Key Points
Question
Is P2Y12 inhibitor monotherapy after 3 months of dual antiplatelet therapy (DAPT; a P2Y12 inhibitor plus aspirin) noninferior to 12 months of DAPT in terms of net adverse clinical events (a composite of major adverse cardiac and cerebrovascular events and major bleeding) after percutaneous coronary intervention?
Findings
In this randomized clinical trial of 1387 patients in South Korea, the net adverse clinical events rate was 1.7% for the P2Y12 inhibitor monotherapy group and 2.6% for the DAPT group. The 1-sided confidence limit of this difference was within the noninferiority margin of 3.0%.
Meaning
These findings suggest that early discontinuation of aspirin and P2Y12 monotherapy was not inferior to 12 months of DAPT, although further research is required in other populations.
Abstract
Importance
P2Y12 inhibitor monotherapy after dual antiplatelet therapy (DAPT; a P2Y12 inhibitor plus aspirin) for a brief duration has recently emerged as an attractive alternative for patients undergoing percutaneous coronary intervention (PCI) with a drug-eluting stent.
Objective
To investigate whether P2Y12 inhibitor monotherapy after 3 months of DAPT was noninferior to 12 months of DAPT following PCI with a drug-eluting stent.
Design, Setting, and Participants
The Short-Term Dual Antiplatelet Therapy After Deployment of Bioabsorbable Polymer Everolimus-Eluting Stent (SHARE) open-label, noninferiority randomized clinical trial was conducted from December 15, 2017, through December 14, 2020. Final 1-year clinical follow-up was completed in January 2022. This study was a multicenter trial that was conducted at 20 hospitals in South Korea. Patients who underwent successful PCI with bioabsorbable polymer everolimus-eluting stents were enrolled.
Interventions
Patients were randomly assigned to receive P2Y12 inhibitor monotherapy after 3 months of DAPT (n = 694) or 12 months of DAPT (n = 693).
Main Outcomes and Measures
The primary outcome was a net adverse clinical event, a composite of major bleeding (based on Bleeding Academic Research Consortium type 3 or type 5 bleeding) and major adverse cardiac and cerebrovascular events (cardiac death, myocardial infarction, stent thrombosis, stroke, or ischemia-driven target lesion revascularization) between 3 and 12 months after the index PCI. The major secondary outcomes were major adverse cardiac and cerebrovascular events and major bleeding. The noninferiority margin was 3.0%.
Results
Of the total 1452 eligible patients, 65 patients were excluded before the 3-month follow-up, and 1387 patients (mean [SD] age, 63.0 [10.7] years; 1055 men [76.1%]) were assigned to P2Y12 inhibitor monotherapy (n = 694) or DAPT (n = 693). Between 3 and 12 months of follow-up, the primary outcome (using Kaplan-Meier estimates) occurred in 9 patients (1.7%) in the P2Y12 inhibitor monotherapy group and in 16 patients (2.6%) in the DAPT group (absolute difference, −0.93 [1-sided 95% CI, −2.64 to 0.77] percentage points; P < .001 for noninferiority). For the major secondary outcomes (using Kaplan-Meier estimates), major adverse cardiac and cerebrovascular events occurred in 8 patients (1.5%) in the P2Y12 inhibitor monotherapy group and in 12 patients (2.0%) in the DAPT group (absolute difference, −0.49 [95% CI, −2.07 to 1.09] percentage points; P = .54). Major bleeding occurred in 1 patient (0.2%) in the P2Y12 inhibitor monotherapy group and in 5 patients (0.8%) in the DAPT group (absolute difference, −0.60 [95% CI, −1.33 to 0.12] percentage points; P = .10).
Conclusions and Relevance
In patients with coronary artery disease undergoing PCI with the latest generation of drug-eluting stents, P2Y12 inhibitor monotherapy after 3-month DAPT was not inferior to 12-month DAPT for net adverse clinical events. Considering the study population and lower-than-expected event rates, further research is required in other populations.
Trial Registration
ClinicalTrials.gov Identifier: NCT03447379
This randomized clinical trial investigates whether P2Y12 inhibitor monotherapy after 3 months of dual antiplatelet therapy (DAPT) was noninferior to 12 months of DAPT following percutaneous coronary intervention with a drug-eluting stent.
Introduction
The optimal duration and regimen of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) remain controversial. Current guidelines suggest that the optimal duration and regimen of DAPT depend on the clinical presentation and the device used for PCI. After drug-eluting stent implantation, DAPT is recommended for 6 months for patients with chronic coronary syndrome and for 12 months for patients with acute coronary syndrome.1,2,3 Nevertheless, current guidelines suggest variations in the duration and regimen of DAPT according to the risk of bleeding or thrombosis in each patient. Therefore, in practice, the duration of DAPT after PCI is at the discretion of the physician. Current trends have shifted toward minimizing the risk of bleeding complications after PCI.4 Various strategies to reduce the risk of bleeding by shortening the duration of DAPT have been evaluated.5
According to recent randomized clinical trials, one of the leading strategies for more efficient management of the risk of bleeding and ischemia is to discontinue aspirin use after a brief period of DAPT and continue P2Y12 inhibitor monotherapy.6,7,8,9,10 The Short-Term Dual Antiplatelet Therapy After Deployment of Bioabsorbable Polymer Everolimus-Eluting Stent (SHARE) trial was performed to compare the results of 3-month DAPT followed by P2Y12 inhibitor monotherapy with 12-month maintenance DAPT in all patients who underwent successful PCI with a drug-eluting stent.
