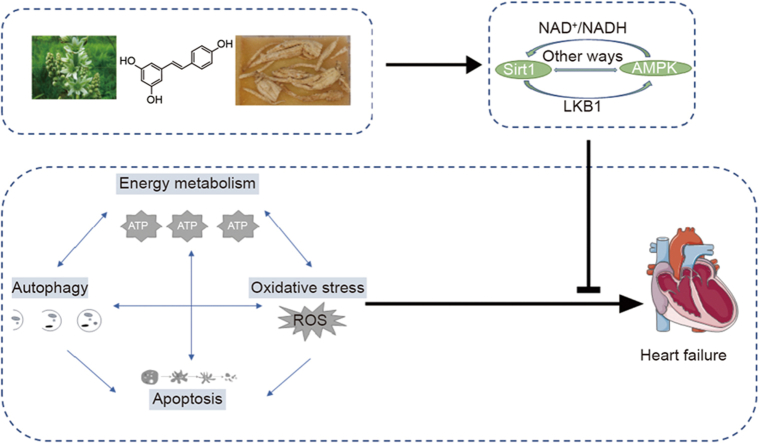
Abstract
Heart failure (HF) is a highly morbid syndrome that seriously affects the physical and mental health of patients and generates an enormous socio-economic burden. In addition to cardiac myocyte oxidative stress and apoptosis, which are considered mechanisms for the development of HF, alterations in cardiac energy metabolism and pathological autophagy also contribute to cardiac abnormalities and ultimately HF. Silent information regulator 1 (Sirt1) and adenosine monophosphate-activated protein kinase (AMPK) are nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases and phosphorylated kinases, respectively. They play similar roles in regulating some pathological processes of the heart through regulating targets such as peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), protein 38 mitogen-activated protein kinase (p38 MAPK), peroxisome proliferator-activated receptors (PPARs), and mammalian target of rapamycin (mTOR). We summarized the synergistic effects of Sirt1 and AMPK in the heart, and listed the traditional Chinese medicine (TCM) that exhibit cardioprotective properties by modulating the Sirt1/AMPK pathway, to provide a basis for the development of Sirt1/AMPK activators or inhibitors for the treatment of HF and other cardiovascular diseases (CVDs).
Keywords: Heart failure, Silent information regulator 1, Adenosine monophosphate-activated protein kinase, Traditional Chinese medicine
Graphical abstract
Highlights
-
•
Sirt1 and AMPK are essential targets for regulating HF.
-
•
Sirt1 and AMPK influence energy metabolism disorders, oxidative stress, autophagy disorders and excessive apoptosis in HF.
-
•
Many traditional Chinese medicines have been demonstrated to exert cardioprotective effect by regulating Sirt1/AMPK pathway.
1. Introduction
One of the leading causes of mortality in the world is heart failure (HF), which is characterized by the inability of the heart to effectively pump enough blood to meet the needs of the body for nourishment and oxygen [1]. Over 64.34 million people were affected by HF in 2020. The annual global economic burden of the disease is more than $300 billion. Between 1992 and 2020, the prevalence of HF increased by 3.9% in elderly people [2]. The median survival of patients with HF was only 2.1 years despite therapeutic advances, and the 5-year mortality hazard ratio of hospitalized patients with HF was approaching 76% [3,4]. People with HF typically show symptoms of exercise intolerance, and some with tension, concern, shortness of breath, and lack of energy [5,6].
HF is a clinical syndrome with complex pathophysiology, characterized by phenotypes including insufficient myocardial contractility, diminished response to stresses, loss of cardiomyocytes, and increased fibrosis [7]. Impaired energy metabolism is one of the cornerstones in the pathophysiology of HF. Compromised mitochondrial oxidative phosphorylation leads to multiple defects in both energy supply and demand within the failing heart, resulting in an organ that is both energy-depleted and dysfunctional [8]. Oxidative stress is also an essential pathophysiological mechanism of HF, abnormalities in the oxidative and antioxidant sates causing oxidative stress were found in HF of various etiologies [9]. Excessive intracellular reactive oxygen species (ROS) accumulation directly impairs the physiological function of cardiomyocytes (including electrophysiology and the contractile machinery), subsequently promoting heart fibrosis [10]. In normal hearts, autophagy can mediate the iteration of organelles by eliminating ROS producers like dysfunctional mitochondria [11]. But impaired autophagy accelerates cardiomyocyte loss, adverse ventricular remodeling, and HF progression [12]. In addition, excessive autophagy has also been proposed as a maladaptive response that contributes to HF progression. An important example is toll-like receptors (TLRs), which are immunoreceptors involved in host defense against invading microbes. Overexpression of TLRs could contribute to persistent autophagy and thus promote the development of HF [13]. Apoptosis, as a genetically programmed event in normal cells, is also another pivotal promoter of cardiac cell fate and plays an important role in the development and progression of HF. The overexpression of apoptosis might create a deadly dilated cardiomyopathy. Apoptotic cells were detected by the number of myocardial nuclei labeled with fluorescein, the levels of apoptosis in cardiomyocytes increased 232 times in HF [14]. In addition, severe HF is associated with elevated levels of apoptosis related factors such as B-cell lymphoma-2 (Bcl-2)-associated x (Bax). Overexpression of the BH4 domain of the anti-apoptosis Bcl-2-related gene long isoform (Bcl-xl) protein could avert the onset of HF [15].
Sirtuins belong to a family of nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases, which include silent information regulator (Sirt1)-7 [16]. Sirt1 is one of the most deeply and widely studied sirtuins in heart and is intimately connected to adenosine monophosphate-activated protein kinase (AMPK) [7]. Under the pathogenic circumstances, Sirt1 was activated to support cardiac cell survival. Levels of HF indicators that include atrial and brain natriuretic peptides are increased in Sirt1 knockout mice, and inhibition of endogenous Sirt1 enhanced apoptosis accumulation [17]. Sirt1 was downregulated in the HF samples compared with healthy cardiomyocytes and was accompanied by an increased oxidative stress index [18]. Similar investigations showed that Sirt1 was connected with autophagy and energy metabolism [19,20]. AMPK, a heterotrimeric complex made up of the three subunits that are α, β and γ, responds to HF by maintaining energy homeostasis, promoting the production of autophagosomes, and limiting cardiac apoptosis and oxidative stress [21,22]. AMPK and Sirt1 are vital in an orchestrated network controlling cellular homeostasis and largely need each other as “transit stations” to act. A prominent example is that AMPK and Sirt1 directly activate proliferator-activated receptor γ coactivator 1α (PGC-1α) through phosphorylation (AMPK) and deacetylation (Sirt1), respectively. However, the PGC-1α mutation that lacks the two AMPK-phosphorylation sites reduces deacetylation of PGC-1α [23]. It may be that phosphorylation of PGC-1α constitutes a priming signal for subsequent deacetylation by Sirt1. Furthermore, the regulatory effect of Sirt1 on mammalian target of rapamycin (mTOR) may be partially mediated by AMPK, which is mentioned in Section 2.3 [24]. In addition, there is a feedforward relationship between AMPK and Sirt1 in the heart. In both sham and ischemia conditions, AMPK silenced hearts revealed significantly decreased Sirt1 activity compared with wild-type rat hearts. FK866, a nicotinamide phosphoribosyl transferase (Nampt) inhibitor that decreases the level of NAD+ to inhibit Sirt1 activity, decreased cardiac AMPK activation in ischemia [25]. Hence, when treatment with AMPK or Sirt1 activators or inhibitors alone is not adequate to treat cardiovascular diseases (CVDs), therapy based on the synergistic effect of AMPK and Sirt1 may make up for the defects. Previous research has confirmed that in the regulation of glucose and lipid metabolism in mice, leucine, a Sirt1 activator, co-treated with metformin (AMPK activator) not only reduced the dosage of metformin by 83% and improved drug tolerability, but also improved efficacy by 66% compared to full-dose metformin (AMPK activator) monotherapy [26]. Although these two targets have been extensively studied and their roles in specific diseases (such as non-alcoholic fatty liver) extensively explained, the magnitude and significance of a synergistic interaction between AMPK and Sirt1 in heart are seldom reported [27,28].
At present, the traditional treatments for HF include angiotensin converting enzyme inhibitors (ACEIs), β-adrenergic blockers, and diuretics. Although these interventions can extend survival, the quality of life of patients still needs to be raised. Clinical trials investigating the treatment of HF have revealed severe side effects such as hypotensive, hyperkaliemia, and dizziness with these drugs [29]. Moreover, patients still face great challenges with treatment or prevention due to the possible conflict between excessive diuretic doses and ACEIs [30]. In essence, at certain stages of development of HF, existing methods and these drugs may not be enough. Traditional Chinese medicine (TCM) is characterized by having muti-components and multi-targets, such as the ginseng in TCM, which not only contains a large number of ginsenosides (including ginsenoside Rb1, ginsenoside Rg1, and ginsenoside Rd), but also acts on various targets to play a role (such as anti-inflammatory by the NOD-like receptor protein 3 (NLRP3) target, and anti-apoptosis by the Bax and Bcl-2 targets [31]. Many TCM formulas have been widely used in the treatment of HF (e.g., Danqi tablets and Qiliqiangxin capsules) [32]. A particular instance is a randomized double-blind controlled trial that indicated that Qiliqiangxin capsule may effectively improve quality of life in patients with chronic HF by decreasing the plasma N-terminal pro-brain natriuretic peptide level and increasing left ventricular ejection fraction [33]. With the development of society, complementary therapy based on TCM can be recommended as an attractive treatment. Herbal medicine and constituents from TCM, such as flavonoids and polyphenols, protect the heart by modulating the Sirt1/AMPK pathway.
Consequently, the retrieved literature was consulted from 2013 to 2023 using the PubMed, Google Scholar, Chinese National Knowledge Infrastructure (CNKI), and Web of Science databases, and considering the following keywords: Sirt1, AMPK, plant extracts, traditional Chinese medicine, and heart failure. We summarized the herbal medicines showing protective properties in CVDs via activation of Sirt1/AMPK signaling. We hope to provide a reference for follow-up studies on the development and utilization of targeted therapy with Sirt1/AMPK and herbal medicines for treating HF and other CVDs.
2. Sirt1 and AMPK collaborate in HF
Sirt1 exhibits a widespread distribution across the heart, brain, and liver [34,35]. In the adult mouse heart, Sirt1, expressed in both the cytoplasm and nucleus, maintains the appropriate subcellular localization by nucleocytoplasmic shuttling, to perform proper functions in a given physiological context. Sirt1 catalytic activity is regulated by post-translational modifications, including small ubiquitin-related modifier (SUMO) ylation at lysine-73 [[36], [37], [38]]. By binding to target proteins and deacetylating acetyl-lysine residues in them, Sirt1 might modulate the activities of target proteins as well as their intracellular localizations and stabilities [39]. It catalyzes NAD+-dependent lysine deacetylation to produce nicotinamide, deacetylated proteins, and 2′-O-acetyl-ADP-ribose (OAADPr, a small signaling metabolic molecule). Nicotinamide is a negative-feedback inhibitor of Sirt1, and OAADPr is converted into adenosine diphosphate ribose to regulate ROS [[40], [41], [42]]. Sirt1 deacetylates several substrates, such as PGC-1α, peroxisome proliferator-activated receptors (PPARs), and forkhead box family and subfamily O of transcription factors (FOXOs), in response to a range of physiological events [[43], [44], [45]].
AMPK is a highly conserved sensor of intracellular adenosine nucleotide levels. Previous research has shown that an increase in adenosine monophosphate (AMP) triggers AMPK γ subunits to bind AMP, boosting the catalytic activity of the complex and enhancing the phosphorylation of key residue (Thr172 in the mice α-subunits) [46,47]. AMPK can also be directly phosphorylated by upstream activators such as liver kinase B1 (LKB1) (at AMPKα-Thr172). In addition, there are many targets involved in cardiac homeostasis and metabolism that are phosphorylated by AMPK, such as acetyl-CoA carboxylase (ACC), PGC-1α and protein kinase B (Akt) [48].
AMPK and Sirt1 play a synergistic function in the metabolic regulation of the heart to a considerable degree in the process of reacting to the pathological alterations of the heart [49,50]. As Sirt1 is a NAD+-dependent histone deacetylase, the abundance of NAD+ directly affects the activity of Sirt1. AMPK can regulate the levels of NAD+ by increasing the expression of Nampt, leading to the activation of Sirt1 [51,52]. Nevertheless, several studies have found that AMPK can activate Sirt1 in a NAD+-independent manner. An important example is that AMPK stimulated Sirt1 with deletion in breast cancer-1 (DBC1, a key Sirt1-associated protein, directly interacts with Sirt1 and limits Sirt1 activity) dissociation. This disassociation leads to an increase in Sirt1 activity without affecting NAD+ levels [53,54]. Furthermore, during glucose deprivation in mouse embryonic fibroblasts, AMPK phosphorylated cytoplasmic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at Ser122, causing GAPDH to redistribute into the nucleus to interact with Sirt1 and dissociate Sirt1 from DBC1 [55]. The Thr172 of AMPK was phosphorylated in response to α-adrenergic agonist phenylephrine stimulus in H9c2 cells, and Sirt1 was likewise activated by AMPK, and the ratio of NAD+/NADH had no alterations [56]. These interactions of AMPK with Sirt1 may be necessary for Sirt1 production in cells experiencing metabolic stress in the absence of alterations in NAD+ levels. Correspondingly, Sirt1 can reduce lysine acetylation of LKB1 (at Lys 48), leading to interaction with and activation of downstream AMPK [57,58]. For instance, Sirt1 activity was suppressed in the ischemic heart, followed by low levels of phosphorylated LKB1 and AMPK [59].
In brief, the parallel regulation of Sirt1 and AMPK encompasses numerous pathways, and this management plays a crucial role in the metabolism of the heart. The interaction between Sirt1 and AMPK is shown in Fig. 1.
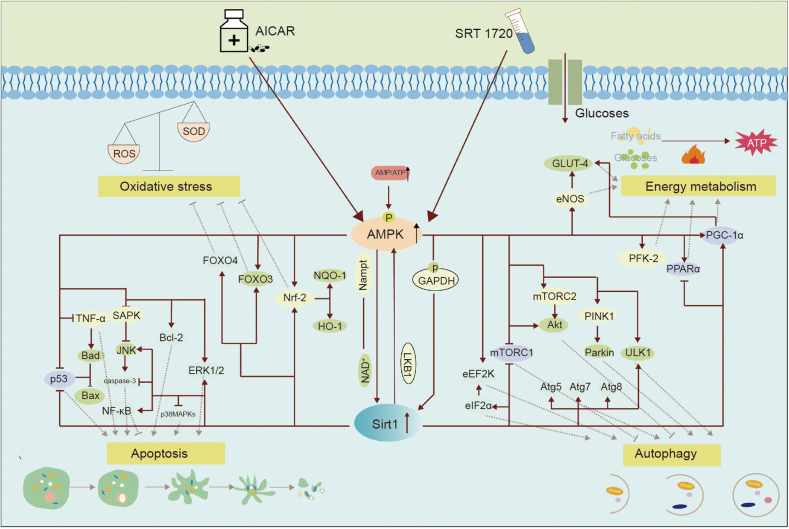
Fig. 1.
