Autoregulation of H(+)/lactate efflux prevents monocarboxylate transport (MCT) inhibitors from reducing glycolytic lactic acid production. Br J Cancer. 2022;127(7):1365‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cortes‐Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, Garcia MA. MCT expression and lactate influx/efflux in tanycytes involved in glia–neuron metabolic interaction. PLoS One. 2011;6(1):e16411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Broer S, Rahman B, Pellegri G, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272(48):30096‐30102. [DOI] [PubMed] [Google Scholar]
- 72. Lopez E, Karattil R, Nannini F, et al. Inhibition of lactate transport by MCT‐1 blockade improves chimeric antigen receptor T‐cell therapy against B‐cell malignancies. J Immunother Cancer. 2023;11(6):e006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Offermans K, Jenniskens JC, Simons CC, et al. Expression of proteins associated with the Warburg‐effect and survival in colorectal cancer. J Pathol Clin Res. 2022;8(2):169‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gallagher FA, Woitek R, McLean MA, et al. Imaging breast cancer using hyperpolarized carbon‐13 MRI. Proc Natl Acad Sci U S A. 2020;117(4):2092‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martins SF, Amorim R, Viana‐Pereira M, et al. Significance of glycolytic metabolism‐related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer. 2016;16:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao H, Yan C, Hu Y, et al. Differentiated cancer cell‐originated lactate promotes the self‐renewal of cancer stem cells in patient‐derived colorectal cancer organoids. Cancer Lett. 2020;493:236‐244. [DOI] [PubMed] [Google Scholar]
- 77. Luz MC, Perez MM, Azzalis LA, et al. Evaluation of MCT1, MCT4 and CD147 genes in peripheral blood cells of breast cancer patients and their potential use as diagnostic and prognostic markers. Int J Mol Sci. 2017;18(4):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang N, Jiang X, Zhang S, et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti‐cancer drug candidates. Cell. 2021;184(2):370‐383.e313. [DOI] [PubMed] [Google Scholar]
- 79. Williams JA. Using PDX for preclinical cancer drug discovery: the evolving field. J Clin Med. 2018;7(3):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Krajnak S, Battista MJ, Hasenburg A, Schmidt M. Metronomic chemotherapy for metastatic breast cancer. Oncol Res Treat. 2022;45(1‐2):12‐17. [DOI] [PubMed] [Google Scholar]
- 81. Palakurthi B, Fross SR, Guldner IH, et al. Targeting CXCL16 and STAT1 augments immune checkpoint blockade therapy in triple‐negative breast cancer. Nat Commun. 2023;14(1):2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pertega‐Gomes N, Felisbino S, Massie CE, et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: a role for monocarboxylate transporters as metabolic targets for therapy. J Pathol. 2015;236(4):517‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soni VK, Shukla D, Kumar A, Vishvakarma NK. Curcumin circumvent lactate‐induced chemoresistance in hepatic cancer cells through modulation of hydroxycarboxylic acid receptor‐1. Int J Biochem Cell Biol. 2020;123:105752. [DOI] [PubMed] [Google Scholar]
- 84. Brown TP, Bhattacharjee P, Ramachandran S, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen‐presenting cells in the tumor microenvironment. Oncogene. 2020;39(16):3292‐3304. [DOI] [PubMed] [Google Scholar]
- 85. Feng J, Yang H, Zhang Y, et al. Tumor cell‐derived lactate induces TAZ‐dependent upregulation of PD‐L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36(42):5829‐5839. [DOI] [PubMed] [Google Scholar]
- 86. Lundo K, Trauelsen M, Pedersen SF, Schwartz TW. Why Warburg works: lactate controls immune evasion through GPR81. Cell Metab. 2020;31(4):666‐668. [DOI] [PubMed] [Google Scholar]