Methods
Study Design and Population
This study was an investigator-initiated, multicenter, open-label, noninferiority randomized clinical trial conducted from December 15, 2017, through December 14, 2020, to demonstrate the noninferiority in the efficacy and safety of P2Y12 inhibitor monotherapy following 3-month DAPT compared with 12-month maintenance DAPT after drug-eluting stent implantation. The trial was conducted at 20 hospitals in South Korea. The study protocol (Supplement 1) was approved by the institutional review board of each participating center, and written informed consent was obtained from all participants. An independent data and safety monitoring board reviewed the trial safety at regular intervals. The study was conducted in accordance with the principles of the Declaration of Helsinki.11 The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.12 Patients with de novo coronary lesions who underwent successful PCI were eligible for enrollment for the study. To minimize bias based on the stent type, only patients treated with the SYNERGY stent (Boston Scientific Corp) were included in this study. The SYNERGY stent is a bioabsorbable polymer everolimus-eluting stent designed to promote rapid reendothelialization by combining a thin-strut (74 μm to 81 μm) platinum-chromium platform with an abluminally coated ultrathin (4-μm), bioabsorbable polymer.13,14 These features of the stent may allow shortened DAPT durations.
The major exclusion criteria for the study were hemodynamic instability or cardiogenic shock; increased risk of bleeding, anemia, or thrombocytopenia; need for oral anticoagulation therapy; noncardiac comorbid conditions with a life expectancy of less than 1 year; history of intracranial hemorrhage; and coronary stent implantation within 12 months before the index procedure. The complete inclusion and exclusion criteria are provided in eTable 1 in Supplement 2.
Randomization and Study Procedures
Patients were randomly assigned in a 1:1 ratio to the P2Y12 inhibitor monotherapy group (aspirin plus a P2Y12 inhibitor for 3 months followed by P2Y12 inhibitor monotherapy) or the DAPT group (aspirin plus a P2Y12 inhibitor for 12 months) stratified by clinical presentation (chronic coronary syndrome or acute coronary syndrome) and enrollment sites. Enrollment and random assignment were performed within 3 months after the index procedure. A web-based response system was used for the randomization.
Clopidogrel was used as a P2Y12 inhibitor in patients with chronic coronary syndrome. For patients with acute coronary syndrome, ticagrelor was the recommended P2Y12 inhibitor; however, clopidogrel was also allowed at the physician’s discretion. Concomitant use of other antiplatelet agents or oral anticoagulants was not permitted. Optimal medical therapy, other than antiplatelet agents, was left to the physician’s discretion. Clinical follow-up was mandatory at 3, 6, and 12 months after index PCI. Telephonic interviews were permitted for patients who missed scheduled outpatient clinic visits.
Outcome Measures and Definitions
The primary outcome was a net adverse clinical event (NACE), defined as a composite of major adverse cardiac and cerebrovascular events (MACCEs) and major bleeding between 3 and 12 months after the index PCI. The MACCEs were defined as cardiac death, myocardial infarction (MI), stent thrombosis, stroke, or ischemia-driven target lesion revascularization. Major bleeding was defined as Bleeding Academic Research Consortium type 3 or type 5 bleeding.15 The major secondary outcomes were MACCEs and major bleeding. Other secondary outcomes included cardiac death, MI, stent thrombosis, stroke, target lesion revascularization, target vessel revascularization, and all-cause death.
All deaths were assumed to be cardiac deaths unless a noncardiac cause could be identified. Myocardial infarction was defined as a creatine kinase–MB fraction or cardiac troponin elevation above the upper reference limit combined with ischemic symptoms, electrocardiographic changes, or a new regional wall motion abnormality. Periprocedural MI was not considered a clinical event. Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium classification.16 Stroke included both ischemic and hemorrhagic strokes. All clinical events were adjudicated by an independent clinical event committee blinded to the treatment assignments.
Statistical Analysis
At the time of the trial design, the annual event rate of the primary end point was expected to be approximately 5%, based on the results of several randomized clinical trials that compared short-term DAPT with 12-month DAPT after coronary PCI using drug-eluting stent implantation.17,18,19,20 A noninferiority margin of 3.0% was chosen, considering that there was no clinical difference between the 2 groups in terms of the primary end point. The number of participants was determined by assuming that the experimental group was not inferior when the upper limit of the 95% CI for the difference in event rate between the 2 groups did not exceed that for the control group. At a significance level of 5% and power of 80%, a total of 1306 participants (653 participants per group) were required. Assuming a 10% dropout rate, a total of 1452 participants (726 participants per group) were required to test our hypothesis.