The interaction between silent information regulator 1 (Sirt1) and adenosine monophosphate-activated protein kinase (AMPK) and the downstream targets regulated by them together. In the presence of AMPK or Sirt1 activators, AMPK can promote the nicotinamide adenine dinucleotide (NAD+)/NADH ratio to activate Sirt1 or GAPDH phosphorylation to enhance Sirt1 expression, and Sirt1 can activate AMPK by deacetylating liver kinase 1 (LKB1), among other ways. AMPK and Sirt1 can also interact in cardiomyocytes. Additionally, through controlling several common targets (such as peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), peroxisome proliferator-activated receptor α (PPARα)), AMPK and Sirt1 may regulate the metabolism of cardiac fatty acids, glucose, and other substrates, improving autophagy (by mechanistic target of rapamycin (mTOR)), reducing oxidative stress (by nuclear factor erythroid2-related factor 2 (Nrf2) and forkhead box family and subfamily O of transcription factors (FOXOs), and reducing apoptosis (by p53 and mitogen-activated protein kinase (MAPK) family). Where represents  indicates stimulation/promoting,
indicates stimulation/promoting,  indicates reduction/inhibition. ROS: reactive oxygen species; SOD: manganese-superoxide dismutase; GLUT4: glucose transporter type 4; eNOS: endothelial nitric oxide synthase; FOXO4: forkhead box family and subfamily O of transcription factors 4; FOXO3: forkhead box family and subfamily O of transcription factors 3; NQO1: NAD(P)H-quinone oxidoreductase 1; HO-1: heme oxygenase-1; SAPK: stress-activated protein kinase; TNF-α: tumor necrosis factor α; BAD: Bcl-2 associated agonist of cell death; Bax: Bcl2-associated x; JNK: c-Jun NH2-terminal kinase; NF-κB: nuclear factor-kappa-B; p38 MAPK: protein 38 mitogen-activated protein kinase; ERK1/2: extracellular signal-regulated kinase; Nampt: nicotinamide phosphoribosyl transferase; eEF2K: elongation factor-2 kinase; eIF2α: eukaryotic initiation factor-2α; mTORC1: mammalian target of rapamycin compound 1; mTORC2: mammalian target of rapamycin compound 2; Akt: protein kinase B; PINK: PTEN-induced kinase 1; ULK1: unc-51-like kinase 1; Atg5: autophagy-related genes 5; Atg7: autophagy-related genes 7; Atg8: autophagy-related genes 8; PFK-2: phosphofructokinase-2; AICAR: aminoimidazole carboxamide ribotide; ATP: adenosine triphosphate; AMP: adenosine monophosphate.
indicates reduction/inhibition. ROS: reactive oxygen species; SOD: manganese-superoxide dismutase; GLUT4: glucose transporter type 4; eNOS: endothelial nitric oxide synthase; FOXO4: forkhead box family and subfamily O of transcription factors 4; FOXO3: forkhead box family and subfamily O of transcription factors 3; NQO1: NAD(P)H-quinone oxidoreductase 1; HO-1: heme oxygenase-1; SAPK: stress-activated protein kinase; TNF-α: tumor necrosis factor α; BAD: Bcl-2 associated agonist of cell death; Bax: Bcl2-associated x; JNK: c-Jun NH2-terminal kinase; NF-κB: nuclear factor-kappa-B; p38 MAPK: protein 38 mitogen-activated protein kinase; ERK1/2: extracellular signal-regulated kinase; Nampt: nicotinamide phosphoribosyl transferase; eEF2K: elongation factor-2 kinase; eIF2α: eukaryotic initiation factor-2α; mTORC1: mammalian target of rapamycin compound 1; mTORC2: mammalian target of rapamycin compound 2; Akt: protein kinase B; PINK: PTEN-induced kinase 1; ULK1: unc-51-like kinase 1; Atg5: autophagy-related genes 5; Atg7: autophagy-related genes 7; Atg8: autophagy-related genes 8; PFK-2: phosphofructokinase-2; AICAR: aminoimidazole carboxamide ribotide; ATP: adenosine triphosphate; AMP: adenosine monophosphate.
2.1. The role of Sirt1/AMPK-mediated energy metabolism in HF
The data from patients and experimental animal models of HF demonstrated that, in comparison to typical cardiac function, HF is characterized by a decrease in mitochondrial oxidative and impaired fatty acid utilization, while glycolysis and ketone body oxidation compensatory increase to meet the high energetic demand of the constantly contracting heart [60,61]. The shift in myocardial energy metabolism occurs sooner than that in cardiac structure and function [62]. Therefore, it may be that early metabolic improvement drives a beneficial effect in HF. For example, a clinical trial found that trimetazidine controlled resting whole body energy metabolism to improve functional class and left ventricular function in patients [63,64]. In addition, metformin (a typical anti-diabetic drug) decreased cardiac remodeling and maintained left ventricular function by improving the myocardial energy metabolic status in HF [65].
PGC-1α and PPARα serve as key transcriptional regulators of cellular energy metabolism [66]. PGC-1α has been shown to regulate the expression of a variety of coactivated genes, including the nuclear receptors (PPARs family and estrogen receptor related receptors (ERRs)) and non-nuclear receptors (nuclear respiratory factors family (Nrfs)), thereby controlling genes that mediate the transfer of myocardial fuel substrates and are involved in mitochondrial oxidative phosphorylation and the electron transport chain [[67], [68], [69], [70]]. Sirt1 can bind to PGC-1α in the central regulatory region between amino acids 200 and 400 and subsequently deacetylate PGC-1α, thereby promoting mitochondrial oxidative phosphorylation and production of adenosine triphosphate (ATP) [71]. After treatment with nicotinamide in cardiomyocytes in vitro, the acetylation level of PGC-1α is increased and its activity is decreased [72]. PGC-1α in the hypoxia-induced H9c2 cells is similarly reliant on the AMPK signaling pathway, since after AMPK knockdown, the upregulation of PGC-1α was abolished [73]. In 2007, with coimmunoprecipitation experiments and mass spectrometry analysis, Jäger et al. [74] found that AMPK directly phosphorylated the PGC-1α protein at threonine-177 and serine-538. The PPAR family of transcription factors, which includes PPARα, PPARβ and PPARγ, stimulates fuel substrate utilization via improving mitochondrial function and fatty acid oxidative pathways [75]. However, the mechanism by which PPAR is regulated is not entirely clear in the context of HF. For instance, PPARα activation promotes fatty acid uptake and oxidation, and fenofibrate (PPARα artificial ligands) protects against endothelin-induced HF [76]. In contrast, persistent PPARα activation induces insulin resistance, lipid accumulation, and liptoxicity [77]. However, cardiomyocyte specific PPARγ knockout and agonist both induced cardiac hypertrophy in mice [78]. Sirt1 mediated regulation of PPAR seems also complex. It was revealed that PPARα bound and recruited Sirt1 to the ERR response element, thereby suppressing ERR target genes, and thereafter facilitating the process of cardiac hypertrophy [40]. ERR are recognized as pleiotropic regulators of heart function. The gene disruption of ERRα in mice led to HF and impaired mitochondrial ATP production [79]. However, another study revealed that in a phenylephrine-induced neonatal cardiomyocyte hypertrophy model, PPARα is activated by Sirt1 overexpression, thereby upregulating fatty acid oxidation and inhibiting cardiomyocyte hypertrophy [80]. All in all, PPAR is necessary for the heart, but too high or too low stock of PPAR will do some damages to the heart. The heart also responds to external stimuli through adaptive regulation, and the full panoply of effector mechanisms remains to be elucidated. In addition, AMPK also seems to modulate the transcriptional activity of PPAR. PPARα and PPARγ agonists significantly enhanced glucose uptake in the papillary muscle of rat hearts, while this effect was blocked after AMPK was inhibited [81]. Whether this effect is mediated by Sirt1 remains to be elucidated. In addition, earlier investigations have revealed that the Compound C (AMPK inhibitor) restricts the protein expression and transcriptional activity of Sirt1, accompanied by the recovery of cardiac hypertrophy and the blocking of fatty acid oxidation, which confirms that AMPK regulates energy metabolism partly through the Sirt1 pathway [82].
In addition, AMPK mediates glucose metabolism in cardiomyocytes potentially through other pathways. For example, phosphofructokinase (PFK-2), as one of the isoforms of PFK, could convert fructose 6-phosphate to 2,6-bisphosphate in glycolysis to promote glucose metabolism [83]. AMPK, in vitro cardiomyocytes, can directly phosphorylate and activate PFK-2 at Ser466 [84]. Furthermore, the regulatory effect of AMPK on glucose metabolism in cardiomyocytes is presumably mediated via modifying glucose transporters. An example of this is the study carried out by Li et al. [85] in which AMPK is involved in the glucose transporter type 4 (GLUT4) translocation and leads to GLUT4 transport from intracellular compartments to the plasma membrane of cardiomyocytes, thereby increasing glucose absorption.
2.2. Regulation of apoptosis by Sirt1 and AMPK in HF
During the progression of HF, cardiomyocyte apoptosis is of great significance [86]. Accumulating evidence established that myocardial apoptosis was one of the critical indicators linked with early symptomatic post-infarction HF [87]. In both in vivo and in vitro experiments, apoptosis was confirmed to positively correlate with the severity of cardiac dysfunction [88]. Cardiomyocyte viability is dependent upon the action of opposing apoptotic and survival pathways. Thus, the possibility of reducing cardiomyocyte loss by inhibiting apoptosis has potentially important implications for the treatment of HF. For example, by suppressing mitochondrial cytochrome c to attenuate activation of the caspase cascade to anti-apoptosis, carvedilol can contribute to the beneficial effects in patients with HF [89].
The upstream targets for regulating cardiomyocyte apoptotic markers (such as Bax and Bcl-2) include p53, MAPKs family, and nuclear factor-kappa-B (NF-κB). p53 is a potent inducer of apoptosis. It can directly bind and inhibit anti-apoptotic Bcl-2 protein and induce transcription of the pro-apoptotic Bax [90]. A study found that replication-defective adenovirus infected cardiomyocytes could result in an increase in p53 transcriptional activity and protein accumulation, eventually causing apoptosis-specific morphological changes [91]. Sirt1 mediated deacetylation antagonizes p53-dependent transcriptional activation, inhibiting p53-dependent apoptosis [92]. An increment of p53 protein acetylation, when the dominant negative form of Sirt1 was overexpressed, activated p53 binding to the promoter of Bax and upregulated Bax expression [17,93]. Furthermore, AMPK was identified to directly phosphorylate p53 at Ser 15. However, conflicting results have been reported regarding the regulation of apoptosis by AMPK/p53 pathway. Jones et al. [94] reported that AMPK improves cell survival through phosphorylation of p53 under energy stress conditions. In contrast, another study found that AMPK dependent phosphorylation of p53 led to cellular apoptosis under similar carbon source depletion [95]. These findings could be due to different cell types and treatment durations. NF-κB super family is an important regulator of apoptosis and consists of some genes encoding the members, such as RelA/p65, RelB, p50 and p52 [96]. Functional loss of NF-κB signaling in cardiac myocytes increases cardiomyocyte susceptibility to tumor necrosis factor-α (TNF-α)-induced apoptosis [97]. Sirt1 can regulate the transcription of NF-κB by combining with the RelA/p65 subunit of NF-κB to deacetylate it at lysine 310, thereby protecting against apoptosis [98]. Although the NF-κB subunits are not direct phosphorylation targets of AMPK, AMPK has a similar inhibitory effect on NF-κB, and the effect of NF-κB by AMPK is partly mediated by Sirt1 [99]. MAPK family members, including p38 MAPK, extracellular signal-regulated kinase (ERK) and c-jun nh2-terminal kinase (JNK), play essential roles in oxidative stress-induced apoptosis and other forms of apoptosis [100]. p38 MAPK activation leads to cell apoptosis through alterations in the expression of proteins involved in apoptosis, including Bcl-2 and Bax. These targets are also involved in the pro-apoptosis function of JNK. Similarly, phosphorylated ERK interacts with downstream effectors Bcl-2 and caspase signaling pathways to adjust cell proliferation and apoptosis. Blockade or deletion of cardiac ERK1/2 will increase the susceptibility of the heart to HF under external stimulation and with an increase in myocyte apoptosis [101]. It has been demonstrated that Sirt1, by enhancing Akt phosphorylation, can inhibit p38 and JNK and enhance ERK1/2 phosphorylation to alleviate apoptosis in ischemia-reperfusion (I/R) rats [102]. The specific mechanisms may be that Sirt1 can activate Akt by binding the pleckstrin homology (PH) domain and deacetylating Lys14 and Lys20, and subsequently, Akt phosphorylates ASK1 that acts upstream of JNK and p38 MAPK. Acetylation of Akt prevents subsequent phosphorylation of Akt [103]. Another study reported that palmitic acid induced cardiomyocyte apoptosis and increased the phosphorylation of stress-activated protein kinase (SAPK)/JNK, and these effects were completely inhibited after treatment with aminoimidazole carboxamide ribotide (AICAR) (AMPK agonist) [104]. It is worth noting that AMPK could directly phosphorylate Akt at Thr308 [105]. Possibly, this effect is also mediated through the Akt pathway. In addition, there are several downstream effector molecules (including autophagy-related mTOR) in tandem with Akt, which is mentioned in Section 2.4.
2.3. The role of Sirt1/AMPK-mediated oxidative stress in HF
ROS refers to all the reactive chemical species derived from oxygen. Superoxide that is generated by mitochondrial respiration is dismasted by manganese-superoxide dismutase (SOD) to form hydrogen peroxide and further originate the highly reactive hydroxyl radical via the Fenton reaction [106]. Excessive ROS buildup may lead to oxidative stress and then result in cardiac damage. Antioxidant defense systems guarantee a balance between the production and neutralization of ROS to maintain a normal heart [107]. The important contribution of ROS to the pathophysiology of HF may indicate that therapeutic strategies aimed at reducing oxidative stress within the myocardium may beneficially influence the course of the disease. For example, vitamin C, as an optimal antioxidant, protects cardiac function and structure by lowering oxidative stress in doxorubicin (DOX)-induced HF [108].
Oxidative stress is one of the driving events of apoptosis, and many upstream effectors share the regulation of oxidative stress and apoptosis. As indicated before, the MAPK family is regulated by Sirt1 and AMPK, playing a role in anti-apoptosis as well as producing the effect of antioxidant stress [109]. In addition, Mn-SOD and catalase are well-characterized targets of the FOXO family of transcription factors, including FOXO1a, FOXO3a, and FOXO4 [110]. FOXO3a contributes to anti-oxidative stress by directly raising the quantities of Mn-SOD messenger RNA and protein [111,112]. Chromatin immunoprecipitation confirmed that FOXO4 binds to and regulates ubiquitin specific peptidase 10 (USP10), leading to a reduction in the content of SOD and an enhancement in the malondialdehyde (reflecting the antioxidant potential) [113]. Some studies have reported that Sirt1 binds and deacetylates FOXO3 and FOXO4 in response to oxidative stress and increases the anti-oxidant stress capabilities of FOXO3 and FOXO4 [[114], [115], [116]]. In vitro kinase assays, AMPK can phosphorylate FOXO3 at serine and threonine, subsequently promoting FOXO3 translocation into the nucleus, then promoting FOXO3 binding and activating thioredoxin (a major antioxidant system) [117]. Nuclear factor erythroid2-related factor 2 (Nrf2) regulates the transcription of enzymatic antioxidant defense proteins, including heme oxygenase 1 (HO-1), SOD, and NAD(P)H dehydrogenase quinone 1 (NQO1) [118]. Sirt1 can physically interact with Nrf2 in vitro, and significantly promote the deacetylation of Nrf2, accompanied by a decrease in the level of oxidative stress [119]. AMPK phosphorylated Nrf2 at the Ser550 residue and promoted gene transactivation driven by the antioxidant response element of Nrf-2 [120]. In the in vitro kinase and peptide competition assays, inhibition of AMPK remarkably reduced the expression of Nrf2 and HO-1 in the diabetic cardiomyocytes, therefore increasing oxidative stress damage [121]. It may be that AMPK was also an upstream target of Nrf2.
2.4. Regulation of autophagy by Sirt1 and AMPK in HF
Autophagy is an endogenous protective process that helps maintain the quality of the intracellular environment by eliminating misfolded proteins and malfunctioning organelles. However, autophagy has been proposed as a “double-edged sword” in heart tissue. The level of autophagy in the heart may determine whether the autophagy will be protective or deleterious. On the one hand, the loss of autophagy can destabilize proteostasis and elevate intracellular oxidative stress, which is critically involved in the development of HF [122]. For instance, injection of Tat-Beclin (a potent inducer of autophagy) attenuated cardiac injury by resorting to mitochondrial autophagy. In a transverse aortic constriction (TAC)-induced HF mouse model, knockout of Eva-1 homologue A (a protein that can activate cell autophagy) generated myocardial contractile dysfunction and cardiac hypertrophy in mice [123,124]. On the other hand, excessive autophagosome production leads to insufficient lysosomes that bind to autophagosomes and to incomplete degradation of autophagosomes, which in turn impairs autophagic flux, causing further damage to cells. An HF mouse model study showed that isoprenaline (ISO) led to a sustained increase in autophagy accompanied cardiac dysfunction [125].
