Graphical abstract
Highlights
-
•
The expression of PDIA3 is differentially disturbed in the BLA, CA3 and DRN of the obesity-depression comorbid mice.
-
•
Celastrol covalently bind and differentially modulate the expression of PDIA3 in above brain nuclei.
-
•
PDIA3 expressed in BLA and CA3 alleviate the comorbid conditions, whereas in DRN deteriorate obesity.
-
•
In BLA and CA3, overexpressed PDIA3 reshape the activated amoeboid microglia into the ramified quiescent ones.
-
•
In DRN, overexpressed PDIA3 may influence 5-HTergic neurons.
Nowadays, roughly 603.7 million people are bothered by obesity [1]. More seriously, obesity brings inflammation to the peripheral and central nervous system, which compromises the comorbidity of obesity, major depression [2], and cognitive deficits [3]. Drug competent in the comorbidity is still lacking. In 2015, Liu et al. [4] reported celastrol (CEL) as a powerful anti-obesity agent. In our previous study, CEL were proved effective in treating obesity mice comorbid with depression and cognitive deficits (COM). Microglia were identified as the target of CEL [5]. Here, we discuss the underlying mechanism by CEL and the covalent binding protein, protein disulfide isomerase A3 (PDIA3). To this aim, (1) the BV2 cell line and the chemical probe for celastrol (CEL-P) was used to focus the covalent binding protein PDIA3; (2) COM mice induced by high fat diet and environment stress were used to verify the expression of PDIA3 in the pathological and pharmacological procedures; (3) protein PDIA3 was overexpressed in the basolateral amygdala (BLA), cornus ammonis 3 of hippocampus (CA3), and dorsal raphe nucleus (DRN) of the COM mice. The association between the overexpressed PDIA3 and the morphology of microglia, as well as the comorbid syndromes was analyzed.
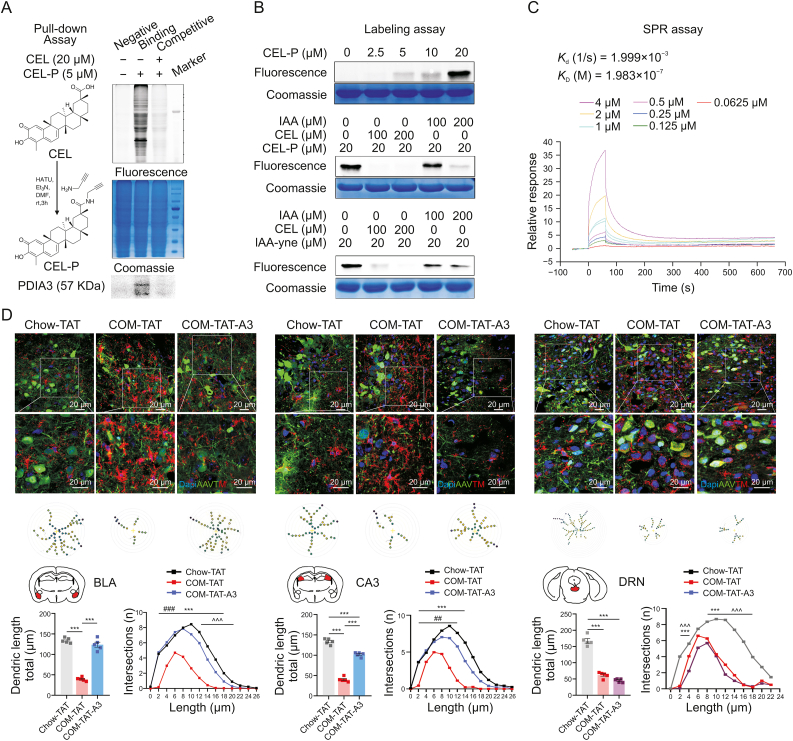
In brief, CEL and its probe (CEL-P) were incubated with the lysates of BV2 cell treated by lipopolysaccharide (LPS) and interferon-γ (IFN-γ). Lysates incubated with nothing was the negative group, incubated with 5 μM CEL-P was the binding group, and incubated with both 5 μM CEL-P and 20 μM CEL was the competitive group. After the incubation, 20 μL sample was reserved for Coomassie blue staining. Others were purified with biotin affinity column, visualized with the fluorescent dye recognizing CEL-P (Fig. 1A), identified with mass spectrometry (MS/MS) (Table S1), and quantified with specific antibody to the target protein (Fig. 1A). In this step, significantly more PDIA3 protein was found in the binding group as compared with the competitive group, indicating PDIA3 is the covalent binding protein of CEL (Fig. 1A). In the labeling assay (Fig. 1B), PDIA3 protein was incubated with excessive CEL and iodoacetamide (IAA, an active alkylating reagent of cysteine), followed by CEL-P/IAA incorporated with an alkyne moiety (IAA-yne) and click reaction with tetramethylrhodamine-biotin-azide (TAMRA). As shown, CEL and IAA competed away CEL-P. Also, CEL and IAA competed away the labeling of PDIA3 by IAA-yne. These in sum indicate that the reactive cysteine residues of PDIA3 are the binding sites for CEL (Fig. 1B). The binding kinetics between CEL and PDIA3 was evaluated by surface plasmon resonance (SPR) assay (Fig. 1C).