The primary analysis was conducted based on the modified intention-to-treat population excluding participants who did not fulfill the enrollment criteria, withdrew their consent within 3 months after the index procedure, or did not attend the 3-month follow-up visit. Additionally, a per-protocol analysis was performed in participants who received the assigned antiplatelet regimen. Subgroup analyses were performed according to age, sex, diabetes, hypertension, body mass index, clinical presentation, presence of multivessel coronary artery disease, left ventricular ejection fraction, and type of P2Y12 inhibitor. In the subgroup analysis, P values for interaction were estimated using the Cox proportional hazards regression model. All of the models were adjusted for clinical presentation and enrollment sites (stratification factors). As the same DAPT treatment was maintained in both groups until 3 months after the index procedure, only outcomes between 3 and 12 months after the index procedure were compared between the 2 groups. Owing to the lack of adjustment for multiple testing of subgroups, the results of the subgroup analyses should be considered exploratory. The findings of the analyses for secondary end points should also be interpreted as exploratory because of the possibility of type I error from multiple comparisons.
Categorical variables are expressed as numbers (percentages) and compared using the χ2 test. Continuous variables are expressed as mean (SD) and compared using a t test. Data were analyzed on a per-patient and per-lesion basis for clinical and angiographic or procedural characteristics, respectively. Cumulative incidences of clinical events were presented as a Kaplan-Meier curve based on the time of performing the index procedure to the occurrence of the first event of interest during follow-up, which was completed January 2022, 1 year after the end of the study. Event rates were compared between the 2 groups using log-rank tests. A noninferiority test was performed for the primary end point. Except for the values of noninferiority testing, all P values were 2-sided, and P < .05 was considered statistically significant. All analyses were performed using SAS, version 9.2 (SAS Institute Inc).
Results
Study Population
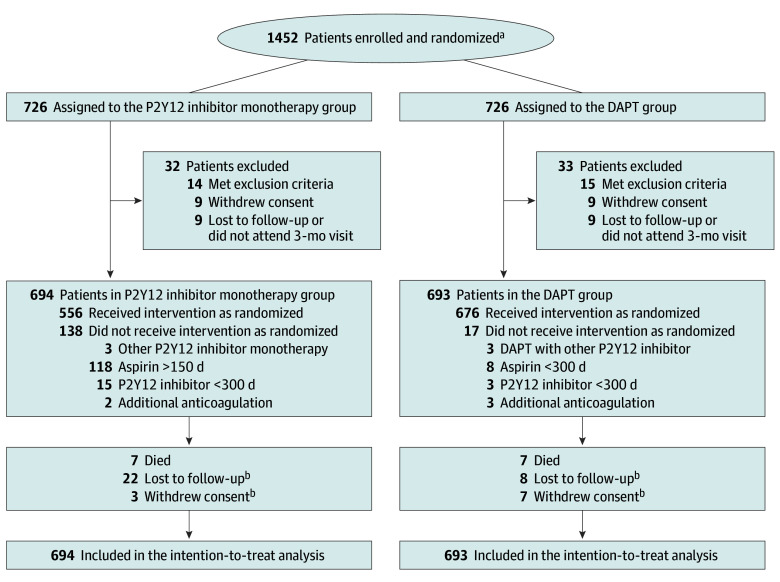
From December 2017 to December 2020, a total of 1452 patients were enrolled, and 726 patients were randomly assigned to each group. Enrollment and random assignment may have been done within 3 months after the index PCI; however, most patients (>90%) were randomized within 3 days after the index PCI. There was no difference in the timing of randomization between the 2 groups. As we only considered events occurring between 3 and 12 months after the index procedure as primary end points, 65 patients were excluded from our analysis for various reasons before completion of the 3-month follow-up period. Therefore, a total of 1387 patients (mean [SD] age, 63.0 [10.7] years; 332 women [23.9%] and 1055 men [76.1%]) were assigned to P2Y12 inhibitor monotherapy (n = 694) or DAPT (n = 693) (Figure 1).
Figure 1. Participant Flowchart.
DAPT indicates dual antiplatelet therapy.
aStudy sites were not required to provide screening logs. Data on the reasons for ineligibility were not available.
bOutcomes of patients who were lost to follow-up or withdrew consent were included at the point of final contact. Time-to-event measurements were censored on the last contact date.
Baseline Characteristics and Medications
The baseline demographic and clinical characteristics are provided in Table 1. Overall, 1023 patients (73.8%) presented with acute coronary syndrome, and 251 (18.1%) had ST-segment elevation MI (STEMI). Ticagrelor was used as a P2Y12 inhibitor in 520 (37.5%) of all patients and in 520 (50.8%) of patients with acute coronary syndrome. The angiographic and procedural characteristics were also well balanced between the 2 groups (eTable 2 in Supplement 2). Both groups received comparable medications upon discharge from index PCI (eTable 3 in Supplement 2). Adherence to the assigned antiplatelet therapy was 80.1% (556 patients) in the P2Y12 inhibitor monotherapy group and 97.5% (676 patients) in the DAPT group. The most prevalent reason for nonadherence was physicians’ discretion based on patients’ risk (eTable 4 in Supplement 2).