The mTOR protein is a serine/threonine kinase that exists in two distinct complexes (mTORC1 and mTORC2). One of the functions of mTORC1 is to negatively regulate autophagy via phosphorylating activators of autophagy, such as phosphatidylinositol 3-kinase catalytic subunit type 3/vacuolar protein sorting 34 [126]. mTORC2, through its downstream target kinase Ypk1, inhibits the Ca2+ and Cmd1/calmodulin-dependent phosphatase, calcineurin, to enable the activation of the amino acid-sensing eukaryotic translation initiation factor 2 subunit alpha (EIF2S1/eIF2a) kinase, Gcn2, and promote autophagy [127]. An investigation discovered that AICAR could increase autophagy by negatively affecting the mTORC1 target and lowering the phosphorylation of the mTORC1 substrates (eukaryotic initiation factor 4E binding protein-1 (4EBP1) and ribosomal S6 kinase (S6K)) [22]. This mechanism of the effect may be that AMPK phosphorylates the tuberous sclerosis complex 2 (TSC2), the upstream suppressor of mTORC1, thereby suppressing mTORC1 [128]. In addition, another study reported that AMPK inhibits mTORC1 via phosphorylating raptor (inhibitory phosphorylation of an essential mTORC1 protein) on two conserved serine residues, Ser 722 and Ser 792 [129]. Noticeably, Sirt1 was a negative regulator of mTORC1 in mouse and human cells; depleting Sirt1 in HeLa cells presented higher phosphorylation of 4EBP1 and S6K, and this change was reversed by a TSC2 knockdown [130]. It may be attributed to the direct interaction of Sirt1 with TSC2 or the regulation of mTORC2 via the AMPK pathway. In addition, another study found that AMPK phosphorylated Akt (the mTORC2 effector) at Ser 473 to subdue autophagy and attenuate cardiac hypertrophy in HF related to mTORC2 activation [107]. The likely explanation for this paradoxical outcome was that AMPK has a bidirectional influence on autophagy regulation, whether AMPK triggers or suppresses autophagy during HF depends on the degree of autophagy. Autophagy is mediated by a cascade reaction of the autophagy-related genes (Atgs), and decreased expression of key Atgs, such as Atg5, Atg6, Atg7, and Atg12, would substantially inhibit the autophagic process [131]. Previous research has found that Sirt1 could bind with Atg5, Atg7, and Atg8 and directly deacetylate them, thus improving autophagy to protect cells [132]. In addition, elongation factor-2 (eEF2) is phosphorylated and activated by eEF2-kinase (eEF2K) and thereby mediates autophagy [133]. Sirt1 could enhance autophagy by promoting eEF2 phosphorylation to enhance cardiomyocyte survival. After Sirt1 expression was inhibited by EX257, phosphorylation of eEF2 by eEF2K returned to the basic level. However, co-immunoprecipitation assay revealed that Sirt1 was not associated with eEF2 or eEF2K, but eukaryotic initiation factor-2α (eIF2α) was co-immunoprecipitated with eEF2K [134]. Previous studies demonstrated that Sirt1 can physically interact with and deacetylate eIF2α at lysine K143 [135]. Therefore, the activation of the eEF2K/eEF2 pathway by Sirt1 may involve eIF2α. By analyzing the labeled peak by nano electrospray ionization tandem mass spectrometry (ESI-MS/MS), Browne et al. [136] found that AMPK can directly phosphorylate eEF2K and that the major site of phosphorylation is Ser-78 in a regulatory domain of eEF2K. Unc-51-like kinase 1 (ULK1) is a protein kinase that is motivated by autophagy; upon autophagy induction, the ULK1 complex translocates for autophagy initiation and regulates the autophagy process [137]. AMPK could bind to the PS domain of ULK1 and promote autophagy [138]. ULK1 may also be one of the upstream targets of Sirt1. In a study, it was found that endothelial nitric oxide synthase may favorably control Sirt1, but this effect was blocked in vascular endothelial cells with ULK1 knockout [139]. This may offer another route for AMPK to influence Sirt1. In addition, mTOR and ULK1 are key pathways in the regulation of mitophagy (a selective autophagy mode that controls mitochondrial quality by removing damaged or aging mitochondria). AMPK and Sirt1 can mediate mitochondrial autophagy through mTOR and ULK1 targets [140]. In addition, PTEN-induced kinase 1 (PINK1), as a molecular sensor of mitochondrial health, is a serine/threonine kinase that recruits and activates parkin and mediates ubiquitination of mitochondrial substrates when it detects mitochondrial damage, then induces mitochondrial autophagy. AMPKα can promote mitochondrial autophagy and prevent the progression of HF via directly phosphorylating and activating PINK1 at Ser495 [141]. Furthermore, in hypoxic cardiomyocytes, activation of Sirt1 could improve autophagic flux and reduce apoptosis, but Compound C abolished the effect of Sirt1 on autophagy activation. This study demonstrated that Sirt1 promotes autophagy partly via AMPK activation, thereby protecting cardiomyocytes [142].
3. Herbal medicines protect against HF by regulating Sirt1/AMPK signaling pathway
More and more reports have comprehensively investigated the effects of herbal medicines on HF. Increasing results illustrated that the extracts or monomers obtained from natural herbal medicines exerted excellent protective effects on HF via activation of Sirt1/AMPK signaling. In the following section, the beneficial effects and molecular mechanisms of herbal medicine and its constituents on HF are detailed.
3.1. Herbal medicine formula
It has been previously established that ischemic heart disease is the primary cause of HF [143]. Ligation of the left anterior descending (LAD) coronary artery is a common method of inducing cardiac ischemic injury in mice. Perfusion is then used to construct various disease models. Permanent ligation is applied to create myocardial ischemia and HF models while ligation and reperfusion are performed to produce an ischemia-reperfusion injury model. The latter differs from HF models. Nevertheless, ischemia-reperfusion injury exacerbates myocardial damage and accelerates the transition to HF. The administration of Danqi tablets upregulated AMPK, Sirt1 and PGC-1α proteins, thereby improving ATP production and heart function in a LAD-induced ischemic injury model [144]. Similarly, Danqi tablets in LAD-induced HF rats restored autophagy and protected against cardiomyocyte injury by upregulating AMPK phosphorylation and reducing mTOR in a rat LAD-induced HF model [145]. Tongxinluo is a Chinese patent medicine that is widely administered to treat various CVDs. Li et al. [146] found that it increased the ejection fraction and improved cardiac function in a LAD-induced myocardial infarction (MI) model. Potential mechanisms of Tongxinluo included the upregulation of AMPK phosphorylation and the downregulation of the mTOR. While Xuefuzhuyu decoction affected hypoxia/reoxygenation (H/R)-induced H9c2 cells injury in the same manner as Danqi tablet and Tongxinluo, its mode of action nonetheless differed from theirs. Xuefuzhuyu decoction reduced AMPK phosphorylation, upregulated mTOR, and inhibited autophagy. This mechanism is consistent with the fact that AMPK bidirectionally regulates autophagy as previously described [147]. In vitro, Xuefuzhuyu oral liquid also prevented ischemia-induced cardiac apoptosis by regulating Sirt1 and its associated genes FOXO1, FOXO2, and NF-κB [148]. In a LAD-induced HF rat model, Linggui Zhugan Decoction improved cardiac energy metabolism by upregulating Sirt1 and AMPK phosphorylation [149]. Shenfu formula consists of ginseng and aconite. In a TAC-induced rat HF model, it improved oxygen consumption and normalized aberrant energy metabolic pathways by modulating the AMPK/PPARα/PGC-1α pathway [150]. Nuanxin capsule consists of red ginseng, Coicis Semen, Citri Exocarpium Rubrum, and Radix Aconite Lateralis Praeparata. It inhibited oxidative stress and apoptosis by activating the AMPK/JNK pathway in the same model [151]. The above herbal medicine formulas effects are summarized in Table 1 [[144], [145], [146], [147], [148], [149], [150], [151]].
Table 1.
Protective effects of herbal medicine formulas on cardiovascular diseases.
| Extracts | Main compositions | Animal/cells | Models | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | |||||||
| DQ | Chinese herbs Salvia miltiorrhiza Bunge and Panax notoginseng | SD rats | LAD-induced ischemic | 1.5 g/kg | Improving heart function and production of ATP; Protecting structure of heart | AMPK, Sirt1, PGC-1α | – | [144] |
| DQ | SD rats | LAD-induced HF | 1.5 g/kg | Improving cardiac function; Increasing the information of autophagosomes | Beclin1, Atg3, AMPK | mTOR, ULK1 | [145] | |
| TXL | Radix ginseng, Buthus martensi, Hirudo, Eupolyphaga seusteleophage, Scolopendra subspinipes, Periostracum cicadae, etc. | SD rats | LAD-induced MI | 4 mg/kg/day | Increasing ejection fraction and cardiac function; Reducing myocardial infarction, fibrosis, inflammation and apoptosis | AMPK, Bcl-2 | mTOR, Bax | [146] |
| XFZY | Chinese Angelica, Dried Rehmannia root, Peachseed, Safflower, Radix Paeoniae Rubra, Radix Bupleuri, etc. | SD Rats, H9c2 cells | H/R-induced cardiomyocyte injury | 4.5, 9, 18, 36, 72 mg/mL | Inhibiting apoptosis and autophagy of cardiomyocytes | mTOR | AMPK, Beclin1 | [147] |
| XFZYOL | H9c2 cells | Cobalt chloride induced H9c2 ischemic | Reducing apoptosis | Sirt1 | FOXO1, FOXO3, NF-κB | [148] | ||
| LGZG | Poria cocos, Neolitsea cassia, Glycyrrhiza and Atractylodes | SD rats | LAD-induced HF | 5.4 g/kg | Improving the cardiac function; Enhancing myocardial antioxidant capacity | Sirt1, AMPK, PGC-1α | – | [149] |
| SFF | Panax Ginseng and Radix Aconitum carmichaelii | C57 mice | TAC-induced HF | 2.5, 5, 10 g/day | Protecting myocardial contraction and diastolic function; Alleviating myocardial necrosis, fibrosis and hypertrophy | AMPKα, PGC-1α, GLUT4, PPARα | – | [150] |
| NX | Red ginseng, Coicis Semen, Citri Exocarpium Rubrum and Radix aconiti lateralis preparata | C57BL/6J mice, H9c2 cells | TAC-induced HF | 0.64 g/kg | Improving cardiac function; Inhibiting apoptosis and oxidative stress; Protecting mitochondrial function | AMPK, Bcl-2 | Bax, JNK | [151] |
SD: Sprague Dawley; DQ: Danqi tablet; TXL: Tongxinluo capsule; XFZY: Xuefuzhuyu decoction; XFZYOL: Xuefuzhuyu oral liquid; LGZG: Lingguizhugan decoction; SF: Shen-fu formulas; NX: Nuanxin capsule; LAD: ligation of the left anterior descending coronary artery; AMPK: adenosine monophosphate activated protein kinase; Sirt1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; HF: heart failure; Atg: autophagy-related genes; mTOR: mammalian target of rapamycin; ULK1: unc-51-like kinase 1; MI: myocardial ischemia; Bcl-2: B-cell lymphoma-2; Bax: Bcl2-associated x; H/R: hypoxia/reoxygenation; FOXO1: forkhead box family and subfamily O of transcription factor 1; FOXO3: forkhead box family and subfamily O of transcription factor 3; NF-κB: nuclear factor-kappa-B; TAC: transverse aortic constriction; GLUT4: glucose transporter type 4; PPARα: peroxisome proliferator-activated receptor α; JNK: c-Jun NH2-terminal kinase.
3.2. Plant extracts
Hongjingtian injection is derived from Rhodiola wallichiana var. cholaensi and inhibited autophagy and lower cardiac cell apoptosis, downregulated AMPK, and upregulated mTOR protein in a LAD-induced myocardial ischemia-reperfusion injury (MI/RI) model [152]. In the same model, Zhao et al. [153] found that total flavonoids from Dracocephalum moldavica alleviated energy metabolism dysregulation via AMPK/Sirt1/PGC-1α pathway. In a mouse LAD-induced MI injury model, saponins from Panax japonicus minimized myocardial necrosis and improved ischemia-induced heart systolic via dose-dependent Sirt1 upregulation and TNF-α downregulation [154]. In a rat LAD-induced HF model, saponins from Panax notoginseng increased cardiomyocyte autophagy by promoting AMPK Thr172 and calmodulin-dependent protein kinase Ⅱ phosphorylation [155]. A Houttuynia cordata herbal extract upregulated AMPK, Nrf-2, and PGC-1α proteins and reduced cardiac oxidative stress in hyperlipidemic mice [156]. In a rat DOX-induced cardiotoxicity model, Glycyrrhiza glabra (licorice) root extract upregulated Sirt1 and PPARα proteins, thereby reducing oxidative stress [157]. In 2016, Chang et al. [158] demonstrated that the TCM drug Alpinate Oxyphyllae Fructus improved rat heart function by upregulating Sirt1, AMPK, and PGC-1α that had been previously downregulated via d-galactose treatment. Pomegranate flower extracts normalized cardiac energy metabolism and mitigated cardiac fatty acid oxidation by regulating the AMPK/PPARα signaling pathway in Zucker diabetic fatty rats [159]. The above plant extracts effects are summarized in Table 2 [[152], [153], [154], [155], [156], [157], [158], [159]], and Fig. 2.
Table 2.
Protective effects of herbal medicine extracts on cardiomyocytes.
| Extracts | Animal/Cells | Models | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | ||||||
| HJT | C57BL/6 J mice; H9c2 cells | LAD-induced MI/RI | 28 μL/10 g | Attenuating I/R-induced infarct size; Alleviating cardiac cell apoptosis; Limiting over-expression of autophagy | mTOR | AMPK | [152] |
| DMF | SD rats | LAD-induced MI/RI | 60 mg/kg/d | Ameliorating heart disease | AMPK, Sirt1, PGC-1α | – | [153] |
| PGS | SD rats | LAD-induced MI | 50, 100 mg/kg | Reducing necrotic myocardium; Ameliorating cardiac function | Sirt1, ERK1/2, p38MAPK, Bcl-2 | Bax, TNF-α, NF-κB | [154] |
| PNs | C57BL/6J mice | LAD-induced HF | 10, 100 μg/mL | Improving survival ratio of MI mice; Preserving cardiac function; Elevating the autophagy of cardiomyocytes | AMPK, CaMKⅡ | ULK1 | [155] |
| HHA | C57BL/6J mice | P407-induced hyperlipidemic | 200 mg/kg/day | Improving cardiac remodeling; Attenuating oxidative stress; Promoting mitochondrial biogenesis | AMPK, PGC-1α | – | [156] |
| Gg | H9c2 cells | DOX-induced cardiotoxicity | 40 μg/mL | Reducing oxidative stress; Improving cardiac morphology | Sirt1, PPARα | – | [157] |
| AOF | SD rats | d-galactose-induced aging mice | 50, 100, 150 mg/kg | Improving heart function; Reducing apoptosis | Bcl-2, PI3K, AMPK, Sirt1, PGC-1α | Bax | [158] |
| PF | Zucker lean rats | Zucker diabetic fatty rats | 500 mg/kg | Reducing cardiac TG content and plasma level | – | PPARα, AMPKα2 | [159] |
SD: Sprague Dawley; HJT: Hongjingtian injection; DMF: dracocephalum moldavica total flavonoids; PGS: saponins extract from panax japonicus; PNs: panax noto ginseng saponins; HHA: Houttuynia cordata herbal extract; Gg: glycyrrhiza glabra; AOF: alpinate oxyphyllae fructus; PF: pomegranate flower; LAD: ligation of the left anterior descending coronary artery; MI/RI: myocardial ischemia-reperfusion injury; mTOR: mammalian target of rapamycin; AMPK: adenosine monophosphate activated protein kinase; Sirt1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; MI: myocardial ischemia; ERK1/2: extracellular signal-regulated kinase; p38 MAPK: protein 38 mitogen-activated protein kinase; Bcl-2: B-cell lymphoma-2; Bax: Bcl2-associated x; TNF-α: tumor necrosis factor α; NF-κB: nuclear factor-kappa-B; HF: heart failure; CaMK Ⅱ: calmodulin dependent protein kinase Ⅱ; ULK1: unc-51-like kinase 1; HF: heart failure.
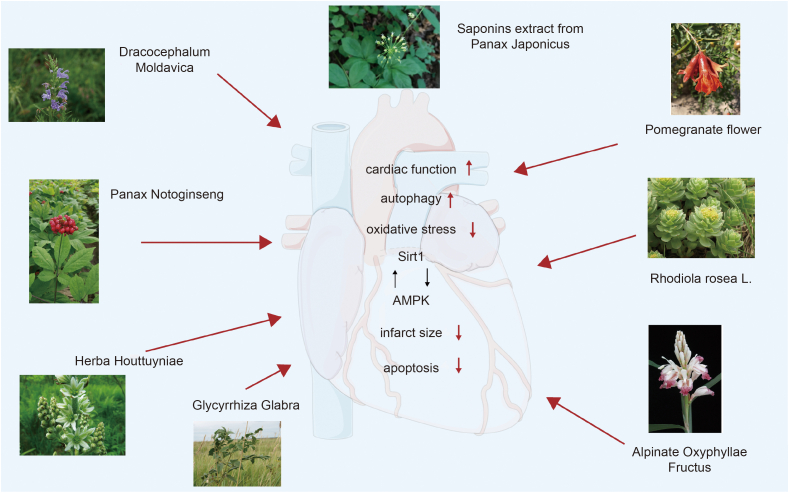
Fig. 2.
The protective effects of traditional Chinese medicine extract on heart through silent information regulator 1 (Sirt1)/adenosine monophosphate activated protein kinase (AMPK) pathway. Some common herbal drugs extracts active ingredients work to improve heart autophagy, lower cardiomyocytes apoptosis, and reduce oxidative stress by regulating the Sirt1/AMPK signal pathways. As a result, cardiac function is improved, the size of heart infract is reduced, and therefore progression of heart failure (HF) is slowed. The above herbal pictures are reprinted from GBIF.org (year) citation guidelines. Available from https://www.gbif.org/citation-guidelines with permission and was partly generated using Servier Medical Art, provided by Servier, licensed under a creative commons attribution 3.0 unported license.
