Abstract
Cross-linking between protein components of whole spinach (Spinacia oleracea var. Nobel) thylakoids and of photosystem I- and II-enriched thylakoid fractions has been produced by reaction with the bifunctional imidoester dimethyl-3,3′-dithiobispropionimidate dihydrochloride as well as by the oxidation of intrinsic sulfydryl groups with an orthophenanthrolinecupric ion complex. The mixture of membrane proteins and their cross-linked products has been analyzed by two-dimensional sodium dodecyl sulfate electrophoresis, with a reductive cleavage step of the cross-linkages before the second dimension. Cross-linked aggregates up to a molecular weight of about 130 kilodaltons (kD) were analyzed, and it was inferred that the polypeptides appearing together in the same aggregates were neighbors within the membrane.
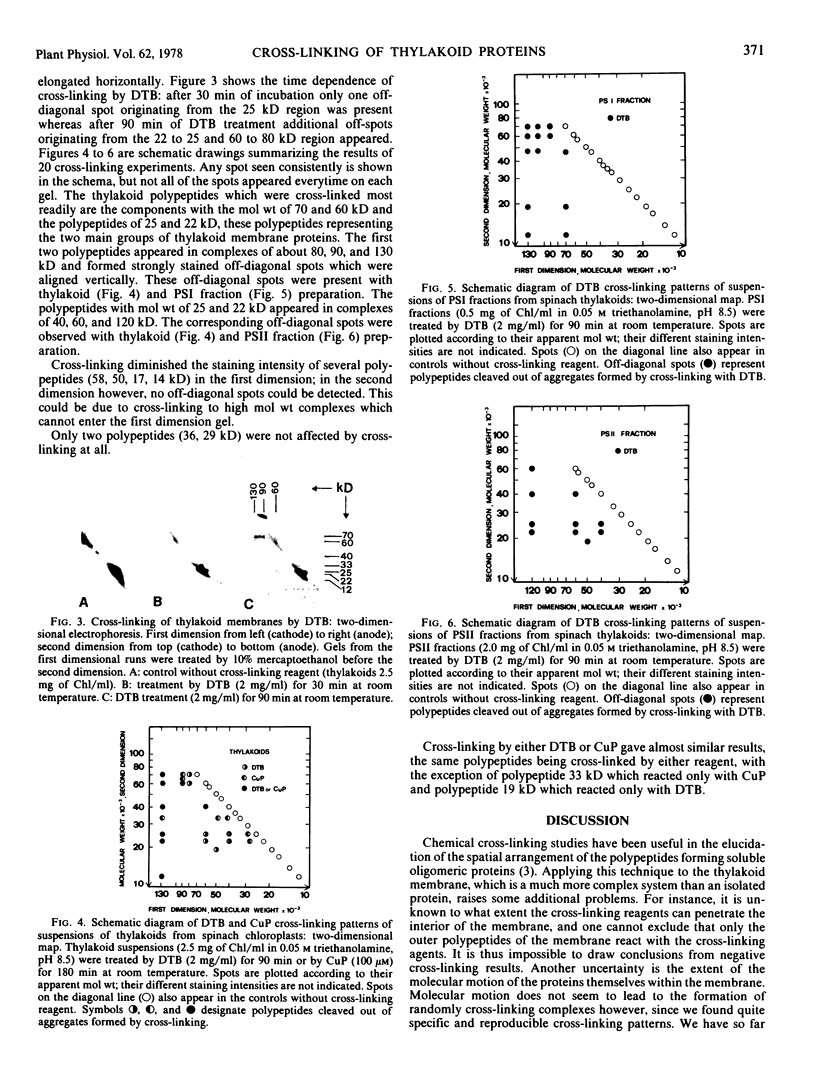
In thylakoids as well as in isolated photosystem fractions, oligomers were formed by cross-linking polypeptides of the 60 to 90 kD range, among them the polypeptides of the chlorophyll-protein complex I. Polypeptides of 46, 19, and 12 kD were cross-linked to these complexes. Polypeptides of 25 and 22 kD, which are related to the chlorophyll-protein complex II, were cross-linked in thylakoids as well as in photosystem II fractions, suggesting that in the membrane these molecules are close together. In photosystem II fractions an oligomer having a molecular weight of about 60 kD was formed by cross-linking several polypeptides of different molecular weights: 40, 25, and 22 kD.
Our cross-linking experiments show that protein interactions in the thylakoid membrane occurred mainly among the polypeptides of the two chlorophyll-protein complexes, thus suggesting an oligomeric nature of these apoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Arntzen C. J., Ditto C. L. Effects of cations upon chloroplast membrane subunit. Interactions and excitation energy distribution. Biochim Biophys Acta. 1976 Nov 9;449(2):259–274. doi: 10.1016/0005-2728(76)90138-9. [DOI] [PubMed] [Google Scholar]
- BIETH R., FREYSZ L., MANDEL P. [Quantitative study of various phosphatidyl and phosphatidal compounds of the rat brain during post-natal growth]. Biochim Biophys Acta. 1961 Nov 11;53:576–578. doi: 10.1016/0006-3002(61)90218-9. [DOI] [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Chemical cross-linking studies of chloroplast coupling factor 1. J Biol Chem. 1976 Nov 25;251(22):6953–6962. [PubMed] [Google Scholar]
- Bickle T. A., Hershey J. W., Traut R. R. Spatial arrangement of ribosomal proteins: reaction of the Escherichia coli 30S subunit with bis-imidoesters. Proc Natl Acad Sci U S A. 1972 May;69(5):1327–1331. doi: 10.1073/pnas.69.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Gross E. L. Protein-protein interactions of the light-harvesting chlorophyll a/b protein. II. Evidence for two stages of cation independent association. Biochim Biophys Acta. 1976 Dec 6;449(3):554–564. doi: 10.1016/0005-2728(76)90164-x. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. Polypeptide composition of chlorophyll-protein complexes from romaine lettuce. Plant Physiol. 1977 Jul;60(1):64–68. doi: 10.1104/pp.60.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak-Hofer I., Siegenthaler P. A. Two-dimensional separation of chloroplast membrane proteins by isoelectric focusing and electrophoresis in sodium dodecyl sulphate. Biochim Biophys Acta. 1977 Aug 1;468(3):461–471. doi: 10.1016/0005-2736(77)90295-4. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A., Depéry F. Influence of unsaturated fatty acids in chloroplasts. Shift of the pH optimum of electron flow and relations to deltapH, thylakoid internal pH and proton uptake. Eur J Biochem. 1976 Jan 15;61(2):573–580. doi: 10.1111/j.1432-1033.1976.tb10052.x. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]