Table 1. Baseline Patient Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| P2Y12i (n = 694) | DAPT (n = 693) | |
| Age, mean (SD), y | 62.8 (10.9) | 63.2 (10.5) |
| Sex | ||
| Women | 156 (22.5) | 176 (25.4) |
| Men | 538 (77.5) | 517 (74.6) |
| BMI, mean (SD) | 25.1 (3.2) | 25.0 (3.5) |
| Comorbidities | ||
| Hypertension | 393 (56.7) | 419 (60.5) |
| Dyslipidemia | 282 (40.7) | 310 (44.8) |
| Diabetes | 227 (32.8) | 243 (35.1) |
| Current smoker | 215 (31.0) | 189 (27.3) |
| Congestive heart failure | 7 (1.0) | 9 (1.3) |
| Chronic kidney disease | 23 (3.3) | 17 (2.5) |
| Previous PCI | 74 (10.7) | 102 (14.7) |
| Previous CABG | 13 (1.9) | 6 (0.9) |
| Previous myocardial infarction | 36 (5.2) | 40 (5.8) |
| Previous stroke | 27 (3.9) | 34 (4.9) |
| Clinical presentation | ||
| Chronic coronary syndrome | 181 (26.1) | 183 (26.4) |
| Acute coronary syndrome | 513 (73.9) | 510 (73.6) |
| P2Y12 inhibitor | ||
| Clopidogrel | 427 (61.5) | 440 (63.5) |
| Ticagrelor | 267 (38.5) | 253 (36.5) |
| Angiographic diagnosis | ||
| 1-Vessel disease | 402 (57.9) | 395 (57.0) |
| 2-Vessel disease | 188 (27.1) | 169 (24.4) |
| 3-Vessel disease | 104 (15.0) | 129 (18.6) |
| Total No. of treated lesions per patient, mean (SD) | 1.3 (0.6) | 1.3 (0.7) |
| Total No. of stents per patient, mean (SD) | 1.3 (0.6) | 1.3 (0.6) |
Abbreviations: BMI (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; DAPT, dual antiplatelet therapy; P2Y12i, P2Y12 inhibitor monotherapy; PCI, percutaneous coronary intervention.
Clinical Outcomes
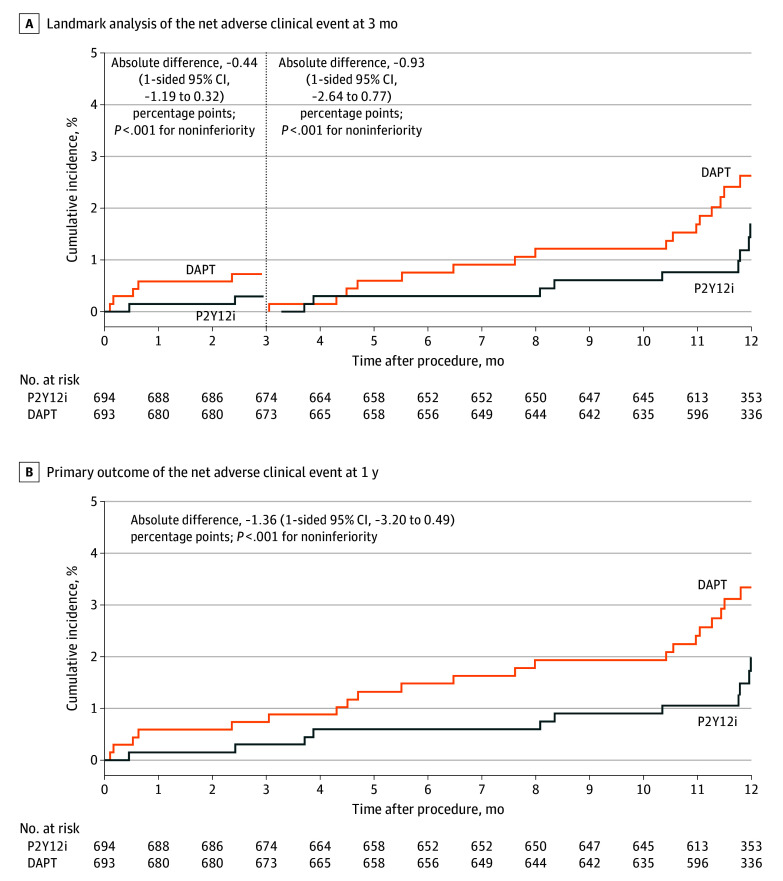
Clinical follow-up for the primary end point was completed in 662 (95.4%) of 694 patients in the P2Y12 inhibitor monotherapy group and in 671 (96.8%) of 693 patients in the DAPT group. Because patients were enrolled within 3 months after the index PCI and both groups received DAPT during the first 3 months, only events between 3 and 12 months after the index PCI were compared between the 2 groups. The primary outcome of NACE occurred in 9 patients in the P2Y12 inhibitor monotherapy group and in 16 patients in the DAPT group (Table 2). Kaplan-Meier estimates of the primary outcome during this period were 1.7% in the P2Y12 inhibitor monotherapy group and 2.6% in the DAPT group (absolute difference, −0.93 [1-sided 95% CI, −2.64 to 0.77] percentage points; P < .001 for noninferiority), thereby meeting the criteria for noninferiority of the P2Y12 inhibitor monotherapy group to the DAPT group (Table 2 and Figure 2). The results were comparable for the entire period, including the first 3 months (absolute difference, −1.36 [1-sided 95% CI, −3.20 to 0.49] percentage points; P < .001 for noninferiority) (Figure 2 and eTable 5 in Supplement 2). The noninferiority of the P2Y12 inhibitor monotherapy group to the DAPT group was also confirmed in the per-protocol analysis (absolute difference, −1.10 [1-sided 95% CI, −2.81 to 0.62] percentage points; P < .001 for noninferiority) (Table 2 and eTable 6 and eFigure 1 in Supplement 2). This finding was consistent for the entire period, including the first 3 months (absolute difference, −1.68 [1-sided 95% CI, −3.48 to 0.12] percentage points; P < .001 for noninferiority) (eTable 7 and eFigure 1 in Supplement 2).