3.3. Constituents from herbal medicines
Flavonoids have a broad range of bioactivity in CVDs. They regulate and normalize oxidative stress, energy metabolism, and apoptosis [160]. In a mouse LAD-induced HF model, tanshinone IIA extracted from Salvia miltiorrhiza upregulated AMPK phosphorylation and Bcl-2 and Beclin1 [161]. It also activated Sirt1 and PGC-1α, thereby ameliorating microvascular and mitochondrial damage [162]. Hence, tanshinone IIA upregulates both AMPK and Sirt1. Acacetin extracted from Acacia farnesiana upregulated AMPK phosphorylation and Sirt1 in a rat angiotensin II (Ang II)-induced HF model. Compound C inhibited acacetin-mediated AMPK phosphorylation but not Sirt1 upregulation. In Sirt1 knockdown cells, however, acacetin failed to upregulate AMPK or PGC-1α. Acacetin also increased the intracellular NAD+/NADH ratio [163]. The anti-inflammatory, anti-cancer, anti-obesity, and cardioprotective effects of acacetin have been reported [164]. Acacetin also upregulated Sirt1 and mediated the AMPK/Nrf2 pathway in a rat DOX-induced cardiomyopathy model and a mouse d-galactose-induced aging model [165,166]. Rhizoma Anemarrhenae contains the diphenyl pyrone flavonoid mangiferin. In HF mice, mangiferin alleviated cardiomyocyte apoptosis by upregulating Sirt1 and promoting FOXO3a deacetylation. However, it produced no such effects in Sirt1 knockout mice [167]. Tilianin is a flavonoid glycoside with an extensive range of biological actions, and it ameliorated a mouse LAD-induced MI/RI model. It upregulated the AMPK, Sirt1, and PGC-1α proteins which, in turn, shrank the cardiac infarcts and minimized cellular swelling and rupture. In contrast, Compound C or EX-527 reversed the foregoing effects [168,169]. Luteolin is a flavonoid in Lonicera japonica thunb and Perillae Folium. It upregulated Sirt1 and downregulated NLRP3 in a mouse LAD-induced MI/RI model [170]. The flavonol quercetin has multiple biological activities. In vivo, it reduced cardiac cell apoptosis by modulating Sirt1 and PGC-1α expression [171]. In an in vitro H/R model, quercetin also ameliorated mitochondrial oxidative stress by upregulating Sirt1. However, these effects were counteracted by Sirt1 silencing [172]. The flavonoid glycoside icariin reduced apoptosis by upregulating Sirt1 in the same model [173]. Similar effects were reported for H2O2 challenged H9c2 cells treated with naringenin [174]. The flavonoid glycoside rutin reduced apoptosis in an H/R-induced myocardial injury model by upregulating Sirt1 [175]. The isoflavone derivative puerarin was detected in the TCM Pueraria. In a LAD-induced MI model, it upregulated Sirt1 and ameliorated myocardial infarction [176].
Sun et al. [177] reported that dihydromyricetin inhibited apoptosis in a rat DOX-induced cardiotoxicity model and the H9c2 cell injury model by upregulating Sirt1. Jaceosidin is an active flavonoid in the TCMs, Artemisiae Argyi Folium and Eupatorii Lindleyani Herba. It mitigated DOX-induced cardiotoxicity in rats by upregulating Sirt1 [178]. Oroxylin A is a major active flavonoid in Scutellariae Radix. It has anti-cancer, anti-inflammatory, and neuroprotectant efficacy [179]. Oroxylin A ameliorated DOX-induced cardiotoxicity by mitigating oxidative damage and apoptosis in vivo through the regulation of Sirt1 and Nrf2 expression [180]. In a mouse Ang II-induced cardiac injury model, kaempferol mitigated cardiac oxidative stress by regulating AMPK expression [181]. It also modulated Sirt1 expression and reduced the ROS levels in an in vitro anoxia/reoxygenation (A/R) model [182]. The propiophenoid phloretin and the flavonoid isorhamnetin in Hippophae fructus ameliorated an in vivo mouse streptozotocin-induced diabetes model and an in vitro A/R-induced cardiomyocyte injury model by upregulating Sirt1 [183,184]. The β-adrenergic agonist ISO induces MI by promoting apoptosis and downregulating Sirt1 and Nrf2. However, hesperetin from Citri Reticulatae Pericarpium reversed these effects and improved myocardial injury in a rat ISO-induced MI model [185]. The flavonoid silibinin in Silybum marianum has multiple modes of action in the treatment of hematological disorders and cancers [186]. In an in vitro ISO-induced MI model, silibinin inhibited cardiac death and protected against mitochondrial dysfunction in myocardial cells by regulating Sirt1 expression [187]. In an H2O2-induced H9c2 cell injury model, liquiritin reduced the ROS levels, attenuated apoptosis and oxidative stress and upregulated AMPK and Sirt1. Nevertheless, Compound C downregulated Sirt1 [188]. Daidzein is extracted from Pueraria lobata root. It upregulated AMPK and Sirt1, protected cardiomyocytes from oxidative damage, and reduced oxidative stress in a rat streptozotocin-induced diabetes model [189].
Terpenoids are the largest and most diverse group of secondary metabolites in plants. They have antitumor, anti-inflammatory, and neuroprotectant efficacy. Triterpenoids have the highest pharmaceutical potential of all terpenoid subclasses [[190], [191], [192], [193], [194]]. However, the monoterpenoid oxypaeoniflorin derived from Paeoniae Radix Alba and Paeoniae Radix Rubra alleviated cardiac damage in a rat LAD-induced MI/RI model possibly by upregulating Sirt1 and downregulating FOXO1 acetylation [195]. The pentacyclic triterpenoid betulin alleviated myocardial inflammation by upregulating Sirt1 in a rat LAD-induced MI/RI model [196]. The monoterpenoid bakuchiol isolated from Psoralea corylifolia seeds reduced apoptosis by activating the Sirt1 and PGC-1α pathways in a rat MI/RI model. The foregoing effects were reversed by Sirtinol (a Sirt1 inhibitor) [197]. Bakuchiol also reduced oxidative stress and upregulated Sirt1 and Nrf2 proteins in a mouse high-fat diet-induced diabetic cardiomyopathy model [198]. The iridoid geniposide isolated from Gardenia jasminoides attenuated high-fat diet-induced myocardial inflammation and apoptosis in rats by upregulating Sirt1 and increasing AMPKα phosphorylation, respectively. Whereas AMPK deficiency did not affect cardiomyocyte pro-inflammatory factor biosynthesis, Sirt1 knockout promoted it [199]. In 2021, Hou et al. [200] demonstrated that geniposide downregulated mitochondrial energy metabolism markers, regulated energy metabolism, and reduced myocardial injury in spontaneously hypertensive rats possibly by upregulating phosphorylation of the AMPK/Sirt1 pathway. AMPK silencing reversed the preceding effects. The sodium salt of isosteviol, a diterpenoid derived from Stevia rebaudiana, reduced oxidative stress by upregulating AMPK phosphorylation and Sirt1 in a rat high fat/high cholesterol-induced myocardial dysfunction model [201]. Isosteviol sodium reversed myocardial hypertrophy in an H9c2 cell model by upregulating AMPK phosphorylation and Sirt1. Sirt1 expression was higher in response to a combination of the polyphenol resveratrol and isosteviol sodium than resveratrol alone. In contrast, EX-257 treatment significantly downregulated Sirt1 [202]. The diterpene lactone ginkgolide B is derived from the dried roots and leaves of Ginkgo biloba. In AngII-treated H9c2 cells, it activated autophagy and reduced oxidative stress by upregulating Sirt1 and FOXO1 proteins [203].
Polyphenols have a wide range of biological and pharmacological effects on CVDs, brain injury, and gut microbiota metabolism [204]. Resveratrol is extracted from giant knotweed rhizome. It significantly upregulated both Sirt1 and AMPK, improved cardiac function, and lowered mortality in a rat LAD-induced HF model. In contrast, nicotinamide reversed the foregoing effects [205]. However, resveratrol neither upregulated AMPK nor protected cardiomyocyte mitochondria in Sirt1 knockout mice [206]. Hence, Sirt1 mediated the action of resveratrol on AMPK. In a rat DOX cardiotoxicity model, the stilbene polyphenol pterostilbene, a resveratrol derivative, alleviated mitochondrial damage and oxidative stress in a dose-dependent manner. Nevertheless, PGC-1α was not activated and the preceding effects were partially reversed in the presence of AMPK knockout or EX257 treatment [207]. Punicalagin counteracted cardiomyocyte apoptosis and inflammation in a rat LAD-induced MI/RI model by upregulating the Sirt1/Nrf2 pathway while downregulating inhibitory kappa B alpha phosphorylation and TNF-α [208]. The low-molecular-weight polyphenol curcumin extracted from Curcuma longa L. reduced cardiac apoptosis and alleviated oxidative stress in MI rats by modulating Sirt1 and FOXO1 expression [209]. Curcumin demonstrated comparable effects in a mouse diabetic cardiomyopathy model [210]. The polyphenolic antioxidant ellagic acid occurs in various fruits and vegetables. It upregulated Sirt1 and FOXO1 deacetylation and prevented oxidative stress in a rat diabetic cardiomyopathy model [211]. In the same model, rosmarinic acid mitigated cardiomyocyte mitochondrial damage by upregulating Sirt1 and PGC-1α. However, Sirt1 knockout counteracted these effects [212].
The saponins in various TCM formulations have neuroprotectant and anti-cancer efficacy and attenuate nerve pain [[213], [214], [215]]. The ginsenosides Rc and Rb2 enhanced cardiac mitochondrial biosynthesis and glucose uptake, improved energy metabolism, and upregulated Sirt1 in a rat I/R model. Both foregoing substances are saponins derived from ginseng (Araliaceae) [216,217]. Araloside C extracted from the root bark of Aralia elata prevented cytotoxicity by upregulating AMPK protein in an in vitro H9c2 cardiomyocyte damage model [218].
Numerous other plant natural products also have cardioprotective efficacy mediated by Sirt1/AMPK signaling. In a mouse arsenic trioxide injection-induced MI model, 6-gingerol derived from Zingiberis Rhizoma Recens (ZRR; Sheng Jiang) suppressed oxidative stress and attenuated myocardial mitochondrial damage by upregulating AMPK, Sirt1, and PGC-1α proteins [219]. In addition, Li et al. [220] reported that the phenolic compound salvianolic acid B from Salviae miltiorrhizae ameliorated cardiac function and reduced myocardial apoptosis in a rat LAD-induced MI model. Nevertheless, EX257 addition reversed the preceding effects and downregulated AMPK phosphorylation and Sirt1 protein. Salvianolic acid B upregulated the AMPK/Phosphoinositide 3-kinas (PI3K) pathway, reduced infarct size, and improved cardiac function. The same study also showed that the mechanism of tanshinone IIA resembled that of salvianolic acid B [221]. In a rat LAD-induced AMI/R model and a myocardial cell oxygen-glucose deprivation-induced injury model, arctigenin inhibited cardiomyocyte apoptosis, oxidative stress, and inflammation and upregulated Sirt1 and AMPK phosphorylation in a dose-dependent manner. Compound C downregulated AMPK and Sirt1 whereas the Sirt1 inhibitor Sirt1-IN-1 did not alter AMPK phosphorylation. Hence, arctigenin upregulated Sirt1 by activating AMPK [222]. The bioactive alkaloid berberine isolated from various TCM formulations has antimicrobial, antidiabetic, and anti-cancer efficacy [223]. LAD-induced I/R injury is associated with excessive autophagy. However, berberine mitigates autophagy by downregulating mTORC2 and AMPK phosphorylation [224]. In a mouse DOX-induced aging heart model, berberine protected against oxidative stress and apoptosis by upregulating Sirt1 [225]. In a rat ISO-induced HF model, the phenylethanol glycoside echinacoside from Cistanche Herba mitigated mitochondrial oxidative damage and apoptosis by upregulating Sirt1 and FOXO3a [226]. The anthraquinone rhein is anti-inflammatory, antitumor, and hepatoprotectant. In a mouse Ang II-induced cardiac remodeling model, it upregulated AMPK and suppressed oxidative stress [227]. Thymoquinone is derived from Nigellae Semen. In an in vitro MI/R model, it improved cardiac function, reduced apoptosis, attenuated oxidative stress damage, and upregulated Sirt1 protein in a concentration-dependent manner [228]. The phenolic hydroxyl honokiol is extracted from Magnoliae Officinalis Cortex. In a rat LAD-induced MI/RI model, it reduced oxidative stress and inhibited cardiac apoptosis by upregulating the Sirt1/Nrf2 pathway [229]. The alkaloid capsaicin is isolated from chili pepper. In an in vitro H/R model, it reduced oxidative stress by upregulating Sirt1 [230]. The ester crocin is extracted from Crocus sativus. Wang et al. [231] reported that in a rat LAD-induced I/R injury model, crocin reduced cardiac apoptosis and endoplasmic reticulum stress and protected myocardial cells against I/R injury by upregulating Sirt1 and Nrf2. However, the preceding effects were reversed by nicotinamide. Above constituents from herbal medicines effects were summarized in Table 3 [[161], [162], [163],[165], [166], [167],[169], [170], [171], [172], [173], [174], [175], [176], [177], [178],[180], [181], [182], [183], [184], [185],[187], [188], [189],[195], [196], [197], [198], [199], [200], [201], [202], [203],[206], [207], [208], [209], [210], [211], [212],[216], [217], [218], [219], [220], [221], [222],[224], [225], [226], [227], [228], [229], [230], [231]], and Fig. 3.
Table 3.
Protective effects of constituents from herbal medicine on cardiomyocytes.