- 87. Cheng WY, Huynh H, Chen P, Pena‐Llopis S, Wan Y. Macrophage PPARgamma inhibits Gpr132 to mediate the anti‐tumor effects of rosiglitazone. Elife. 2016;5:e18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nii T, Prabhu VV, Ruvolo V, et al. Imipridone ONC212 activates orphan G protein‐coupled receptor GPR132 and integrated stress response in acute myeloid leukemia. Leukemia. 2019;33(12):2805‐2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen P, Zuo H, Xiong H, et al. Gpr132 sensing of lactate mediates tumor‐macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114(3):580‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lahvic JL, Ammerman M, Li P, et al. Specific oxylipins enhance vertebrate hematopoiesis via the receptor GPR132. Proc Natl Acad Sci U S A. 2018;115(37):9252‐9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vadevoo SMP, Gunassekaran GR, Lee C, et al. The macrophage odorant receptor Olfr78 mediates the lactate‐induced M2 phenotype of tumor‐associated macrophages. Proc Natl Acad Sci U S A. 2021;118(37):e2102434118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Galle E, Wong CW, Ghosh A, et al. H3K18 lactylation marks tissue‐specific active enhancers. Genome Biol. 2022;23(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Fan W, Li N, et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation‐mediated upregulation of FGF2. Genome Biol. 2023;24(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yu J, Chai P, Xie M, et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang Z, Yan C, Ma J, et al. Lactylome analysis suggests lactylation‐dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. 2023;5(1):61‐79. [DOI] [PubMed] [Google Scholar]
- 96. Chen J, Zhang M, Liu Y, et al. Histone lactylation driven by mROS‐mediated glycolytic shift promotes hypoxic pulmonary hypertension. J Mol Cell Biol. 2023;14(12):mjac073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang JL, Dou XD, Cheng J, et al. Functional screening and rational design of compounds targeting GPR132 to treat diabetes. Nat Metab. 2023;5(10):1726‐1746. [DOI] [PubMed] [Google Scholar]
- 98. Jenniskens JCA, Offermans K, Simons C, et al. Energy balance‐related factors in childhood and adolescence and risk of colorectal cancer expressing different levels of proteins involved in the Warburg‐effect. Int J Cancer. 2022;150(11):1812‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Al‐Azzam N. Sirtuin 6 and metabolic genes interplay in Warburg effect in cancers. J Clin Biochem Nutr. 2020;66(3):169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pan RY, He L, Zhang J, et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer's disease. Cell Metab. 2022;34(4):634‐648 e636. [DOI] [PubMed] [Google Scholar]
- 101. Zdralevic M, Vucetic M, Daher B, Marchiq I, Parks SK, Pouyssegur J. Disrupting the ‘Warburg effect’ re‐routes cancer cells to OXPHOS offering a vulnerability point via ‘ferroptosis’‐induced cell death. Adv Biol Regul. 2018;68:55‐63. [DOI] [PubMed] [Google Scholar]
- 102. Sun S, Xu X, Liang L, et al. Lactic acid‐producing probiotic Saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front Immunol. 2021;12:777665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu J, Hu M, Jiang H, et al. Endothelial cell‐derived lactate triggers bone mesenchymal stem cell histone lactylation to attenuate osteoporosis. Adv Sci (Weinh). 2023;10(31):e2301300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Susser LI, Nguyen MA, Geoffrion M, et al. Mitochondrial fragmentation promotes inflammation resolution responses in macrophages via histone lactylation. Mol Cell Biol. 2023;43(10):531‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang N, Wang W, Wang X, et al. Histone lactylation boosts reparative gene activation post‐myocardial infarction. Circ Res. 2022;131(11):893‐908. [DOI] [PubMed] [Google Scholar]
- 106. Du S, Zhang X, Jia Y, et al. Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis‐mediated HMGB1 lactylation and secretion of hepatocytes. Theranostics. 2023;13(11):3856‐3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Traxler L, Herdy JR, Stefanoni D, et al. Warburg‐like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer's disease. Cell Metab. 2022;34(9):1248‐1263 e1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Flores A, Schell J, Krall AS, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19(9):1017‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Faget DV, Ren Q, Stewart SA. Unmasking senescence: context‐dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19(8):439‐453. [DOI] [PubMed] [Google Scholar]