Table 2. Clinical Outcomes Between 3 and 12 Months.
| Outcome | Patients, No. (%)a | Absolute difference (95% CI) percentage points | P value | |
|---|---|---|---|---|
| P2Y12i (n = 674) | DAPT (n = 673) | |||
| Primary | ||||
| Net adverse clinical eventb | ||||
| ITT analysis | 9 (1.7) | 16 (2.6) | −0.93 (−2.64 to 0.77) | <.001c |
| PP analysisd | 6 (1.4) | 15 (2.5) | −1.10 (−2.81 to 0.62) | <.001c |
| Secondary | ||||
| Death | 3 (0.5) | 3 (0.5) | 0 (−0.73 to 0.72) | .99 |
| Cardiac | 1 (0.2) | 0 | 0.15 (−0.14 to 0.44) | .32 |
| Noncardiac | 2 (0.3) | 3 (0.5) | −0.15 (−0.82 to 0.51) | .65 |
| Myocardial infarction | 1 (0.2) | 2 (0.3) | −0.12 (−0.76 to 0.52) | .71 |
| Stent thrombosis | 1 (0.2) | 1 (0.2) | 0.03 (−0.55 to 0.60) | .93 |
| Stroke | 3 (0.5) | 2 (0.3) | 0.16 (−0.50 to 0.82) | .64 |
| Ischemic | 2 (0.3) | 2 (0.3) | 0.01 (−0.59 to 0.60) | .99 |
| Hemorrhagic | 1 (0.2) | 0 | 0.15 (−0.15 to 0.46) | .32 |
| Any revascularization | 19 (3.7) | 23 (4.3) | −0.56 (−2.99 to 1.87) | .65 |
| TLR | 4 (0.9) | 7 (1.2) | −0.27 (−1.56 to 1.01) | .68 |
| TVR | 8 (1.7) | 10 (1.9) | −0.27 (−1.93 to 1.39) | .75 |
| Other vessel PCI | 11 (2.1) | 13 (2.4) | −0.3 (−2.14 to 1.53) | .75 |
| Major bleedinge | 1 (0.2) | 5 (0.8) | −0.6 (−1.33 to 0.12) | .10 |
| MACCEf | 8 (1.5) | 12 (2.0) | −0.49 (−2.07 to 1.09) | .54 |
Abbreviations: DAPT, dual antiplatelet therapy; ITT, intention-to-treat; MACCE, major adverse cardiac and cerebrovascular event; P2Y12i, P2Y12 inhibitor monotherapy; PCI, percutaneous coronary intervention; PP, per protocol; TLR, target lesion revascularization; TVR, target vessel revascularization.
Percentages are Kaplan-Meier estimates.
A composite of MACCEs and major bleeding.
P value for noninferiority.
The PP population was 556 for the P2Y12i group and 676 for the DAPT group.
Based on Bleeding Academic Research Consortium type 3 or type 5 bleeding.
A composite of cardiac death, myocardial infarction, stent thrombosis, stroke, or TLR.
Figure 2. Time-to-Event Curves for the Primary Outcomes.
A net adverse clinical event was defined as a composite of major bleeding (based on Bleeding Academic Research Consortium type 3 or type 5 bleeding) or major adverse cardiac and cerebrovascular events. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses. Vertical dashed line indicates 3-month point (after which 1 group received P2Y12 inhibitor monotherapy [P2Y12i] and the other received dual antiplatelet therapy [DAPT]).
For the major secondary outcomes (using Kaplan-Meier estimates), MACCEs occurred in 8 patients (1.5%) in the P2Y12 inhibitor monotherapy group and 12 patients (2.0%) in the DAPT group (absolute difference, −0.49 [95% CI, −2.07 to 1.09] percentage points; P = .54) (Table 2 and eFigure 2 in Supplement 2). Major bleeding occurred in 1 patient (0.2%) in the P2Y12 inhibitor monotherapy group and in 5 patients (0.8%) in the DAPT group (absolute difference, −0.60 [95% CI, −1.33 to 0.12] percentage points; P = .10) (Table 2 and eFigure 3 in Supplement 2). According to the per-protocol analysis, the incidence of MACCEs did not differ between the 2 groups, but only 4 of 676 patients (0.6%) in the DAPT group experienced major bleeding (eTable 6 in Supplement 2). Significant differences between the 2 groups were not seen in terms of other secondary outcomes (Table 2).
The treatment effect of P2Y12 inhibitor monotherapy on the primary outcome was consistent without significant interactions across various subgroups, except for sex (Figure 3). P2Y12 inhibitor monotherapy was more favored over DAPT in men than in women (P = .04 for interaction). In the subgroup analyses of the major secondary outcomes, the results were consistent across the same subgroups (eFigure 4 in Supplement 2).
Figure 3. Subgroup Analyses for the Primary Outcome Between 3 and 12 Months After the Index Procedure.