Fig. 1.
The identification of protein disulfide isomerase A3 (PDIA3) as the covalent binding target of celastrol (CEL) and the modulator of the morphology of microglia in comorbid brain. (A) The pull-down assay verifying the binding between the chemical probe for celastrol (CEL-P) and PDIA3. (B) The labeling assay showing the reactive cysteine residues of PDIA3 is the binding sites for CEL. CEL, CEL-P, active alkylating reagent of cysteine (IAA) and IAA incorporated with an alkyne moiety (IAA-yne) were used in the labeling. (C)The surface plasmon resonance (SPR) assay revealing the binding kinetics of CEL and PDIA3. (D) The graphical abstract showing mice comorbid of obesity, depression, and cognitive deficits (COM) and CEL mediated modulation of PDIA3 in brain. The immunofluorescence labeling of Dapi (405-blue), adeno-associated virus (AAV9, green-488), transmembrane protein 119 (TMEM119, 594-red), in the basolateral amygdala (BLA), cornus ammonis 3 of hippocampus (CA3), and dorsal raphe nucleus (DRN). Data are presented as mean ± standard error of mean (SEM). P values are determined by one-way analysis of variance (ANOVA) and Tukey's post tests in the charts showing dendritic length, ∗∗∗P < 0.001. In the charts showing the dendritic intersections, ∗∗∗P < 0.001 significant difference between chow diet mice expressing trans-activator of transcription (Chow-TAT) and COM mice expressing trans-activator of transcription (COM-TAT), ##P < 0.005 and ###P < 0.001 significant difference between COM mice expressing trans-activator of transcription-PDIA3 (COM-TAT-A3) and COM-TAT, ˆˆˆP < 0.001 significant difference between Chow-TAT and COM-TAT-A3.
The expressions of PDIA3 in the chow diet (Chow), COM and CEL treated (CEL-L/M/H, 0.5/1/2 mg/kg) groups were quantified by the double staining of the microglia marker transmembrane protein 119 (TMEM119), 5-HTergic marker tryptophan 5-hydroxylase 2 (TPH2), and PDIA3 (Fig. S1). In BLA and CA3, approximately 80% PDIA3 was expressed in TMEM119-positive microglia (Figs. S1A and B). In DRN, merely 20% of PDIA3 were expressed in microglia, meanwhile 90% of PDIA3 positive spots were found colocalized with the 5-HTergic marker TPH2 (Fig. S1C). In BLA and CA3, qualified by the amount of TMEM119-PDIA3 double positive spots, significant decreases in COM were found as compared with the Chow, while CEL mediated the upregulation in dose-dependent manner (R2 = 0.7973, R2 = 0.6726) (Figs. S1A and B). In DRN, increased TPH2-PDIA3 double positive spots were found in COM mice, which were downregulated by CEL in dose-dependent manner (R2 = 0.3537) (Fig. S1C).