| Classification/Constituents | Chemical structure | Animal/Cells | Models | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | |||||||
| Tanshinone IIA |  |
SD rats; H9c2 cells | LAD-induced HF | 1.5 mg/kg; 5 μM | Reversing pathological changes; Reducing the ratio of apoptosis; Upregulating autophagy | AMPK, Bcl-2, Beclin1 | Bax | [161] |
| Tanshinone IIA | C57BL/6 mice | LAD-induced HF | 5, 25 mg/kg | Reducing microvascular damage, apoptosis and mitochondrial damage of cardiomyocytes | Sirt1, PGC-1α | – | [162] | |
| Acacetin |  |
SD rats; H9c2 cells | Ang II-induced HF | 10 mg/kg; 0.3, 1, 3 μM | Protecting cardiomyocyte hypertrophy; Reducing ROS production and apoptosis; Increasing anti-oxidation | PGC-1α, PPARα, AMPK, Sirt1 | BNP | [163] |
| Acacetin | C57BL/6 mice; H9c2 cells | Dox-induced cardiomyopathy rats | 15 mg/kg; 0.3, 1, 3 μM | Improving cardiac function and cell viability; Reducing ROS levels | Sirt1, AMPK, Nrf2, HO-1, | Bax, cleaved caspase-3 | [165] | |
| Acacetin | C57BL/6 mice; H9c2 cells | D-galactose induced aging mouse | 10, 20, 50 mg/kg; 0.3, 1, 3 μM | Improving heart function; Decreasing myocardial senescence markers | PINK1, Parkin, Sirt6, Sirt1, AMPK | – | [166] | |
| Mangiferin |  |
C57BL/6 mice; H9c2 cells | LAD-induced HF | 40 mg/kg/day; 20 μM | Alleviating cardiomyocyte apoptosis; Improving cell viability | Sirt1, FOXO3α | – | [167] |
| Tilianin | 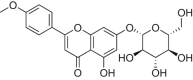 |
SD rats | LAD-induced MI/RI | 5 mg/kg | Reducing cardiac infarct size; Improving swollen and ruptured of cells | AMPK, Sirt1, PGC-1α, FOXO1 | – | [169] |
| Luteolin | 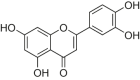 |
SD rats | LAD-induced MI/RI | 20, 40 mg/kg | Reducing myocardial infarction area; Improving myocardial pathological | Sirt1 | TNF-α, IL-6, NLRP3 | [170] |
| Quercetin |  |
SD rats | LAD-induced I/R | 25, 50, 100 mL/L | Reducing the pathological changes and apoptosis | Sirt1, PGC-1α, Bcl-2 | Bax | [171] |
| Quercetin | Human cardiomyocytes | Vitro H/R model | 50, 100, 150, 200, 250 mg/L | Improving the vulnerability of cardiomyocytes; Ameliorating mitochondrial oxidative stress | Sirt1, PINK1, Atg5, TMBIM6, Bcl-2 | – | [172] | |
| Icariin | 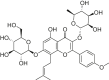 |
C57BL/6 mice | Vitro H/R model | 2, 4, 8 μM | Reducing cell death and apoptosis; Inhibiting oxidative stress; Restoring mitochondrial dysfunction | Sirt1, FOXO1, Bcl-2, Sirt6 | Bax, caspase-3 | [173] |
| Naringenin | 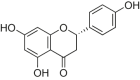 |
H9c2 cells | H2O2 induced cardiomyocyte injury | 10, 30, 100, 300 μM | Reducing ROS levels and inflammatory; Alleviating cardiac fibrotic remodeling | Sirt1 | TNF-α, IL-6 | [174] |
| Rutin |  |
H9c2 cells | Vitro H/R model | 50 μM | Protecting cell viability; Attenuating myocardial injury and apoptosis | Sirt1 | caspase-3 | [175] |
| Puerarin | 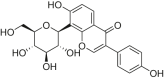 |
C57BL/6 mice | LAD-induced MI | 100 mg/kg | Reducing myocardial infarct size; Improving cardiac structural damage | Sirt1 | NLRP3, NF-κB, IL-18, IL-1β | [176] |
| Dihydromyricetin | 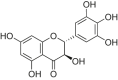 |
SD rats; H9c2 cells | Dox-induced cardiotoxicity rats | 100, 200 mg/kg/day; 0.5, 1, 2, 5, 10 μM | Preventing cardiac damage; Attenuating the left ventricle dysfunction; Inhibiting apoptosis | Bcl-2, Sirt1 | Bax, NLRP3, IL-1β | [177] |
| Jaceosidin |  |
C57BL/6 mice; neonatal rat cardiac myocytes | Dox-induced cardiotoxicity rats | 4 mg/kg; 2.5, 5, 10, 15 μM | Suppressing cardiac ROS; Inhibiting inflammation and cell loss; Attenuating cardiac injury and oxidative stress | Sirt1, Bax | NF-κB, TNF-α, MCP-1, Bcl-2 | [178] |
| Oroxylin A |  |
C57BL/6 mice; H9c2 cells | Dox-induced cardiotoxicity rats | 40 mg/kg; 40 μM | Suppressing cardiac injury and cardiac dysfunction; Alleviating oxidative damage and apoptosis | Sirt1, Nrf2, HO-1, NQO1 | TNF-α, IL-6, IL-1β, MMP-2, MMP-9 | [180] |
| Kaempferol |  |
C57BL/6 mice | Ang II-induced mice cardiac injury | 2.5, 10 mM | Attenuating cardiac disfunction; Reducing inflammation and oxidative stress of cardiac | AMPK, Nrf-2 | ERK, TNF-α, IL-6 |
[181] |
| Kaempferol | H9c2 cells | Vitro A/R model | 20 μM | Increasing the viability of cardiac; Decreasing ROS level | Sirt1, Bcl-2 | Caspase-3 | [182] | |
| Isorhamnetin | 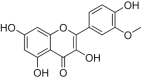 |
SD rats; H9c2 cells |
Vivo A/R model | 10, 20, 40 mM | Improving cell viability; Reducing oxidative stress and apoptosis | Sirt1 | caspase-3 | [183] |
| Phloretin |  |
rats | Streptozotocin-induced diabetic mouse model | 20 mg/kg | Preventing cardiomyocyte injury; Attenuating fibrotic response, apoptotic death | Sirt1 | IL-6, TNF-α, ANP, TGF-β |
[184] |
| Hesperetin |  |
Kunming mice | ISO-induced MI | 25, 50 mg/kg/day | Antagonizing the myocardial injury; Improving myocardial injury | Bcl-2, Nrf-2, Sirt1, NQO-1, HO-1 |
IL-6, TNF-α, Bax, caspase-3 |
[185] |
| Silibinin |  |
H9c2 cells | Vitro ISO induced myocardial cells injury | 0.3, 0.5 mM | Inhibiting cardiac death; Protecting mitochondrial function | Bcl2, Sirt1 | – | [187] |
| Liquiritin | 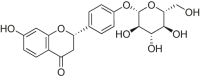 |
H9c2 cells | H2O2 induced cardiomyocyte injury | 5, 10, 20 μM | Promoting cells proliferation; Reducing ROS level and oxidative stress | AMPK, Sirt1 | NF-κB | [188] |
| Daidzein |  |
SD rats | Streptozotocin-induced diabetes rats | 25, 50, 100 mg/kg | Improving cardiac function; Reducing oxidative stress | AMPK, Sirt1 | – | [189] |
| Oxypaeoniflorin | 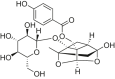 |
C57BL/6 mice | LAD-induced MI/RI | 10, 20, 40 mg/kg | Improving cardiac function; Ameliorating cardiac damage; Protecting cardiomyocytes | Sirt1, Bcl-2 |
Bax, cleaved caspase-3, FOXO1 | [195] |
| Betulin |  |
Wistar rats | LAD-induced MI/RI | 20, 40 mg/kg | Alleviating myocardial inflammatory; Relieving myocardial function changes | Sirt1, NLRP3 | IL-6, TNF-α, IL-18 |
[196] |
| Bakuchiol | 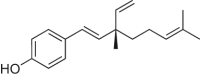 |
C57BL/6 mice | LAD-induced MI/RI | 0.25, 0.5, 1 μM | Reducing myocardial dysfunction and cardiac hypertrophy; Alleviating oxidative stress | Sirt1, PGC-1α, Bcl2 | Bax, cleaved Caspase3 | [197] |
| Bakuchiol | SD rats | High-fat diet-induced diabetic rats | 60 mg/kg | Improving the functional recovery; Reducing apoptosis and oxidative stress | Sirt1, Nrf2 | TGF-β1 | [198] | |
| Geniposide | 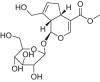 |
Wistar–Kyoto and SHR rats | High-fat diet-induced diabetic rats | 25, 50 mg/kg/day | Reducing myocardial injury; Improving the systolic function of the left ventricle and myocardial hypertrophy | AMPK, FOXO1, Sirt1 | Bax | [199] |
| Geniposide | C57/B6J mice | Spontaneously hypertensive rats | 50 μM | Improving left ventricular function; Inhibiting myocardial inflammation and apoptosis | AMPK, Sirt1 | cleaved caspase-3, NF-κB, TNF-α, IL-1 |
[200] | |
| Isosteviol sodium |  |
SD rats | High fat/high cholesterol-induced rats | 1, 10, 20 mg/kg/day | Improving cardiac function; Relieving myocardial fibrosis and oxidative stress | AMPK, Sirt1 | – | [201] |
| Isosteviol sodium | H9c2 cells | High glucose and ISO induced H9c2 | 5, 10, 50 μM | Reducing ROS accumulation; Protecting cells against cardiomyocyte hypertrophy | Sirt1, PGC-1α, AMPK | – | [202] | |
| Ginkgolide B |  |
H9c2 cells | AngII-induced H9c2 cells hypertrophy | 10, 30, 50, 100 μM | Preventing cardiomyocyte hypertrophy; Promoting autophagy; Inhibiting oxidative stress | Beclin-1, Sirt1, FOXO1 | – | [203] |
| Resveratrol | 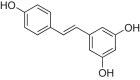 |
SD rats; H9c2 cells | LAD-induced HF | 2.5 mg/kg; 50, 100 μM | Improving cardiac function; Reducing mortality of HF | AMPK, Sirt1 | – | [206] |
| Pterostilbene | 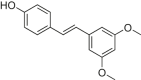 |
C57BL/6 mice; H9c2 cells | Dox-induced cardiotoxicity | 10 mg/kg/day; 10 μM | Alleviating cell viability inhibition, mitochondrial damage and oxidative stress | AMPK, Sirt1, PGC-1α, NRF1, UCP2 | – | [207] |
| Punicalagin | 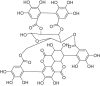 |
SD rats | LAD-induced MI/RI | 20, 40, 80 mg/kg/day | Reducing apoptosis, inflammation and myocardial infarct size | Sirt1, Nrf2, HO-1, NQO-1 |
caspase-3 | [208] |
| Curcumin | 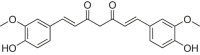 |
SD rats; H9c2 cells | LAD-induced MI | 100 mg/kg/day | Reducing apoptosis and oxidative stress; Improving cardiac function | Sirt1, PI3K, Akt, FOXO1, Bcl-2 |
Bax | [209] |
| Curcumin | C57BL/6J mice | Streptozotocin-induced diabetes rats | 100 mg/kg/day | Alleviating cardiac fibrosis | Sirt1 | MMP-2, MMP-9 | [210] | |
| Ellagic acid |  |
Wistar rats | High-glucose induced diabetic cardiomyopathy mice | 20, 50, 100, 200 mg/kg | Preserving cardiac systolic and diastolic function; Preventing oxidative stress | Sirt1, FOXO1 | IL-6, NF-κB |
[211] |
| Rosmarinic acid | 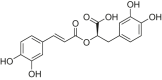 |
C57BL/6 mice | High-glucose induced diabetic cardiomyopathy mice | 20 mM | Ameliorating cardiac function; Preventing mitochondrial injury | Sirt1, PGC-1α | cleaved caspase-3 | [212] |
| Ginsenoside Rc | 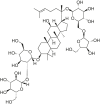 |
SD rats | LAD-induce I/R | 10 mg/kg | Enhancing mitochondrial biosynthesis and glucose uptake of cardiac; Inhibiting mitochondrial damage | Sirt1, GLUT4, HK-Ⅰ/Ⅱ, PFK, PGC-1α | Bax | [216] |
| Ginsenoside Rb2 |  |
Wistar rats | LAD-induce I/R | 10, 20 mg/kg | Reducing oxidative stress and inflammation response; Improving cardiac dysfunction | Sirt1, Bcl-2 |
IL-1β, IL-6, TNF-α |
[217] |
| Araloside C |  |
H9c2 cells | H2O2 induced cardiomyocyte injury | 6.5, 12.5, 25 μM | Inhibiting H9c2 cardiomyocytes mitochomyocytes damage and cytotoxicity of H2O2-induced | AMPK, Bcl2 | Bax | [218] |
| 6-Gingerol | 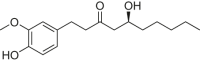 |
KunMing mice | As2O3-induced MI | 10, 20 mg/kg | Ameliorating heart injury; Suppressing oxidative stress; Attenuating myocardial mitochondrial damage | Bcl-2, AMPK, Sirt1, PGC-1α | TNF-α, IL-6, Bax |
[219] |
| Salvianolic acid B | 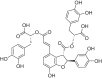 |
SD rats; H9c2 cells | LAD-induced MI | 8, 32 mg/kg | Ameliorating cardiac function; Reducing infarct size and myocardial apoptosis | Sirt1, AMPK, PGC-1α | NLRP3, Caspase-1 | [220] |
| Salvianolic acid B | C57BL/6 mice | LAD-induced MI | 40 mg/kg | Attenuating cardiac infraction sizes; Improving cardiac function | AMPK, eNOS, PI3K, Akt | – | [221] | |
| Arctigenin |  |
SD rats; H9c2 cells | LAD-induce AMI/R | 100 μmol/kg; 0, 25, 50, 100, 200, 400 μM | Inhibiting apoptosis of cardiomyocytes, oxidative stress and inflammatory; Improving cardiac functions | AMPK, Sirt1, Bcl-2, I-κB |
NF-κB, Caspase3 | [222] |
| Berberine | 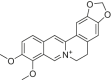 |
C57BL/6 mice; H9c2 cells | LAD-induced I/R | 5, 10 mg/kg; 5, 20 μM | Restoring cardiac function; Protecting cell death and inhibiting autophagy | – | AMPK, mTORC2, Sirt1 | [224] |
| Berberine | SD rats; H9c2 cells | Dox-induced aging mice | 100, 200 mg/kg | Reducing ratio of senescent cells; Improving cell viability | Bcl-2, Sirt1 | Bax | [225] | |
| Echinacoside | 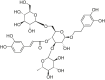 |
SD rats; AC16 cells | ISO-induced HF | 20 μg/g; 50 μM | Reversing myocardial remodeling; Inhibiting mitochondrial oxidative damage and apoptosis | Sirt1, FOXO3a | – | [226] |
| Rhein | 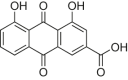 |
C57BL/6 J mice | Ang II-induced cardiac remodeling mice | 50, 100 mg/kg | Inhibiting cardiac hypertrophy and cardiac fibroblasts; Improving cardiac function; Suppressing oxidative stress | AMPK | FGF-23 | [227] |
| Thymoquinone | 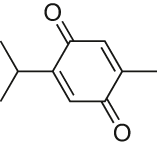 |
SD rats | LAD- induce MI/R | 2.5, 5, 10 μM | Improving cardiac function; Reducing apoptosis oxidative stress damage | Sirt1, Bcl-2 |
Bax, cleaved caspase-3 | [228] |
| Honokiol | 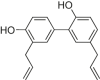 |
SD rats; H9c2 cells | LAD-induced MI/RI | 5 mg/kg/day; 5 μM | Alleviating cardiac damage; Reducing oxidative stress apoptosis | Sirt1, Nrf2, Bcl-2 | Bax | [229] |
| Capsaicin | 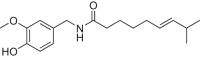 |
Sprague Dawley rats | Vitro H/R model | 20 μM | Improving viabilities of cardiomyocytes; Reducing oxidative stress | Bcl-2, Sirt1 | Caspase-3 | [230] |
| Crocin | 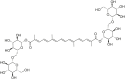 |
newborn C57BL/6 mice | LAD-induced I/R | 50 mg/kg/day; 10 μM | Reducing apoptosis and ER stress | Bcl-2, Sirt1, Nrf2, HO-1 | Caspase-3, Bax | [231] |
SD: Sprague Dawley; LAD: ligation of the left anterior descending coronary artery; HF: heart failure; AMPK: adenosine monophosphate activated protein kinase; Bcl-2: B-cell lymphoma-2; Bax: Bcl2-associated x; Sirt1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; Ang II: angiotensin II; PPARα: peroxisome proliferator-activated receptor α; BNP: brain natriuretic peptide; DOX: doxorubicin; Nrf2: nuclear factor erythroid2-related factor 2; HO-1: heme oxygenase-1; PINK: PTEN-induced kinase 1; Sirt6: silent information regulator 6; FOXO3: forkhead box family and subfamily O of transcription factor 3; MI/RI: myocardial ischemia-reperfusion injury; FOXO1: forkhead box family and subfamily O of transcription factor 1; TNF-α: tumor necrosis factor α; IL-6: interleukin 6; NLRP3: NOD-like receptor protein 3; I/R: ischemia-reperfusion; H/R: hypoxia/reoxygenation; Atg5: autophagy-related gene 5; TMBIM6: transmembrane BAX inhibitor motif containing 6; NF-κB: nuclear factor-kappa-B; IL-1: interleukin 1; IL-18: interleukin 18; MCP-1: monocyte chemotactic protein-1; NQO1: NAD(P)H-quinone oxidoreductase 1; MMP-2: matrix metalloproteinase 2; MMP-9: matrix metalloproteinase 9; ERK: extracellular signal-regulated kinase; ANP: atrial natriuretic peptide; TGF-β: transforming growth factor-β; NRF-1: nuclear respiratory factor-1; UCP2: uncoupling protein 2; PI3K: phosphoinositide 3-kinase; Akt: protein kinase B; GLUT4: glucose transporter type 4; HK-Ⅰ/Ⅱ: hexokinase-Ⅰ/Ⅱ; PFK: phosphofructokinase; eNOS: endothelial nitric oxide synthase; I-κB: inhibitor kappa B; mTORC2: mammalian target of rapamycin compound 2; ISO: isoprenaline; FGF-23: fibroblast growth factor.
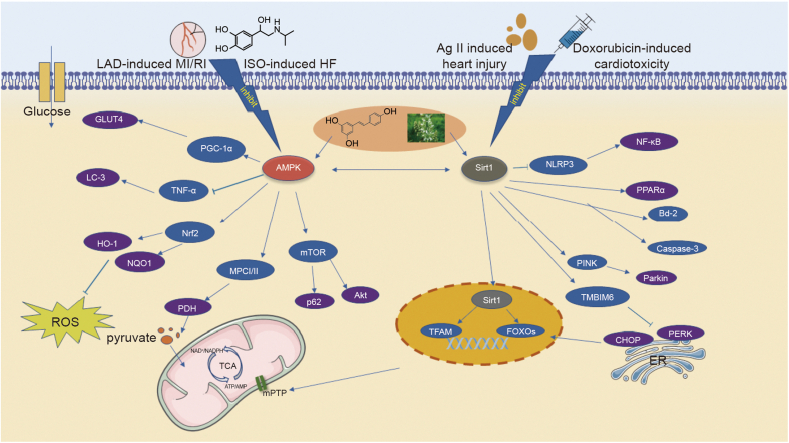
Fig. 3.