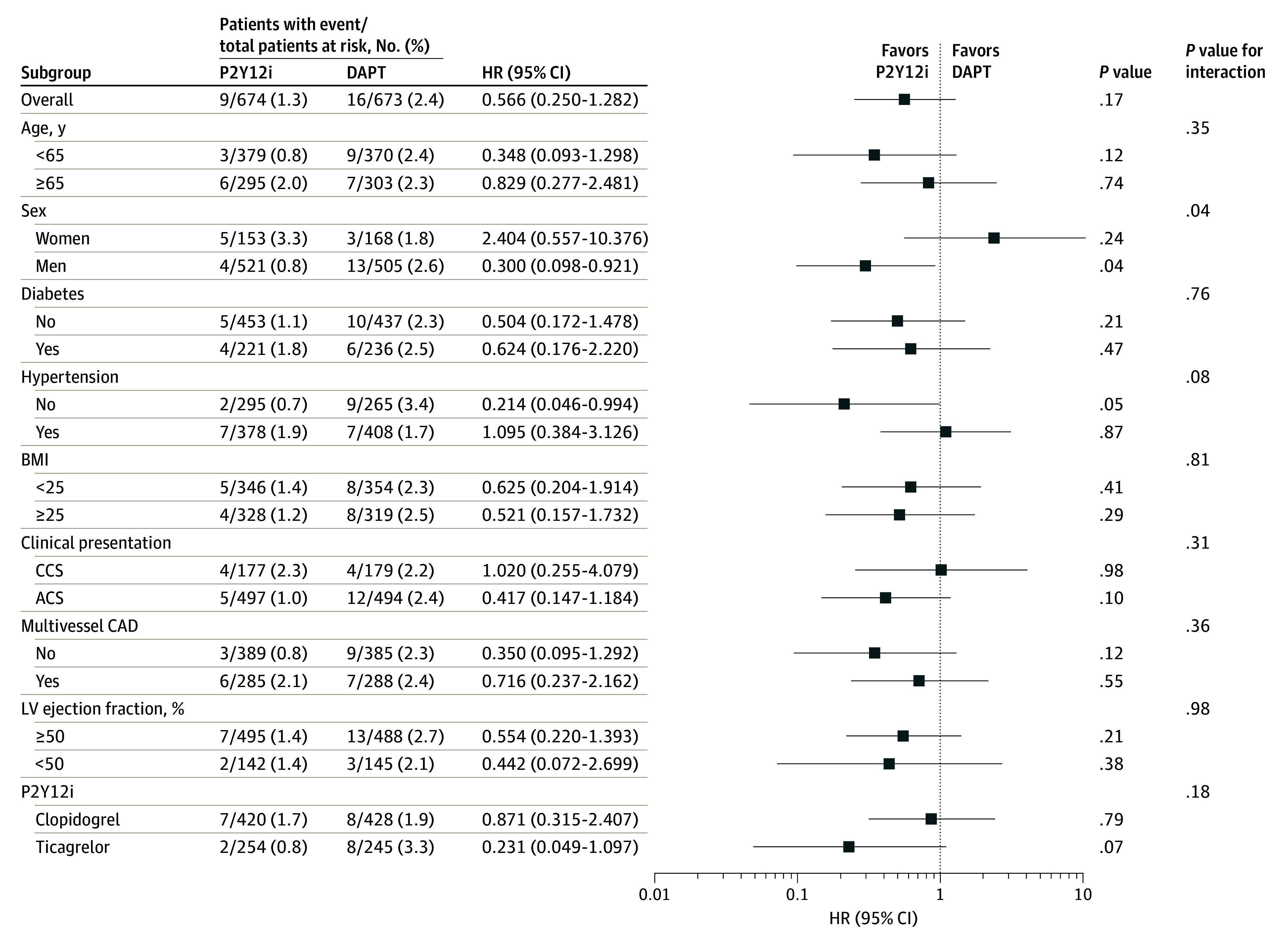
Squares indicate hazard ratios (HRs), with horizontal lines indicating 95% CIs. ACS indicates acute coronary syndrome; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAD, coronary artery disease; CCS, chronic coronary syndrome; DAPT, dual antiplatelet therapy; LV, left ventricular; P2Y12i, P2Y12 inhibitor monotherapy.
Discussion
In the SHARE randomized clinical trial, we demonstrated that P2Y12 inhibitor monotherapy after 3-month DAPT was not inferior to 12-month DAPT in terms of the primary outcome of NACE in patients who underwent PCI. This study recruited patients with chronic coronary syndrome and acute coronary syndrome, including those with STEMI, who underwent successful PCI. Our findings suggest that P2Y12 inhibitor monotherapy after 3-month DAPT could be considered as a treatment option in a wide range of patients, including those with STEMI, who have undergone PCI using the latest generation of drug-eluting stents.
Among various strategies to reduce the risk of post-PCI bleeding, discontinuation of aspirin after short-term DAPT and switching to P2Y12 inhibitor monotherapy have been the main focus of recent clinical trials.6,7,8,9,10 These studies varied in terms of study population, type of P2Y12 inhibitor, and timing of transition or duration of P2Y12 inhibitor monotherapy. Therefore, there are some discrepancies in their results regarding ischemic or bleeding risk reduction.