To verify the roles of PDIA3 assumed in above brain nuclei, trans-activator of transcription (TAT) tag and TAT tag fused protein PDIA3 were overexpressed in the BLA, CA3 and DRN of COM mice, and the compromising morphological alternations of microglia were quantified by the dendrites stained with TMEM119. In the BLA, CA3, and DRN, compared to the chow mice expressing TAT (Chow-TAT), significant retraction of the dendrites, shown by the decreases of total length and intersections, was found in the comorbid mice expressing TAT (COM-TAT), indicating the activation of microglia in COM-TAT (Fig. 1D). In the BLA and CA3, compared to the COM-TAT, the transformation into resting state, presented by the increases of length and intersections was achieved in the comorbid mice expressing TAT-PDIA3 (COM-TAT-A3) (Fig. 1D). However, in DRN, PDIA3 brought no difference to the morphology of microglia in the COM-TAT-A3 group, as compared with COM-TAT (Fig. 1D).
In BLA injection groups, PDIA3 mediated alleviation of obesity was clarified by the significant decreases of weight, energy intake, and white adipose, as well as improved glucose tolerance (shown by decreased area under the receiver operating characteristic curve (AUC)) in COM-TAT-A3, as compared with COM-TAT (Fig. 2A). Depression was quantified by the behaviors in the forced swimming (FST) and tail suspension tests (TST). Compared with Chow-TAT, COM-TAT was significantly depressive, as shown by the increased immobility time in FST and TST tests (Fig. 2B). Compared with COM-TAT, the depressive behaviors were significantly relieved by PDIA3, which were presented by the decreases of immobility time in COM-TAT-A3 (Fig. 2B).
Fig. 2.
The validation of the pharmacological consequences of protein disulfide isomerase A3 (PDIA3) in the basolateral amygdala (BLA), cornus ammonis 3 of hippocampus (CA3) and dorsal raphe nucleus (DRN). (A) Quantification of the metabolic indexes of mice with overexpressed TAT/TAT-A3 in the BLA. (B) Forced swimming (FST) and tail suspension tests (TST) quantifying the depression behaviors of mice with overexpressed TAT/TAT-PDIA3 in the BLA. (C) Quantification of the metabolic indexes of mice with overexpressed trans-activator of transcription (TAT)/TAT-PDIA3 (TAT-A3) in the CA3. (D) FST and TST quantifying the depression behaviors, the Morris water maze testing the cognitive functions of mice with overexpressed TAT/TAT-A3 in the CA3. (E) Quantification of the metabolic indexes of mice with overexpressed TAT/TAT-A3 in the DRN. (F) TST quantifying the depression behaviors of mice with overexpressed TAT/TAT-A3 in the DRN. In (A) (C) (E), ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001 significant difference between chow diet mice expressing TAT (Chow-TAT) and comorbid mice expressing TAT (COM-TAT), #P < 0.05, ###P < 0.001 significant difference between comorbid mice expressing TAT-PDIA3 (COM-TAT-A3) and COM-TAT, ˆP < 0.05, ˆˆˆP < 0.001 significant difference between Chow-TAT and COM-TAT-A3. In (B) (D) (F), ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001.
In CA3 injection groups, obesity, depression, and cognitive deficits were analyzed (Figs. 2C and D). Compared with COM-TAT, significant decreases of weight, energy intake, white adipose, but not the blood glucose, were found in COM-TAT-A3 (Fig. 2C). Compared with the efficient downregulation of blood glucose in BLA, the PDIA3 in CA3 showed inferior effects (Fig. 2C). Meanwhile, significant decreases of the immobility time in the TST and FST were achieved by COM-TAT-A3 as compared with COM-TAT, indicating the effective alleviation of depression by PDIA3 in CA3 (Fig. 2D). The cognitive behaviors were quantified by the Morris water maze. In detail, mice were treated with a 3-day training and 1-day test. In the training day, mice were trained to find the platform in quarter 2 (Q2). On the test day, the platform was removed, time and the distance spent in each quarter (Q1-4) were recorded. In Chow-TAT, significantly more time and distance were spent in Q2 than others, indicating the undisturbed spatial learning and memory ability (Fig. 2D). In COM-TAT, the time and distance in Q2 were not different from others, indicating the injured cognitive function (Fig. 2D). In COM-TAT-A3 ones, significant increases of time and distance were spent in Q2 as compared with Q1, indicating that PDIA3 in CA3 is helpful for the cognitive functions (Fig. 2D).