- 110. Li X, Chen Y, Wang T, et al. GPR81‐mediated reprogramming of glucose metabolism contributes to the immune landscape in breast cancer. Discov Oncol. 2023;14(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ma R, Li X, Gong S, et al. Dual roles of lactate in EGFR‐TKI‐resistant lung cancer by targeting GPR81 and MCT1. J Oncol. 2022;2022:3425841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Menendez JA, Joven J, Cufi S, et al. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle. 2013;12(8):1166‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Miao Z, Zhao X, Liu X. Hypoxia induced beta‐catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Exp Cell Res. 2023;422(1):113439. [DOI] [PubMed] [Google Scholar]
- 114. Li L, Chen K, Wang T, et al. Glis1 facilitates induction of pluripotency via an epigenome–metabolome–epigenome signalling cascade. Nat Metab. 2020;2(9):882‐892. [DOI] [PubMed] [Google Scholar]
- 115. Hermans D, Gautam S, Garcia‐Canaveras JC, et al. Lactate dehydrogenase inhibition synergizes with IL‐21 to promote CD8(+) T cell stemness and antitumor immunity. Proc Natl Acad Sci U S A. 2020;117(11):6047‐6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xing BC, Wang C, Ji FJ, Zhang XB. Synergistically suppressive effects on colorectal cancer cells by combination of mTOR inhibitor and glycolysis inhibitor, Oxamate. Int J Clin Exp Pathol. 2018;11(9):4439‐4445. [PMC free article] [PubMed] [Google Scholar]
- 117. Yu H, Yin Y, Yi Y, et al. Targeting lactate dehydrogenase A (LDHA) exerts antileukemic effects on T‐cell acute lymphoblastic leukemia. Cancer Commun (Lond). 2020;40(10):501‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin J, Liu G, Chen L, Kwok HF, Lin Y. Targeting lactate‐related cell cycle activities for cancer therapy. Semin Cancer Biol. 2022;86(pt 3):1231‐1243. [DOI] [PubMed] [Google Scholar]
- 119. Kim EY, Chung TW, Han CW, et al. A novel lactate dehydrogenase inhibitor, 1‐(phenylseleno)‐4‐(trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci Rep. 2019;9(1):3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Friberg A, Rehwinkel H, Nguyen D, et al. Structural evidence for isoform‐selective allosteric inhibition of lactate dehydrogenase A. ACS Omega. 2020;5(22):13034‐13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Daneshmandi S, Wegiel B, Seth P. Blockade of lactate dehydrogenase‐A (LDH‐A) improves efficacy of anti‐programmed cell death‐1 (PD‐1) therapy in melanoma. Cancers (Basel). 2019;11(4):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jiang X, Chen Z, Zhu J, et al. E2F1 promotes Warburg effect and cancer progression via upregulating ENO2 expression in Ewing sarcoma. Mol Med Rep. 2022;26(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pereira‐Vieira J, Azevedo‐Silva J, Preto A, Casal M, Queiros O. MCT1, MCT4 and CD147 expression and 3‐bromopyruvate toxicity in colorectal cancer cells are modulated by the extracellular conditions. Biol Chem. 2019;400(6):787‐799. [DOI] [PubMed] [Google Scholar]
- 124. Singh M, Afonso J, Sharma D, et al. Targeting monocarboxylate transporters (MCTs) in cancer: how close are we to the clinics? Semin Cancer Biol. 2023;90:1‐14. [DOI] [PubMed] [Google Scholar]
- 125. Sprowl‐Tanio S, Habowski AN, Pate KT, et al. Lactate/pyruvate transporter MCT‐1 is a direct Wnt target that confers sensitivity to 3‐bromopyruvate in colon cancer. Cancer Metab. 2016;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ghafouri‐Fard S, Shabestari FA, Vaezi S, et al. Emerging impact of quercetin in the treatment of prostate cancer. Biomed Pharmacother. 2021;138:111548. [DOI] [PubMed] [Google Scholar]
- 128. Eichner R, Heider M, Fernandez‐Saiz V, et al. Immunomodulatory drugs disrupt the cereblon–CD147–MCT1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016;22(7):735‐743. [DOI] [PubMed] [Google Scholar]
- 129. Afonso J, Santos LL, Miranda‐Goncalves V, et al. CD147 and MCT1‐potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog. 2015;54(11):1451‐1466. [DOI] [PubMed] [Google Scholar]