In the GLOBAL LEADERS trial,10 ticagrelor monotherapy after 1 month of DAPT failed to demonstrate superiority in all-cause mortality or new Q-wave MI reduction compared with aspirin monotherapy after 12 months of DAPT. Moreover, it did not reduce the risk of major bleeding. In contrast, in the STOPDAPT-2 trial,6 which used clopidogrel as a P2Y12 inhibitor and enrolled patients with relatively low ischemic risk, 1 month of DAPT followed by clopidogrel monotherapy reduced the bleeding end point and NACE compared with 12 months of DAPT. The SMART-CHOICE trial7 demonstrated that P2Y12 inhibitor monotherapy after 3-month DAPT was not inferior to 12-month DAPT for MACCEs. The bleeding rate was lower in the P2Y12 monotherapy group.
The TICO trial,9 performed only in patients with acute coronary syndrome, showed that ticagrelor monotherapy after 3-month DAPT, compared with 12-month DAPT, significantly reduced NACE at 1 year, mainly owing to the reduction in major bleeding. However, in the STOPDAPT-2 acute coronary syndrome trial,21 clopidogrel monotherapy following 1 to 2 months of DAPT failed to prove noninferiority to 12-month DAPT for NACE despite a reduction in bleeding events in patients with acute coronary syndrome. Therefore, at least in patients with acute coronary syndrome, using a more potent P2Y12 inhibitor as monotherapy is expected to help reduce NACE; however, using DAPT for less than 3 months still needs to be sufficiently validated.
In our study’s subgroup analyses, there was a significant interaction between the antiplatelet strategy and sex for the occurrence of the primary outcome. Although several studies have demonstrated that women tend to have a higher risk of bleeding during DAPT compared with men,22,23,24 it is difficult to draw conclusions from our study due to the low incidence of major bleeding.
In our study, only 1 type of drug-eluting stent, the SYNERGY stent, was used in the PCI to avoid difficulties in interpretation of the results from different stent types. Pathologically, third-generation drug-eluting stents have been reported to be superior to the second-generation ones in animal models and autopsy samples.25 However, to our knowledge, no clinical data yet support the superiority of specific types of drug-eluting stents over others for early discontinuation of DAPT after PCI.26
Current guidelines recommend shortening the DAPT duration after drug-eluting stent implantation in patients at high risk of bleeding.1,2,3 Several drug-eluting stents, including the one used in our study, have been approved for use in these situations.26 According to a meta-analysis of recent trials, early discontinuation of aspirin and maintenance of P2Y12 inhibitor monotherapy did not increase the risk of major adverse cardiovascular events and reduced the risk of bleeding compared with standard, 12-month DAPT.27 In light of recent clinical trials and our study, early discontinuation of aspirin and P2Y12 monotherapy after PCI with the latest drug-eluting stents may be applied to a broader patient population beyond that with a high risk of bleeding.
Limitations
This study had several limitations. First, this was an open-label trial, which could have resulted in bias owing to nonadherence to the study drug. The low adherence rate in the P2Y12 inhibitor monotherapy group may have influenced the findings. However, the results of the per-protocol analysis were consistent with those of the intention-to-treat analysis, suggesting that potential biases attributable to nonadherence to the study drug may be minimal. Second, the calculation of study power was based on the occurrence of NACE, the composite outcome. Thus, any comparison made about the occurrence of individual components may be underpowered. Third, the actual event rate of the primary outcome was lower than that expected when the study was designed. Therefore, the noninferiority margin of 3.0% (corresponding to a 60% increase in the expected event rate and a 115.4% increase in the observed event rate) was relatively wide, and this study may have been underpowered. Considering the observed event rate of 2.6% in the control group and allowing for a 40% increase in risk instead of 60%, a recalculated noninferiority margin would be reduced to 1.04% instead of 3.0%. However, even when the noninferiority margin was set to 1.04%, the noninferiority of the P2Y12 inhibitor monotherapy group compared with the DAPT group was still met (P = .01 for noninferiority). Noninferiority was also met with a revised margin of 1.04% (P = .007 for noninferiority) in the per-protocol analysis. Fourth, randomization was performed within 3 months after the index PCI, not at 3 months after the PCI. However, only the events between 3 and 12 months after the index PCI were compared between the 2 groups as the main outcomes. Fifth, in nearly 50% of the patients with acute coronary syndrome, clopidogrel was used instead of ticagrelor. This finding can be attributed to the less frequent use of potent P2Y12 inhibitors in South Korea, likely due to concerns regarding high bleeding risk. The rate of clopidogrel use in patients with acute coronary syndrome was comparable to that reported in other published studies7,28 performed in South Korea. Sixth, this study was conducted in South Korea only; therefore, caution should be exercised when extrapolating these results to other populations.
Conclusions
This randomized clinical trial found that among patients with coronary artery disease undergoing PCI with the latest generation of drug-eluting stents, P2Y12 inhibitor monotherapy after 3-month DAPT was not inferior to 12-month DAPT for NACE. Further research is required to analyze the impact of this strategy on individual outcomes, including bleeding events.