The possible molecular mechanisms for constituents from herbal medicines to treat HF via activation of AMPK/Sirt1 signaling. Some monomer compounds of traditional Chinese medicine, such as resveratrol, up-regulate the expression of the GLUT4 protein to promote glucose transport, promote the expression of Bcl-2 to inhibit apoptosis, and up-regulate the expression of the Nrf2 protein to reduce the level of oxidative stress in the heart by activating Sirt1 and AMPK. In addition, these compounds also regulate the tricarboxylic acid cycle through the Sirt1/AMPK pathway to combat the imbalance of myocardial energy metabolism, regulate the opening of mPTP channels to maintain mitochondrial function, regulate autophagy, and inhibit endoplasmic reticulum oxidative stress to maintain normal cell physiological function. Where represents  indicates stimulation/promoting,
indicates stimulation/promoting,  indicates reduction/inhibition. LAD: ligation of the left anterior descending coronary artery; MI/RI: myocardial ischemia-reperfusion injury; ISO: isoprenaline; HF: heart failure; Ang II: angiotensin II; GLUT4: glucose transporter type 4; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; AMPK: adenosine monophosphate activated protein kinase; LC-3: light chain 3; TNF-α: tumor necrosis factor α; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid2-related factor 2; NQO1: NAD(P)H-quinone oxidoreductase 1; PDH: pyruvate dehydrogenase; MPC Ⅰ/Ⅱ: mitochondrial pyruvate carrier Ⅰ/Ⅱ; p62: sequestosome 1; mTOR: mammalian target of rapamycin; Akt: protein kinase B; Sirt1: silent information regulator 1; NLRP3: NOD-like receptor protein 3; NF-κB: nuclear factor-kappa-B; PPARα: peroxisome proliferator-activated receptor α; JNK: c-Jun NH2-terminal kinase; Bcl-2: B-cell lymphoma-2; PINK: PTEN-induced kinase 1; TMBIM6: transmembrane BAX inhibitor motif containing 6; CHOP: C/EBP-homologous protein; PERK: protein kinase R (PKR)-like endoplasmic reticulum kinase; TFAM: mitochondrial transcription factor A; FOXOs: forkhead box family and subfamily O of transcription factors; TCA: tricarboxylic acid; mPTP: mitochondrial permeability transition pore; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated x. The above herbal pictures are reprinted from Reprinted from GBIF.org (year) citation guidelines. Available from https://www.gbif.org/citation-guidelines with permission and was partly generated using Servier Medical Art, provided by Servier, licensed under a creative commons attribution 3.0 unported license.
indicates reduction/inhibition. LAD: ligation of the left anterior descending coronary artery; MI/RI: myocardial ischemia-reperfusion injury; ISO: isoprenaline; HF: heart failure; Ang II: angiotensin II; GLUT4: glucose transporter type 4; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; AMPK: adenosine monophosphate activated protein kinase; LC-3: light chain 3; TNF-α: tumor necrosis factor α; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid2-related factor 2; NQO1: NAD(P)H-quinone oxidoreductase 1; PDH: pyruvate dehydrogenase; MPC Ⅰ/Ⅱ: mitochondrial pyruvate carrier Ⅰ/Ⅱ; p62: sequestosome 1; mTOR: mammalian target of rapamycin; Akt: protein kinase B; Sirt1: silent information regulator 1; NLRP3: NOD-like receptor protein 3; NF-κB: nuclear factor-kappa-B; PPARα: peroxisome proliferator-activated receptor α; JNK: c-Jun NH2-terminal kinase; Bcl-2: B-cell lymphoma-2; PINK: PTEN-induced kinase 1; TMBIM6: transmembrane BAX inhibitor motif containing 6; CHOP: C/EBP-homologous protein; PERK: protein kinase R (PKR)-like endoplasmic reticulum kinase; TFAM: mitochondrial transcription factor A; FOXOs: forkhead box family and subfamily O of transcription factors; TCA: tricarboxylic acid; mPTP: mitochondrial permeability transition pore; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated x. The above herbal pictures are reprinted from Reprinted from GBIF.org (year) citation guidelines. Available from https://www.gbif.org/citation-guidelines with permission and was partly generated using Servier Medical Art, provided by Servier, licensed under a creative commons attribution 3.0 unported license.
4. Discussion and perspectives
The present review demonstrated that the numerous target molecules can affect the fuel-sensing Sirt1/AMPK pathway that is evolutionarily conserved among different cell types. Sirt1 and AMPK regulate PGC-1α, thereby improving cardiac fatty acid and glucose metabolism, regulating p53 targets, and alleviating apoptosis. They also decrease ROS by regulating FOXOs and improve autophagy by mediating mTOR. Sirt1 and AMPK may also protect against HF by modulating other targets. Here, we summarized the natural constituents including flavonoids, terpenoids, polyphenols, and saponins in herbal medicines and their extracts that have demonstrated prophylactic efficacy against HF. Most of these substances alleviate apoptosis and oxidative stress, increase autophagy, and improve energy metabolism in HF. The primary mechanism common to all these therapeutic effects is the activation of the Sirt1/AMPK signaling pathway. The present review provided a theoretical and practical reference for the development of natural products that treat cardiovascular disorders by targeting Sirt1/AMPK.
AMPK is a heterotrimeric complex comprising ≤12 distinct proteins that are formed by assorting various subunits. In humans, γ2 missense mutations cause glycogen-storage cardiomyopathy. In pigs, however, a γ3 mutation causes muscle glycogen accumulation [232]. Thus, the arrangement of AMPK subunits in humans may differ from those in other animals. The use of suitable and appropriate animal models may determine whether the AMPK activators being investigated could, in practice, improve cardiovascular function in vivo. Sirt1 is known as a longevity gene. In mice, both Sirt1 overexpression and silencing downregulated nuclear-encoded mitochondrial genes [233]. Hence, only a low Sirt1 expression level may be required to induce the foregoing genes in cardiac mitochondria. Future research should investigate agonists and inhibitors targeting AMPK or Sirt1 for the treatment of HF and other CVDs. Nevertheless, Sirt1 and AMPK expression levels may change in response to aging and/or disease [93,227,234]. Thus, it might be more successful to combine with treatment of Sirt1 and AMPK activators or inhibitors when the endogenous Sirt1 or AMPK is too high or too low. This gives us a promise. In the foregoing overview of TCM, some drugs play their role through Sirt1-mediated AMPK, some regulate Sirt1 through AMPK. Thus, the compatibility of combinations of multiple medications may have to be evaluated. A Sirt1 activator might upregulate AMPK just as an AMPK activator might upregulate Sirt1. A prior study showed that Sirt1 downregulation impeded AMPK activation in aging hearts [25]. Moreover, Sirt1 and AMPK may operate in tandem to treat various conditions and disorders. They are indispensable targets in the treatment of acute renal injury, renal fibrosis, and other kidney diseases [235]. They also play important roles in the chemotherapeutic resistance of patients with gastric cancer, Sirt1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop [236]. HF is a complex clinical syndrome. Except when it is the result of mechanical injury, its co-morbidities may include vascular and skeletal muscle dysfunction. The compounds described herein demonstrated protective efficacy against certain heart diseases. Nonetheless, the pleiotropic effects of these substances on multiple organ systems merit further exploration.
The relative efficacies of the aforementioned natural compounds as Sirt1/AMPK activators in the prevention and therapy of HF and other CVDs are widely variable. Some of the flavonoids described in this paper have considerable disparities in bioavailability because of their distinct forms of existence. On the one hand, due to their hydrophilicity, flavonoid glycosides could not rapidly diffuse across the intestinal membrane during ingestion. On the other hand, although flavonoid aglycons have high permeability, their oral bioavailability is low due to their poor solubility [237]. Other phenolic compounds confront the same problems in cardiovascular disease, they not only have low oral bioavailability, but also have poor water solubility. Therefore, in order to expand their clinical application, one alternative method, just as some other TCM constituents also face low bioavailability is to make derivatives (structural modifications) and modify dosage forms [238]. Such as pterostilbene, a dimethyl derivative of resveratrol, which has improved bioavailability and similar pharmacological effects [207]. Another study revealed that solid orally delivered lipid resveratrol-loaded nanoparticles had 8.035-fold higher bioavailability than resveratrol suspension [239]. Nevertheless, the pharmacological effects and efficacy of structurally and dosage form-modified drugs must be carefully evaluated. Furthermore, many of these natural plant products are pleiotropic. For this reason, it is difficult to determine whether it is their direct biological modes of action or their side effects that ameliorate cardiopathy. In addition, it remains to be established whether it is the parent compounds or their metabolites that have prophylactic or therapeutic efficacy against CVDs. For example, although the protective effects of polyphenols on HF have been reviewed, polyphenols are extensively metabolized by cytochrome P450 and UDP-glucose pyro-phosphorylase in the liver and intestinal epithelium [240,241].
Steady progress has been made in identifying the constituents of natural plant products that are cardioprotective by activating Sirt1/AMPK signaling. However, preclinical and clinical trials will be required to detect and validate the safety and efficacy of novel plant secondary metabolites for the prevention and treatment of HF.
CRediT author statement
TaoZhang: Investigation, Data curation, Writing - Original draft preparation, Reviewing and Editing; Lei Xu: Writing - Original draft preparation; Xiaowei Guo: Investigation, Data curation; Honglin Tao: Investigation, Data curation; Yue Liu: Supervision; Xianfeng Liu: Writing -Reviewing and Editing; Yi Zhang: Resources, Writing - Reviewing and Editing, Supervision, Funding acquisition; Xianli Meng: Resources, Writing - Reviewing and Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No.: 82130113), the “Xinglin Scholars” Research Promotion Program of Chengdu University of Traditional Chinese Medicine (Program No.: ZDZX2022005), the China Postdoctoral Science Foundation (Grant No.: 2021MD703800), and the Science Foundation for Youths of Science & Technology Department of Sichuan Province (Grant No.: 2022NSFSC1449). Figs. 2 and 3 and graphical abstract were partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license”. The herbal durgs shown in Figs. 2 and 3 and graphical abstract available from: https://www.gbif.org.
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
Contributor Information
Xianfeng Liu, Email: 1697339184@qq.com.
Yi Zhang, Email: zhangyi@cdutcm.edu.cn.
Xianli Meng, Email: xlm999@cdutcm.edu.cn.
References
- 1.Shugg T., Hudmon A., Overholser B.R. Neurohormonal regulation of IKs in heart failure: Implications for ventricular arrhythmogenesis and sudden cardiac death. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippi G., Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med. J. 2020;5 [Google Scholar]
- 3.Warraich H.J., Xu H., DeVore A.D., et al. Trends in hospice discharge and relative outcomes among medicare patients in the get with the guidelines-heart failure registry. JAMA Cardiol. 2018;3:917–926. doi: 10.1001/jamacardio.2018.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah Kevin S., Xu H.L., Matsouaka R.A., et al. Heart Failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 5.Del Buono M.G., Arena R., Borlaug B.A., et al. Exercise intolerance in patients with heart failure. J. Am. Coll. Cardiol. 2019;73:2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Zambroski C.H., Moser D.K., Bhat G., et al. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur. J. Cardiovasc. Nurs. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Tanno M., Kuno A., Horio Y., et al. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012;107 doi: 10.1007/s00395-012-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopaschuk G.D., Karwi Q.G., Tian R., et al. Cardiac energy metabolism in heart failure. Circ. Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacCarthy P.A., Shah A.M. Oxidative stress and heart failure. Coron. Artery Dis. 2003;14:109–113. doi: 10.1097/00019501-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 10.van der Pol A., van Gilst W.H., Voors A.A., et al. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei Y., Thompson M.D., Cohen R.A., et al. Autophagy and oxidative stress in cardiovascular diseases. Biochim. Biophys. Acta. 2015;1852:243–251. doi: 10.1016/j.bbadis.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oka T., Hikoso S., Yamaguchi O., et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao T., Zhang S.P., Wang J.F., et al. TLR3 contributes to persistent autophagy and heart failure in mice after myocardial infarction. J. Cell. Mol. Med. 2018;22:395–408. doi: 10.1111/jcmm.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivetti G., Abbi R., Quaini F., et al. Apoptosis in the failing human heart. N Engl J. Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 15.Cowan J., Longman M.R., Snabaitis A.K. Regulation of cardiomyocyte DNA damage and cell death by the type 2A protein phosphatase regulatory protein alpha4. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-85616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R., Mohan N., Upadhyay A.D., et al. Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell. 2014;13:975–980. doi: 10.1111/acel.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu W.L., Zhang Q.J., Tang X.Q., et al. Overexpression of a dominant-negative mutant of SIRT1 in mouse heart causes cardiomyocyte apoptosis and early-onset heart failure. Sci. China Life Sci. 2014;57:915–924. doi: 10.1007/s11427-014-4687-1. [DOI] [PubMed] [Google Scholar]
- 18.Akkafa F., Altiparmak I.H., Erkus M.E., et al. Reduced SIRT1 expression correlates with enhanced oxidative stress in compensated and decompensated heart failure. Redox Biol. 2015;6:169–173. doi: 10.1016/j.redox.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W.C., Wang L.L., Yang M.Y., et al. Circ-SIRT1 inhibits cardiac hypertrophy via activating SIRT1 to promote autophagy. Cell Death Dis. 2021;12 doi: 10.1038/s41419-021-04059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima A., Lopaschuk G.D. Acetylation control of cardiac fatty acid β-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim. Biophys. Acta. 2016;1862:2211–2220. doi: 10.1016/j.bbadis.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Chen C., Dong B., et al. AMPK attenuates ventricular remodeling and dysfunction following aortic banding in mice via the Sirt3/Oxidative stress pathway. Eur. J. Pharmacol. 2017;814:335–342. doi: 10.1016/j.ejphar.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Chen C., Yao F., et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch. Biochem. Biophys. 2014;558:79–86. doi: 10.1016/j.abb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Cantó C., Gerhart-Hines Z., Feige J.N., et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantó C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Quan N., Sun W., et al. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018;114:805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagon Y., Mantzoros C.S., Kim Y.B. AMPK↔Sirt1: From a signaling network to a combination drug. Metabolism. 2016;65:1692–1694. doi: 10.1016/j.metabol.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Liang Y., Vanhoutte P.M. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 28.Anggreini P., Kuncoro H., Sumiwi S.A., et al. Role of the AMPK/SIRT1 pathway in non-alcoholic fatty liver disease (review) Mol Med Rep. 2023;27 doi: 10.3892/mmr.2022.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bœuf-Gibot S., Pereira B., Imbert J., et al. Benefits and adverse effects of ACE inhibitors in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2021;77:321–329. doi: 10.1007/s00228-020-03018-4. [DOI] [PubMed] [Google Scholar]
- 30.Ter Maaten J.M., Martens P., Damman K., et al. Higher doses of loop diuretics limit uptitration of angiotensin-converting enzyme inhibitors in patients with heart failure and reduced ejection fraction. Clin. Res. Cardiol. 2020;109:1048–1059. doi: 10.1007/s00392-020-01598-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiefer D., Pantuso T. Panax ginseng. Am. Fam. Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 32.Jia Q., Wang L., Zhang X., et al. Prevention and treatment of chronic heart failure through traditional Chinese medicine: Role of the gut microbiota. Pharmacol. Res. 2020;151 doi: 10.1016/j.phrs.2019.104552. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Zhang J., Huang J., et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of Qili Qiangxin capsules in patients with chronic heart failure. J. Am. Coll. Cardiol. 2013;62:1065–1072. doi: 10.1016/j.jacc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzen J.M., Martino F., Thum T. Epigenetic modifications in cardiovascular disease. Basic Res. Cardiol. 2012;107 doi: 10.1007/s00395-012-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueiras R., Habegger K.M., Chaudhary N., et al. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanno M., Sakamoto J., Miura T., et al. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 37.Bolasco G., Calogero R., Carrara M., et al. Cardioprotective mIGF-1/SIRT1 signaling induces hypertension, leukocytosis and fear response in mice. Aging. 2012;4:402–416. doi: 10.18632/aging.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zee R.S., Yoo C.B., Pimentel D.R., et al. Redox regulation of sirtuin-1 by S-glutathiolation, Antioxid. Redox Signal. 2010;13:1023–1032. doi: 10.1089/ars.2010.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu C.P., Odewale I., Alcendor R.R., et al. Sirt1 protects the heart from aging and stress. Biol. Chem. 2008;389:221–231. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 40.Borra M.T., O’Neill F.J., Jackson M.D., et al. Conserved enzymatic production and biological effect of O-acetyl-ADP-ribose by silent information regulator 2-like NAD+-dependent deacetylases. J. Biol. Chem. 2002;277:12632–12641. doi: 10.1074/jbc.M111830200. [DOI] [PubMed] [Google Scholar]