- 130. Xiong J, He J, Zhu J, et al. Lactylation‐driven METTL3‐mediated RNA m(6)A modification promotes immunosuppression of tumor‐infiltrating myeloid cells. Mol Cell. 2022;82(9):1660‐1677.e1610. [DOI] [PubMed] [Google Scholar]
- 131. Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH‐A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30(6):2983‐2991. [DOI] [PubMed] [Google Scholar]
- 132. Granchi C, Roy S, Giacomelli C, et al. Discovery of N‐hydroxyindole‐based inhibitors of human lactate dehydrogenase isoform A (LDH‐A) as starvation agents against cancer cells. J Med Chem. 2011;54(6):1599‐1612. [DOI] [PubMed] [Google Scholar]
- 133. Lin X, Zhang F, Bradbury CM, et al. 2‐Deoxy‐D‐glucose‐induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63(12):3413‐3417. [PubMed] [Google Scholar]
- 134. Michel KA, Zielinski R, Walker CM, et al. Hyperpolarized pyruvate MR spectroscopy depicts glycolytic inhibition in a mouse model of glioma. Radiology. 2019;293(1):168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kraus D, Reckenbeil J, Veit N, et al. Targeting glucose transport and the NAD pathway in tumor cells with STF‐31: a re‐evaluation. Cell Oncol (Dordr). 2018;41(5):485‐494. [DOI] [PubMed] [Google Scholar]
- 136. Wu Q, Ba‐Alawi W, Deblois G, et al. GLUT1 inhibition blocks growth of RB1‐positive triple negative breast cancer. Nat Commun. 2020;11(1):4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Clem B, Telang S, Clem A, et al. Small‐molecule inhibition of 6‐phosphofructo‐2‐kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7(1):110‐120. [DOI] [PubMed] [Google Scholar]
- 138. Thirusangu P, Ray U, Sarkar Bhattacharya S, et al. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene. 2022;41(33):4003‐4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kashyap A, Umar SM, Dev JRA, et al. Combination of 3PO analog PFK15 and siPFKL efficiently suppresses the migration, colony formation ability, and PFK‐1 activity of triple‐negative breast cancers by reducing the glycolysis. J Cell Biochem. 2023;124(9):1259‐1272. [DOI] [PubMed] [Google Scholar]
- 140. Park JH, Kundu A, Lee SH, et al. Specific pyruvate kinase M2 inhibitor, compound 3K, induces autophagic cell death through disruption of the glycolysis pathway in ovarian cancer cells. Int J Biol Sci. 2021;17(8):1895‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ni M, Zhou J, Zhu Z, et al. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe(2+) accumulation. Phytomedicine. 2023;112:154701. [DOI] [PubMed] [Google Scholar]
- 142. Wang Y, Zhang J, Wang F, Chen W, Ma J, Wang H. Alkannin inhibits the development of ovarian cancer by affecting miR‐4461. Evid Based Complement Alternat Med. 2021;2021:5083302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jia Z, Xie Y, Wu H, et al. Phlorizin from sweet tea inhibits the progress of esophageal cancer by antagonizing the JAK2/STAT3 signaling pathway. Oncol Rep. 2021;46(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hung MH, Chen YL, Chen LJ, et al. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose‐influx‐induced beta‐catenin activation. Cell Death Dis. 2019;10(6):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Okada J, Yamada E, Saito T, et al. Dapagliflozin inhibits cell adhesion to collagen I and IV and increases ectodomain proteolytic cleavage of DDR1 by increasing ADAM10 activity. Molecules. 2020;25(3):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yao X, Xie R, Cao Y, et al. Simvastatin induced ferroptosis for triple‐negative breast cancer therapy. J Nanobiotechnology. 2021;19(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Amorim R, Pinheiro C, Miranda‐Goncalves V, et al. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5‐fluorouracil in colorectal cancer cells. Cancer Lett. 2015;365(1):68‐78. [DOI] [PubMed] [Google Scholar]
- 148. George K, Thomas NS, Malathi R. 4,4′‐Diisothiocyanatostilbene‐2,2′‐disulfonate modulates voltage‐gated K(+) current and influences cell cycle arrest in androgen sensitive and insensitive human prostate cancer cell lines. Toxicol Mech Methods. 2020;30(5):358‐369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available from the authors upon request.