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Angiographic and Procedural Characteristics for Treated Lesions
eTable 3. Discharge Medication
eTable 4. Reasons for Nonadherence to the Allocated Treatment
eTable 5. Clinical Outcomes at 12 Months
eTable 6. Per-Protocol Analyses for Clinical Outcomes Between 3 and 12 Months
eTable 7. Per-Protocol Analyses for Clinical Outcomes at 12 Months
eFigure 1. Time-to-Event Curves for the Primary Outcomes in Per-Protocol Analysis
eFigure 2. Time-to-Event Curves for the Major Adverse Cardiac and Cerebrovascular Events
eFigure 3.Time-to-Event Curves for the Major Bleeding
eFigure 4. Subgroup Analyses for the Major Adverse Cardiac and Cerebrovascular Events
Nonauthor Collaborators. The SHARE Investigators
Data Sharing Statement
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. doi: 10.1016/j.jacc.2016.03.513 [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 3.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 pt A):2915-2931. doi: 10.1016/j.jacc.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 4.Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of coronary stent technology and implications for duration of dual antiplatelet therapy. Prog Cardiovasc Dis. 2018;60(4-5):478-490. doi: 10.1016/j.pcad.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 5.Capodanno D, Baber U, Bhatt DL, et al. P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary intervention. Nat Rev Cardiol. 2022;19(12):829-844. doi: 10.1038/s41569-022-00725-6 [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Domei T, Morimoto T, et al. ; STOPDAPT-2 Investigators . Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321(24):2414-2427. doi: 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn JY, Song YB, Oh JH, et al. ; SMART-CHOICE Investigators . Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321(24):2428-2437. doi: 10.1001/jama.2019.8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032-2042. doi: 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, Hong SJ, Cho YH, et al. ; TICO Investigators . Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323(23):2407-2416. doi: 10.1001/jama.2020.7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson GJ, Marks A, Berg KJ, et al. The SYNERGY biodegradable polymer everolimus eluting coronary stent: porcine vascular compatibility and polymer safety study. Catheter Cardiovasc Interv. 2015;86(6):E247-E257. doi: 10.1002/ccd.25993 [DOI] [PubMed] [Google Scholar]
- 14.Torii S, Jinnouchi H, Sakamoto A, et al. Drug-eluting coronary stents: insights from preclinical and pathology studies. Nat Rev Cardiol. 2020;17(1):37-51. doi: 10.1038/s41569-019-0234-x [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 17.Kim BK, Hong MK, Shin DH, et al. ; RESET Investigators . A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation). J Am Coll Cardiol. 2012;60(15):1340-1348. doi: 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 18.Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505-513. doi: 10.1161/CIRCULATIONAHA.111.059022 [DOI] [PubMed] [Google Scholar]
- 19.Feres F, Costa RA, Abizaid A, et al. ; OPTIMIZE Trial Investigators . Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510-2522. doi: 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 20.Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086-2097. doi: 10.1016/j.jacc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Morimoto T, Natsuaki M, et al. ; STOPDAPT-2 ACS Investigators . Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 2022;7(4):407-417. doi: 10.1001/jamacardio.2021.5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chichareon P, Modolo R, Kerkmeijer L, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol. 2020;5(1):21-29. doi: 10.1001/jamacardio.2019.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehran R, Chandrasekhar J, Urban P, et al. ; LEADERS FREE Investigators . Sex-based outcomes in patients with a high bleeding risk after percutaneous coronary intervention and 1-month dual antiplatelet therapy: a secondary analysis of the LEADERS FREE randomized clinical trial. JAMA Cardiol. 2020;5(8):939-947. doi: 10.1001/jamacardio.2020.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ten Haaf ME, van Geuns RJ, van der Linden MMJM, et al. Sex-related bleeding risk in acute coronary syndrome patients receiving dual antiplatelet therapy with aspirin and a P2Y12 inhibitor. Med Princ Pract. 2023;32(3):200-208. doi: 10.1159/000529863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migita S, Kitano D, Li Y, et al. Pathological findings after third- and second-generation everolimus-eluting stent implantations in coronary arteries from autopsy cases and an atherosclerotic porcine model. Sci Rep. 2021;11(1):6281. doi: 10.1038/s41598-021-85740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capodanno D, Bhatt DL, Gibson CM, et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol. 2022;19(2):117-132. doi: 10.1038/s41569-021-00598-1 [DOI] [PubMed] [Google Scholar]
- 27.O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation. 2020;142(6):538-545. doi: 10.1161/CIRCULATIONAHA.120.046251 [DOI] [PubMed] [Google Scholar]
- 28.Han JK, Hwang D, Yang S, et al. Comparison of 3- to 6-month versus 12-month dual antiplatelet therapy after coronary intervention using the contemporary drug-eluting stents with ultrathin struts: the HOST-IDEA randomized clinical trial. Circulation. 2023;147(18):1358-1368. doi: 10.1161/CIRCULATIONAHA.123.064264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Angiographic and Procedural Characteristics for Treated Lesions
eTable 3. Discharge Medication
eTable 4. Reasons for Nonadherence to the Allocated Treatment
eTable 5. Clinical Outcomes at 12 Months
eTable 6. Per-Protocol Analyses for Clinical Outcomes Between 3 and 12 Months
eTable 7. Per-Protocol Analyses for Clinical Outcomes at 12 Months
eFigure 1. Time-to-Event Curves for the Primary Outcomes in Per-Protocol Analysis
eFigure 2. Time-to-Event Curves for the Major Adverse Cardiac and Cerebrovascular Events
eFigure 3.Time-to-Event Curves for the Major Bleeding
eFigure 4. Subgroup Analyses for the Major Adverse Cardiac and Cerebrovascular Events
Nonauthor Collaborators. The SHARE Investigators
Data Sharing Statement