- 41.Lee S., Tong L., Denu J.M. Quantification of endogenous sirtuin metabolite O-acetyl-ADP-ribose. Anal. Biochem. 2008;383:174–179. doi: 10.1016/j.ab.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong L., Lee S., Denu J.M. Hydrolase regulates NAD+ metabolites and modulates cellular redox. J. Biol. Chem. 2009;284:11256–11266. doi: 10.1074/jbc.M809790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 44.Oka S., Alcendor R., Zhai P., et al. PPARα-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011;14:598–611. doi: 10.1016/j.cmet.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cattelan A., Ceolotto G., Bova S., et al. NAD(+)-dependent SIRT1 deactivation has a key role on ischemia-reperfusion-induced apoptosis. Vasc. Pharmacol. 2015;70:35–44. doi: 10.1016/j.vph.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardie D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 48.Li X., Liu J., Lu Q., et al. AMPK: a therapeutic target of heart failure-not only metabolism regulation. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruderman N.B., Julia Xu X., Nelson L., et al. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiNicolantonio J.J., McCarty M.F., Assanga S.I., et al. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart. 2022;9 doi: 10.1136/openhrt-2021-001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y., Huang W., Zhang C., et al. Energy metabolism disorders and potential therapeutic drugs in heart failure. Acta Pharm. Sin. B. 2021;11:1098–1116. doi: 10.1016/j.apsb.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menssen A., Hydbring P., Kapelle K., et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E187–E196. doi: 10.1073/pnas.1105304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J.E., Chen J., Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 54.Nin V., Escande C., Chini C.C., et al. Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. J. Biol. Chem. 2012;287:23489–23501. doi: 10.1074/jbc.M112.365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang C., Su H., Zhang D., et al. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell. 2015;60:930–940. doi: 10.1016/j.molcel.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 56.Passariello C.L., Zini M., Nassi P.A., et al. Upregulation of SIRT1 deacetylase in phenylephrine-treated cardiomyoblasts. Biochem. Biophys. Res. Commun. 2011;407:512–516. doi: 10.1016/j.bbrc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 57.Lan F., Cacicedo J.M., Ruderman N., et al. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potenza M.A., Sgarra L., Nacci C., et al. Activation of AMPK/SIRT1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia-reperfusion (I/R) injury in rats. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sack M.N., Rader T.A., Park S., et al. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 61.Azevedo P.S., Minicucci M.F., Santos P.P., et al. Energy metabolism in cardiac remodeling and heart failure. Cardiol. Rev. 2013;21:135–140. doi: 10.1097/CRD.0b013e318274956d. [DOI] [PubMed] [Google Scholar]
- 62.Wu J., Lu J., Huang J., et al. Variations in energy metabolism precede alterations in cardiac structure and function in hypertrophic preconditioning. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/fcvm.2020.602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fragasso G., Palloshi A., Puccetti P., et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J. Am. Coll. Cardiol. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 64.Fragasso G., Salerno A., Lattuada G., et al. Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart Br. Card. Soc. 2011;97:1495–1500. doi: 10.1136/hrt.2011.226332. [DOI] [PubMed] [Google Scholar]
- 65.Salvatore T., Galiero R., Caturano A., et al. Effects of metformin in heart failure: From pathophysiological rationale to clinical evidence. Biomolecules. 2021;11 doi: 10.3390/biom11121834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai S., Armstrong C.M., Kaeberlein M., et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 67.Di W., Lv J., Jiang S., et al. PGC-1: The energetic regulator in cardiac metabolism. Curr. News. Mol. Biol. 2018;28:29–46. doi: 10.21775/cimb.028.029. [DOI] [PubMed] [Google Scholar]
- 68.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Dillon L.M., Rebelo A.P., Moraes C.T. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowe G.C., Jiang A., Arany Z. PGC-1 coactivators in cardiac development and disease. Circ. Res. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schilling J., Lai L., Sambandam N., et al. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor γ coactivator-1 signaling. Circ. Heart Fail. 2011;4:474–482. doi: 10.1161/CIRCHEARTFAILURE.110.959833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldman M., Cohen K., Yadin D., et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1α. Cardiovasc. Diabetol. 2018;17 doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu L., Wang Q., Zhang L., et al. Hypoxia induces PGC-1α expression and mitochondrial biogenesis in the myocardium of TOF patients. Cell Res. 2010;20:676–687. doi: 10.1038/cr.2010.46. [DOI] [PubMed] [Google Scholar]
- 74.Jäger S., Handschin C., St-Pierre J., et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montaigne D., Butruille L., Staels B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021;18:809–823. doi: 10.1038/s41569-021-00569-6. [DOI] [PubMed] [Google Scholar]
- 76.Irukayama-Tomobe Y., Miyauchi T., Sakai S., et al. Endothelin-1-induced cardiac hypertrophy is inhibited by activation of peroxisome proliferator-activated receptor-alpha partly via blockade of c-Jun NH2-terminal kinase pathway. Circulation. 2004;109:904–910. doi: 10.1161/01.CIR.0000112596.06954.00. [DOI] [PubMed] [Google Scholar]
- 77.Park S.-Y., Cho Y.-R., Finck B.N., et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 78.Duan S.Z., Ivashchenko C.Y., Russell M.W., et al. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ. Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 79.Huss J.M., Imahashi K.I., Dufour C.R., et al. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 80.Planavila A., Iglesias R., Giralt M., et al. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011;90:276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 81.Xiao X., Su G., Brown S.N., et al. Peroxisome proliferator-activated receptors gamma and alpha agonists stimulate cardiac glucose uptake via activation of AMP-activated protein kinase. J. Nutr. Biochem. 2010;21:621–626. doi: 10.1016/j.jnutbio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Dong H.W., Zhang L.F., Bao S.L. AMPK regulates energy metabolism through the SIRT1 signaling pathway to improve myocardial hypertrophy. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2757–2766. doi: 10.26355/eurrev_201805_14973. [DOI] [PubMed] [Google Scholar]
- 83.Tran D.H., Wang Z.V. Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marsin A.S., Bertrand L., Rider M.H., et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 85.Li J., Hu X., Selvakumar P., et al. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287:E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 86.Williams R.S. Apoptosis and heart failure. N. Engl. J. Med. 1999;341:759–760. doi: 10.1056/NEJM199909023411012. [DOI] [PubMed] [Google Scholar]
- 87.Abbate A., Biondi-Zoccai G.G.L., Bussani R., et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003;41:753–760. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- 88.Garg S., Narula J., Chandrashekhar Y. Apoptosis and heart failure: Clinical relevance and therapeutic target. J. Mol. Cell. Cardiol. 2005;38:73–79. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Rössig L., Haendeler J., Mallat Z., et al. Congestive heart failure induces endothelial cell apoptosis: Protective role of carvedilol. J. Am. Coll. Cardiol. 2000;36:2081–2089. doi: 10.1016/s0735-1097(00)01002-0. [DOI] [PubMed] [Google Scholar]
- 90.Fujita T., Ishikawa Y. Apoptosis in heart failure.-The role of the β-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes- Circ. J. 2011;75:1811–1818. doi: 10.1253/circj.cj-11-0025. [DOI] [PubMed] [Google Scholar]
- 91.Long X., Boluyt M.O., Hipolito M.L., et al. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J. Clin. Investig. 1997;99:2635–2643. doi: 10.1172/JCI119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long X., Crow M.T., Sollott S.J., et al. Enhanced expression of p53 and apoptosis induced by blockade of the vacuolar proton ATPase in cardiomyocytes. J. Clin. Investig. 1998;101:1453–1461. doi: 10.1172/JCI345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C., Feng Y., Qu S., et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011;90:538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- 94.Jones R.G., Plas D.R., Kubek S., et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 95.Okoshi R., Ozaki T., Yamamoto H., et al. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J. Biol. Chem. 2008;283:3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 96.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 97.Gordon J.W., Shaw J.A., Kirshenbaum L.A. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ. Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 98.Yeung F., Hoberg J.E., Ramsey C.S., et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salminen A., Hyttinen J.M.T., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen B., Zhang H., Zhu Z., et al. Baicalin relieves LPS-induced lung inflammation via the NF-κB and MAPK pathways. Molecules. 2023;28 doi: 10.3390/molecules28041873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yokota T., Wang Y. p38 MAP kinases in the heart. Gene. 2016;575:369–376. doi: 10.1016/j.gene.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becatti M., Taddei N., Cecchi C., et al. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell. Mol. Life Sci. 2012;69:2245–2260. doi: 10.1007/s00018-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sundaresan N.R., Pillai V.B., Wolfgeher D., et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci. Signal. 2011;4 doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 104.Adrian L., Lenski M., Tödter K., et al. AMPK prevents palmitic acid-induced apoptosis and lipid accumulation in cardiomyocytes. Lipids. 2017;52:737–750. doi: 10.1007/s11745-017-4285-7. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., Wang Y., Zou M., et al. AMPK blunts chronic heart failure by inhibiting autophagy. Biosci. Rep. 2018;38 doi: 10.1042/BSR20170982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiyuna L.A., Albuquerque R.P.E., Chen C., et al. Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free. Radic. Biol. Med. 2018;129:155–168. doi: 10.1016/j.freeradbiomed.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmed Z., Tang W.H. Pharmacologic strategies to target oxidative stress in heart failure. Curr. Heart Fail. Rep. 2012;9:14–22. doi: 10.1007/s11897-011-0081-5. [DOI] [PubMed] [Google Scholar]
- 108.Akolkar G., da Silva Dias D., Ayyappan P., et al. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H795–H809. doi: 10.1152/ajpheart.00253.2017. [DOI] [PubMed] [Google Scholar]
- 109.Ruan Y., Dong C., Patel J., et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 2015;35:1116–1124. doi: 10.1159/000373937. [DOI] [PubMed] [Google Scholar]
- 110.Olmos Y., Sánchez-Gómez F.J., Wild B., et al. Sirt1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kops G.J., Dansen T.B., Polderman P.E., et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 112.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 113.Huang J., Liu Y., Wang M., et al. FoxO4 negatively modulates USP10 transcription to aggravate the apoptosis and oxidative stress of hypoxia/reoxygenation-induced cardiomyocytes by regulating the Hippo/YAP pathway. J. Bioenerg. Biomembr. 2021;53:541–551. doi: 10.1007/s10863-021-09910-7. [DOI] [PubMed] [Google Scholar]
- 114.Brunet A., Sweeney L.B., Sturgill J.F., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 115.Lai L., Yan L., Gao S., et al. Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of manganese superoxide dismutase via the SIRT1/FoxO3a pathway. Circulation. 2013;127:1692–1701. doi: 10.1161/CIRCULATIONAHA.112.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobayashi Y., Furukawa-Hibi Y., Chen C., et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 2005;16:237–243. [PubMed] [Google Scholar]
- 117.Li X., Song J., Zhang L., et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hong Z.X., Cao J., Liu D.D., al. ta. Celastrol targeting Nedd4 reduces Nrf2-mediated oxidative stress in astrocytes after ischemic stroke. J. Pharm. Anal. 2023;13:156–169. doi: 10.1016/j.jpha.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu J., Cui J., Lin Q., et al. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2021;342:82–93. doi: 10.1016/j.ijcard.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 120.Joo M.S., Kim W.D., Lee K.Y., et al. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Wu D., Tian Y. Fibroblast growth factor 19 protects the heart from oxidative stress-induced diabetic cardiomyopathy via activation of AMPK/Nrf2/HO-1 pathway. Biochem. Biophys. Res. Commun. 2018;502:62–68. doi: 10.1016/j.bbrc.2018.05.121. [DOI] [PubMed] [Google Scholar]
- 122.Nishida K., Kyoi S., Yamaguchi O., et al. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 123.Shirakabe A., Zhai P., Ikeda Y., et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation. 2016;133:1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang S., Lin X., Li G., et al. Knockout of Eva1a leads to rapid development of heart failure by impairing autophagy. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liao M., Xie Q., Zhao Y., et al. Main active components of Si-Miao-Yong-An decoction (SMYAD) attenuate autophagy and apoptosis via the PDE5A-AKT and TLR4-NOX4 pathways in isoproterenol (ISO)-induced heart failure models. Pharmacol. Res. 2022;176 doi: 10.1016/j.phrs.2022.106077. [DOI] [PubMed] [Google Scholar]
- 126.Sciarretta S., Forte M., Frati G., et al. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vlahakis A., Powers T. A role for TOR complex 2 signaling in promoting autophagy. Autophagy. 2014;10:2085–2086. doi: 10.4161/auto.36262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Inoki K., Zhu T., Guan K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 129.Gwinn D.M., Shackelford D.B., Egan D.F., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ghosh H.S., McBurney M., Robbins P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mizushima N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2020;63:1–10. doi: 10.1016/j.ceb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 132.Lee I.H., Cao L., Mostoslavsky R., et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hait W.N., Wu H., Jin S., et al. Elongation factor-2 kinase: Its role in protein synthesis and autophagy. Autophagy. 2006;2:294–296. doi: 10.4161/auto.2857. [DOI] [PubMed] [Google Scholar]
- 134.Da Silva J.P., Monceaux K., Guilbert A., et al. SIRT1 protects the heart from ER stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells. 2020;9 doi: 10.3390/cells9020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prola A., Pires Da Silva J., Guilbert A., et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017;24:343–356. doi: 10.1038/cdd.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Browne G.J., Finn S.G., Proud C.G. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J. Biol. Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 137.Xu P., Xu S., Pan H., et al. Differential effects of the LncRNA RNF157-AS1 on epithelial ovarian cancer cells through suppression of DIRAS3- and ULK1-mediated autophagy. Cell Death Dis. 2023;14 doi: 10.1038/s41419-023-05668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee J.W., Park S., Takahashi Y., et al. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xing J., Liu H., Yang H., et al. Upregulation of Unc-51-like kinase 1 by nitric oxide stabilizes SIRT1, independent of autophagy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoo S.M., Jung Y.K. A molecular approach to mitophagy and mitochondrial dynamics. Mol. Cells. 2018;41:18–26. doi: 10.14348/molcells.2018.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang B., Nie J., Wu L., et al. AMPKα2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ. Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luo G., Jian Z., Zhu Y., et al. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019;43:2033–2043. doi: 10.3892/ijmm.2019.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lala A., Desai A.S. The role of coronary artery disease in heart failure. Heart Fail. Clin. 2014;10:353–365. doi: 10.1016/j.hfc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 144.Meng H., Wang Q., Li N., et al. Danqi Tablet regulates energy metabolism in ischemic heart rat model through AMPK/SIRT1-PGC-1α pathway. Chin. J. Integr. Med. 2021;27:597–603. doi: 10.1007/s11655-019-3040-8. [DOI] [PubMed] [Google Scholar]
- 145.Wang X., Jiang Y., Zhang Q., et al. Autophagy as a novel insight into mechanism of Danqi pill against post-acute myocardial infarction heart failure. J. Ethnopharmacol. 2021;266 doi: 10.1016/j.jep.2020.113404. [DOI] [PubMed] [Google Scholar]
- 146.Li Q., Li N., Cui H., et al. Tongxinluo exerts protective effects via anti-apoptotic and pro-autophagic mechanisms by activating AMPK pathway in infarcted rat hearts. Exp. Physiol. 2017;102:422–435. doi: 10.1113/EP086192. [DOI] [PubMed] [Google Scholar]
- 147.Shi X., Zhu H., Zhang Y., et al. XuefuZhuyu Decoction protected cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. BMC Complementary Altern. Med. 2017;17 doi: 10.1186/s12906-017-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen M., Yao K., Liu Z., et al. Xuefu Zhuyu oral liquid prevents apoptosis of ischemic myocardium cells in rats by regulating SIRT1 and its pathway-related genes. Chin. J. Integr. Med. 2020;26:442–447. doi: 10.1007/s11655-019-3076-9. [DOI] [PubMed] [Google Scholar]
- 149.Yu S., Qian H., Tian D., et al. Linggui Zhugan Decoction activates the SIRT1-AMPK-PGC1α signaling pathway to improve mitochondrial and oxidative damage in rats with chronic heart failure caused by myocardial infarction. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1074837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang Y., Zhang K., Jiang M., et al. Regulation of energy metabolism by combination therapy attenuates cardiac metabolic remodeling in heart failure. Int. J. Biol. Sci. 2020;16:3133–3148. doi: 10.7150/ijbs.49520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lou T., Ma J., Xie Y., et al. Nuanxin capsule enhances cardiac function by inhibiting oxidative stress-induced mitochondrial dependent apoptosis through AMPK/JNK signaling pathway. Biomed Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111188. [DOI] [PubMed] [Google Scholar]
- 152.Zhao J., Zhang J., Liu Q., et al. Hongjingtian injection protects against myocardial ischemia reperfusion-induced apoptosis by blocking ROS induced autophagic- flux. Biomed Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111205. [DOI] [PubMed] [Google Scholar]