In DRN injection groups, obesity and depression were analyzed (Figs. 2E and F). Compared with COM-TAT, significant increases of weight, energy intake, white and brown adipose, energy expenditure, and AUC were found in COM-TAT-A3 (Fig. 2E). PDIA3 in DRN brought increases of both the intake and expenditure of energy, whereas the increases of intake were greater than expenditure (Fig. 2E). Meanwhile, significant decreases of the immobility time in the TST were achieved by COM-TAT-A3 as compared with COM-TAT (Fig. 2F). The overexpressed PDIA3 in the 5-HTergic DRN neurons seems protective for the depressive behaviors (Fig. S1C).
In conclusion, by probe-based chemical proteomics, protein PDIA3 was identified as a covalently binding protein of CEL when treating the activated BV2 cells. In COM mice, CEL mediate the dose-dependent upregulation of PDIA3 in microglia in the BLA and CA3, as well as the downregulation of PDIA3 in 5-HTergic neurons in DRN. Shown by the results of morphological and behavioral tests, PDIA3 in BLA and CA3 inactivate microglia, correct the metabolic, depressive, and cognitive dysfunctions. However, in DRN, PDIA3 do not contribute to the modulation of microglia. Overexpressed PDIA3 in DRN brings aggravation to the metabolism but alleviation to depression. Further study is still needed to clarify the brain nucleus and cell specific roles of PDIA3 in the complicated obesity brain.
CRediT author statement
Chunyan Zhu: Design, Writing - Original draft preparation, and Funding acquisition; Xuemin Yao: Experiments, Writing - Original draft preparation; Dandan Liu: Methodology; Guoxin Zhang: Project administration; Yongping Zhu: Project administration; Hongyu Chi: Project administration; Shuangpan Zhang: Project administration; Jun Yang: Project administration; Ying Liu: Project administration; Jigang Wang: Writing - Reviewing and Editing; Na Lin: Design and Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the grants from the Natural Science Foundation of Beijing Municipality (Grant No.: 7212185), the Scientific and Technological Innovation project of China Academy of Chinese Medical Sciences (Grant No.: CI2021A03808), the National Natural Science Foundation of China (Grant Nos.: 82330124, 81974526, and 82274176), the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine, China (Program No.: ZYYCXTD-C-202002), the Special Project for Training Outstanding Young Scientific and Technological Talents (innovative type) of Necessary Scientific Research Business Expenses of China Academy of Chinese Medical Sciences (Project Nos.: ZZ13-YQ-051, and ZZ15-YQ-063), and the Fundamental Research Funds for the Central public welfare research institutes (Grant No.: ZXKT21010).
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.10.002.
Contributor Information
Jigang Wang, Email: jgwang@icmm.ac.cn.
Na Lin, Email: linna888@163.com, nlin@icmm.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira-Miranda E., Costa P.R.F., Queiroz V.A.O., et al. Overweight and obesity associated with higher depression prevalence in adults: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017;36:223–233. doi: 10.1080/07315724.2016.1261053. [DOI] [PubMed] [Google Scholar]
- 3.Atti A.R., Valente S., Iodice A., et al. Metabolic syndrome, mild cognitive impairment, and dementia: A meta-analysis of longitudinal studies. Am. J. Geriatr. Psychiatry. 2019;27:625–637. doi: 10.1016/j.jagp.2019.01.214. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Lee J., Salazar Hernandez M.A., et al. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu C., Yang J., Zhu Y., et al. Celastrol alleviates comorbid obesity and depression by directly binding amygdala HnRNPA1 in a mouse model. Clin. Transl. Med. 2021;11 doi: 10.1002/ctm2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.