- 153.Zhao Y.L., Yuan Y., Ma X.L., et al. Study on protective mechanism of Dracocephalum moldavica total flavonoids against myocardial ischemia-reperfusion injury in rats based on AMPK/SIRT1/PGC-1α signaling pathway. Chinese Pharmacy. 2021;32:278–283. [Google Scholar]
- 154.Wei N., Zhang C., He H., et al. Protective effect of saponins extract from Panax japonicus on myocardial infarction: Involvement of NF-κB, Sirt1 and mitogen-activated protein kinase signalling pathways and inhibition of inflammation. J. Pharm. Pharmacol. 2014;66:1641–1651. doi: 10.1111/jphp.12291. [DOI] [PubMed] [Google Scholar]
- 155.Wang D., Lv L., Xu Y., et al. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111287. [DOI] [PubMed] [Google Scholar]
- 156.Cao K., Lv W., Liu X., et al. Herba Houttuyniae extract benefits hyperlipidemic mice via activation of the AMPK/PGC-1α/Nrf2 cascade. Nutrients. 2020;12:164. doi: 10.3390/nu12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Upadhyay S., Mantha A.K., Dhiman M. Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112690. [DOI] [PubMed] [Google Scholar]
- 158.Chang Y.M., Chang H.H., Kuo W.W., et al. Anti-apoptotic and pro-survival effect of alpinate oxyphyllae fructus (AOF) in a d-galactose-induced aging heart. Int. J. Mol. Sci. 2016;17:466. doi: 10.3390/ijms17040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang T.H., Peng G., Kota B.P., et al. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: Role of lowering circulating lipids. Br. J. Pharmacol. 2005;145:767–774. doi: 10.1038/sj.bjp.0706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Syahputra R.A., Harahap U., Dalimunthe A., et al. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: A review. Molecules. 2022;27 doi: 10.3390/molecules27041320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang X., Wang Q., Wang X., et al. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108599. [DOI] [PubMed] [Google Scholar]
- 162.Zhong J., Ouyang H., Sun M., et al. Tanshinone IIA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1α-mitochondrial apoptosis pathway, Cell Stress. Chaperones. 2019;24:991–1003. doi: 10.1007/s12192-019-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cui Y., Hong Y., Wu W., et al. Acacetin ameliorates cardiac hypertrophy by activating Sirt1/AMPK/PGC-1α pathway. Eur. J. Pharmacol. 2022;920 doi: 10.1016/j.ejphar.2022.174858. [DOI] [PubMed] [Google Scholar]
- 164.Wu C., Yan J., Li W. Acacetin as a potential protective compound against cardiovascular diseases. Evid. Based Complementary Altern. Med. 2022;2022 doi: 10.1155/2022/6265198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu W., Cui Y., Hong Y., et al. Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J. Cell. Mol. Med. 2020;24:12141–12153. doi: 10.1111/jcmm.15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hong Y., Wu W., Song F., et al. Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy. Aging. 2021;13:16381–16403. doi: 10.18632/aging.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen L., Li S., Zhu J., et al. Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J. Cell. Mol. Med. 2021;25:2944–2955. doi: 10.1111/jcmm.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khattulanuar F.S., Sekar M., Fuloria S., et al. Tilianin: A potential natural lead molecule for new drug design and development for the treatment of cardiovascular disorders. Molecules. 2022;27 doi: 10.3390/molecules27030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tian L., Cao W., Yue R., et al. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019;139:352–360. doi: 10.1016/j.jphs.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 170.Zhao L., Zhou Z., Zhu C., et al. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-κB pathway. Int. Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106680. [DOI] [PubMed] [Google Scholar]
- 171.Tang J., Lu L., Liu Y., et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J. Cell. Biochem. 2019;120:9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 172.Chang X., Zhang T., Meng Q., et al. Quercetin improves cardiomyocyte vulnerability to hypoxia by regulating SIRT1/TMBIM6-related mitophagy and endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/5529913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu B., Feng J., Yu L., et al. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br. J. Pharmacol. 2018;175:4137–4153. doi: 10.1111/bph.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Testai L., Piragine E., Piano I., et al. The Citrus flavonoid naringenin protects the myocardium from ageing-dependent dysfunction: Potential role of SIRT1. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang H., Wang C., Zhang L., et al. Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial cells by up-regulating SIRT1 expression. Chem. Biol. Interact. 2019;297:44–49. doi: 10.1016/j.cbi.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 176.Wang Z.K., Chen R.R., Li J.H., et al. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107086. [DOI] [PubMed] [Google Scholar]
- 177.Sun Z., Lu W., Lin N., et al. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem Pharmacol. 2020;175 doi: 10.1016/j.bcp.2020.113888. [DOI] [PubMed] [Google Scholar]
- 178.Liu Y., Zhou L., Du B., et al. Protection against doxorubicin-related cardiotoxicity by jaceosidin involves the Sirt1 signaling pathway. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9984330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu L., Guo Q., Zhao L. Overview of oroxylin A: A promising flavonoid compound. Phytother. Res. 2016;30:1765–1774. doi: 10.1002/ptr.5694. [DOI] [PubMed] [Google Scholar]
- 180.Zhang W., Zheng Y., Wu Y. Protective effects of oroxylin A against doxorubicin-induced cardiotoxicity via the activation of Sirt1 in mice. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Du Y., Han J., Zhang H., et al. Kaempferol prevents against ang II-induced cardiac remodeling through attenuating ang II-induced inflammation and oxidative stress. J. Cardiovasc. Pharmacol. 2019;74:326–335. doi: 10.1097/FJC.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo Z., Liao Z., Huang L., et al. Kaempferol protects cardiomyocytes against anoxia/reoxygenation injury via mitochondrial pathway mediated by SIRT1. Eur. J. Pharmacol. 2015;761:245–253. doi: 10.1016/j.ejphar.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 183.Huang L., He H., Liu Z., et al. Protective effects of isorhamnetin on cardiomyocytes against Anoxia/reoxygenation-induced injury is mediated by SIRT1. J. Cardiovasc. Pharmacol. 2016;67:526–537. doi: 10.1097/FJC.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 184.Ying Y., Jiang C., Zhang M., et al. Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging. 2019;11:2822–2835. doi: 10.18632/aging.101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu P., Li J., Liu M., et al. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111552. [DOI] [PubMed] [Google Scholar]
- 186.Zou H., Zhu X., Zhang G., et al. Silibinin: An old drug for hematological disorders. Oncotarget. 2017;8:89307–89314. doi: 10.18632/oncotarget.19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou B., Wu L.J., Li L.H., et al. Silibinin protects against isoproterenol-induced rat cardiac myocyte injury through mitochondrial pathway after up-regulation of SIRT1. J. Pharmacol. Sci. 2006;102:387–395. doi: 10.1254/jphs.fpj06005x. [DOI] [PubMed] [Google Scholar]
- 188.Tang T., Wang X., Wang L., et al. Liquiritin inhibits H2O2-induced oxidative stress injury in H9c2 cells via the AMPK/SIRT1/NF-κB signaling pathway. J. Food Biochem. 2022;46 doi: 10.1111/jfbc.14351. [DOI] [PubMed] [Google Scholar]
- 189.Laddha A.P., Kulkarni Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119664. [DOI] [PubMed] [Google Scholar]
- 190.Moses T., Pollier J., Thevelein J.M., et al. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. N. Phytol. 2013;200:27–43. doi: 10.1111/nph.12325. [DOI] [PubMed] [Google Scholar]
- 191.Kamran S., Sinniah A., Abdulghani M.A.M., et al. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers. 2022;14:1100. doi: 10.3390/cancers14051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bergman M.E., Davis B., Phillips M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules. 2019;24:3961. doi: 10.3390/molecules24213961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hortelano S., González-Cofrade L., Cuadrado I., et al. Current status of terpenoids as inflammasome inhibitors. Biochem. Pharmacol. 2020;172 doi: 10.1016/j.bcp.2019.113739. [DOI] [PubMed] [Google Scholar]
- 194.Agatonovic-Kustrin S., Kustrin E., Gegechkori V., et al. Anxiolytic terpenoids and aromatherapy for anxiety and depression. Adv. Exp. Med. Biol. 2020;1260:283–296. doi: 10.1007/978-3-030-42667-5_11. [DOI] [PubMed] [Google Scholar]
- 195.Wang K., Hu W. Oxypaeoniflorin improves myocardial ischemia/reperfusion injury by activating the Sirt1/Foxo1 signaling pathway. Acta Biochim. Pol. 2020;67:239–245. doi: 10.18388/abp.2020_5206. [DOI] [PubMed] [Google Scholar]
- 196.Yu C., Cai X., Liu X., et al. Betulin alleviates myocardial ischemia-reperfusion injury in rats via regulating the Siti1/NLRP3/NF-κB signaling pathway. Inflammation. 2021;44:1096–1107. doi: 10.1007/s10753-020-01405-8. [DOI] [PubMed] [Google Scholar]
- 197.Feng J., Yang Y., Zhou Y., et al. Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: The role of silent information regulator 1. Apoptosis. 2016;21:532–545. doi: 10.1007/s10495-016-1225-6. [DOI] [PubMed] [Google Scholar]
- 198.Ma W., Guo W., Shang F., et al. Bakuchiol alleviates hyperglycemia-induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/3732718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ma Z., Kong C., Song P., et al. Geniposide protects against obesity-related cardiac injury through AMPKα- and Sirt1-dependent mechanisms. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/6053727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hou Y., Yuan P., Fu Y., et al. Geniposide from Gardenia jasminoides var. radicans makino attenuates myocardial injury in spontaneously hypertensive rats via regulating apoptotic and energy metabolism signalling pathway. Drug Des. Dev. Ther. 2021;15:949–962. doi: 10.2147/DDDT.S292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mei Y., Hu H., Deng L., et al. Isosteviol sodium attenuates high fat/high cholesterol-induced myocardial dysfunction by regulating the Sirt1/AMPK pathway. Biochem. Biophys. Res. Commun. 2022;621:80–87. doi: 10.1016/j.bbrc.2022.06.044. [DOI] [PubMed] [Google Scholar]
- 202.Mei Y., Liu B., Su H., et al. Isosteviol sodium protects the cardiomyocyte response associated with the SIRT1/PGC-1α pathway. J. Cell. Mol. Med. 2020;24:10866–10875. doi: 10.1111/jcmm.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang Q., Lu M., Li J., et al. Ginkgolide B protects cardiomyocytes from angiotensin II-induced hypertrophy via regulation of autophagy through SIRT1-FoxO1. Cardiovasc. Ther. 2021;2021 doi: 10.1155/2021/5554569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fraga C.G., Croft K.D., Kennedy D.O., et al. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/c8fo01997e. [DOI] [PubMed] [Google Scholar]
- 205.Gu X.S., Wang Z.B., Ye Z., et al. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet. Mol. Res. 2014;13:323–335. doi: 10.4238/2014.January.17.17. [DOI] [PubMed] [Google Scholar]
- 206.Price N.L., Gomes A.P., Ling A.J., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu D., Ma Z., Xu L., et al. PGC1α activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging. 2019;11:10061–10073. doi: 10.18632/aging.102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yu L.M., Dong X., Xue X.D., et al. Protection of the myocardium against ischemia/reperfusion injury by punicalagin through an SIRT1-NRF-2-HO-1-dependent mechanism. Chem. Biol. Interact. 2019;306:152–162. doi: 10.1016/j.cbi.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 209.Xiao J., Sheng X., Zhang X., et al. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des. Dev. Ther. 2016;10:1267–1277. doi: 10.2147/DDDT.S104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ren B.C., Zhang Y.F., Liu S.S., et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020;24:12355–12367. doi: 10.1111/jcmm.15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Altamimi J.Z., Alfaris N.A., Alshammari G.M., et al. Ellagic acid protects against diabetic cardiomyopathy in rats by stimulating cardiac silent information regulator 1 signaling. J. Physiol. Pharmacol. 2020;71:891–904. doi: 10.26402/jpp.2020.6.12. [DOI] [PubMed] [Google Scholar]
- 212.Diao J., Zhao H., You P., et al. Rosmarinic acid ameliorated cardiac dysfunction and mitochondrial injury in diabetic cardiomyopathy mice via activation of the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2021;546:29–34. doi: 10.1016/j.bbrc.2021.01.086. [DOI] [PubMed] [Google Scholar]
- 213.Tan B., Wu X., Yu J., et al. The role of saponins in the treatment of neuropathic pain. Molecules. 2022;27 doi: 10.3390/molecules27123956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rao A.V., Sung M.K. Saponins as anticarcinogens. J. Nutr. 1995;125:717S–724S. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- 215.Sun A., Xu X., Lin J., et al. Neuroprotection by saponins. Phytother. Res. 2015;29:187–200. doi: 10.1002/ptr.5246. [DOI] [PubMed] [Google Scholar]
- 216.Huang Q., Su H., Qi B., et al. A SIRT1 activator, ginsenoside rc, promotes energy metabolism in cardiomyocytes and neurons. J. Am. Chem. Soc. 2021;143:1416–1427. doi: 10.1021/jacs.0c10836. [DOI] [PubMed] [Google Scholar]
- 217.Xue Y., Fu W., Liu Y., et al. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J. Food Sci. 2020;85:4039–4049. doi: 10.1111/1750-3841.15505. [DOI] [PubMed] [Google Scholar]
- 218.Wang M., Wang R., Xie X., et al. Araloside C protects H9c2 cardiomyoblasts against oxidative stress via the modulation of mitochondrial function. Biomed Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109143. [DOI] [PubMed] [Google Scholar]
- 219.Han X., Yang Y., Zhang M., et al. Protective effects of 6-gingerol on cardiotoxicity induced by arsenic trioxide through AMPK/SIRT1/PGC-1α signaling pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.868393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li Q., Zuo Z., Pan Y., et al. Salvianolic acid B alleviates myocardial ischemia injury by suppressing NLRP3 inflammasome activation via SIRT1-AMPK-PGC-1α signaling pathway. Cardiovasc. Toxicol. 2022;22:842–857. doi: 10.1007/s12012-022-09760-8. [DOI] [PubMed] [Google Scholar]
- 221.Pan C., Lou L., Huo Y., et al. Salvianolic acid B and tanshinone IIA attenuate myocardial ischemia injury in mice by NO production through multiple pathways. Ther. Adv. Cardiovasc. Dis. 2011;5:99–111. doi: 10.1177/1753944710396538. [DOI] [PubMed] [Google Scholar]
- 222.Liu C.Y., Zhou Y., Chen T., et al. AMPK/SIRT1 pathway is involved in arctigenin-mediated protective effects against myocardial ischemia-reperfusion injury. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.616813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang K., Feng X., Chai L., et al. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017;49:139–157. doi: 10.1080/03602532.2017.1306544. [DOI] [PubMed] [Google Scholar]
- 224.Huang Z., Han Z., Ye B., et al. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur. J. Pharmacol. 2015;762:1–10. doi: 10.1016/j.ejphar.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 225.Li C., Jiang S., Wang H., et al. Berberine exerts protective effects on cardiac senescence by regulating the Klotho/SIRT1 signaling pathway. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113097. [DOI] [PubMed] [Google Scholar]
- 226.Ni Y., Deng J., Liu X., et al. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J. Cell. Mol. Med. 2021;25:203–216. doi: 10.1111/jcmm.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lu W., Zhu H., Wu J., et al. Rhein attenuates angiotensin II-induced cardiac remodeling by modulating AMPK-FGF23 signaling. J. Transl. Med. 2022;20 doi: 10.1186/s12967-022-03482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lu Y., Feng Y., Liu D., et al. Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of SIRT1 signaling. Cell. Physiol. Biochem. 2018;47:1193–1206. doi: 10.1159/000490216. [DOI] [PubMed] [Google Scholar]
- 229.Zhang B., Zhai M., Li B., et al. Honokiol ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by reducing oxidative stress and apoptosis through activating the SIRT1-Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/3159801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.He H., Zhou Y., Huang J., et al. Capsaicin protects cardiomyocytes against Anoxia/reoxygenation injury via preventing mitochondrial dysfunction mediated by SIRT1. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1035702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang X., Yuan B., Cheng B., et al. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway, Shock. Augusta Ga. 2019;51:123–130. doi: 10.1097/SHK.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 232.Arad M., Seidman C.E., Seidman J.G. AMP-activated protein kinase in the heart: Role during health and disease. Circ. Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 233.Oka S., Zhai P., Alcendor R., et al. Suppression of ERR targets by a PPARα/Sirt1 complex in the failing heart, Cell Cycle Georget. Tex. 2012;11:856–864. doi: 10.4161/cc.11.5.19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Julien C., Tremblay C., Emond V., et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Clark A.J., Parikh S.M. Targeting energy pathways in kidney disease: The roles of sirtuins, AMPK, and PGC1α. Kidney Int. 2021;99:828–840. doi: 10.1016/j.kint.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.An Y., Wang B., Wang X., et al. SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop. Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ma Y., Zeng M., Sun R., et al. Disposition of flavonoids impacts their efficacy and safety. Curr. Drug Metab. 2014;15:841–864. doi: 10.2174/1389200216666150206123719. [DOI] [PubMed] [Google Scholar]
- 238.Liu Y., Luo Q.L., Jia X.B., et al. Multidisciplinary strategies to enhance therapeutic effects of flavonoids from Epimedii Folium: Integration of herbal medicine, enzyme engineering, and nanotechnology. J. Pharm. Anal. 2023;13:239–254. doi: 10.1016/j.jpha.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pandita D., Kumar S., Poonia N., et al. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014;62:1165–1174. [Google Scholar]
- 240.Najjar R.S., Feresin R.G. Protective role of polyphenols in heart failure: Molecular targets and cellular mechanisms underlying their therapeutic potential. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hoda M., Hemaiswarya S., Doble M. Role of Phenolic Phytochemicals in Diabetes Management. Springer; Singapore: 2019. Pharmacokinetics and pharmacodynamics of polyphenols; pp. 159–173. [Google Scholar]