Key Points
Question
Does functional laryngectomy (FL) improve voice, swallowing, functional oral intake, quality of life, and mood states in head and neck cancer survivors with profound dysphagia and intractable aspiration?
Findings
This cohort study including 20 patients with swallowing dysfunction and intractable aspiration after head and neck cancer treatment found that FL was associated with improvements in voice, swallowing, oral intake, quality of life, and mood states. Six months post-FL, 63% of patients dependent on a feeding tube underwent removal, and 85% of patients were using a tracheoesophageal voice prosthesis for alaryngeal communication.
Meaning
These findings indicate that FL may effectively improve function—voice, swallowing, and oral intake—as well as overall quality of life and well-being in patients experiencing profound dysphagia and intractable aspiration after head and neck cancer treatment.
Abstract
Importance
Late effects of head and neck cancer (HNC) treatment include profound dysphagia, chronic aspiration, and death. Functional laryngectomy (FL) can improve patient survival and quality of life (QoL); however, removing a failing larynx for a noncancer reason is a difficult decision. Data regarding the ability of FL to improve self-perceptions of voice, swallowing, and QOL in survivors of HNC with intractable aspiration are inconclusive.
Objective
To investigate the association of FL with changes in self-reported perceptions of voice, swallowing, oral intake, QoL, and mood in survivors of HNC experiencing profound dysphagia and intractable aspiration.
Design, Settings, and Participants
This cohort study was conducted at a single academic institution and included survivors of HNC with profound swallowing dysfunction and intractable aspiration who underwent FL from July 2016 through March 2022. Of the initial 22 patients enrolled, 2 patients (15%) died of aspiration pneumonia before receiving FL. Data analyses were performed from July 2016 through March 2023.
Main Outcomes and Measures
Self-reported measures of voice using the VHI (30-item Voice Handicap Index), swallowing using the EAT-10 (10-item Eating Assessment Tool), functional oral intake scale using the FOIS (Functional Oral Intake Scale), and quality of life using the FACT-H&N (Functional Assessment of Cancer Therapy−Head & Neck) were assessed before FL and at 1, 3, and 6 months after FL. Mood states were evaluated using the POMS (Profile of Mood States, second edition), before FL and at 6 months after FL.
Results
The study analyses included 20 patients (mean [SD] age, 72.4 (7.0) years; 19 [95%] males and 1 [5%] female) who underwent FL and had complete data across all time points. Among these, 12 patients (60%) had received chemoradiation for oropharyngeal, 7 (35%) for laryngeal, or 1 (5%) for nasopharyngeal cancer. The mean (SD) time from completion of oncologic treatment to FL was 15.5 (5.5) years. Mean (SD) score on the EAT-10 improved from 33.2 (7.4) to 23.1 (10.8) at 1 month; 12.1 (9.1) at 3 months; and 8.3 (7.4) at 6 months, with a large effect size (η2 = 0.72; 95% CI, 0.54-0.80). Mean (SD) score on the FOIS improved from 2.0 (1.5) to 2.9 (1.7) at 1 month; 4.8 (2.5) at 3 months; and 5.2 (1.7) at 6 months, with a large effect size (η2 = 0.6; 95% CI, 0.38-0.71). Improvement in oral intake was achieved in 19 patients (95%), and feeding tubes were removed in 10 of 16 patients (63%) who were feeding tube−dependent; 6 patients (27%) continued to require supplemental tube feedings. Mean (SD) score on the VHI improved from 63.6 (34.0) to 86.9 (33.7) at 1 month; 71.3 (36.1) at 3 months; and 39.7 (26.9) at 6 months, with a large effect size (η2 = 0.42; 95% CI, 0.19-0.56). Seventeen patients (85%) were able to use a tracheoesophageal voice prosthesis for alaryngeal communication. Mean (SD) score on the FACT-H&N improved from 86.2 (17.8) to 93.6 (18.4) at 1 month; 109.0 (18.4) at 3 months; and 121.0 (16.8) at 6 months, with a large effect size (η2 = 0.64; 95% CI, 0.42-0.74). Mean (SD) score on the POMS improved from 58.9 (13.2) at baseline to 44.5 (9.9) at 6 months, with a large effect size (Cohen d = 1.04; 95% CI, 0.48-1.57). None of the patients experienced major complications of FL; 1 patient (5%) had a postoperative pharyngocutaneous fistula.
Conclusions and Relevance
The findings of this cohort study indicate that FL was associated with marked improvements in self-perception of voice and swallowing, functional oral intake, QoL, and mood state among survivors of HNC. These findings can serve as a framework for FL counseling among HNC survivors experiencing profound dysphagia and intractable aspiration.
This cohort study assesses the association of functional laryngectomy with self-perceptions of voice, swallowing, oral intake, quality of life, and mood among survivors of head and neck cancer experiencing intractable aspiration.
Introduction
Late radiation effects of head and neck cancer (HNC) treatment include severe swallowing dysfunction, aspiration, and adverse consequences that negatively affect overall health and quality of life.1 Adverse effects of long-term radiation may lead to radiation fibrosis syndrome, the progressive sclerosing of tissues, including bone, nerve, muscle, connective tissue, and visceral structures.2 The clinical presentation of radiation fibrosis syndrome includes atrophy, denervation, and lower cranial neuropathies of the swallowing apparatus and may render a dysfunctional larynx.3
Late radiation-associated dysphagia (swallowing impairment from long-term radiation toxicity) frequently has an insidious onset, with affected patients demonstrating functional swallowing for long intervals before symptoms of dysphagia present.1 Although advancements in radiation planning and recognition of dysphagia- and aspiration-related structures now limit radiation dosage to swallowing organs at risk, curative radiation doses to the base of tongue; superior-, middle-, and inferior-pharyngeal constrictors; glottic and supraglottic larynx; upper esophageal sphincter; and esophagus contribute to the development of late radiation dysphagia.4 Sensory motor impairments can lead to chronic airway invasion. In patients with feeding tube−dependent dysphagia from late radiation-associated effects, 80% were found to have absent laryngopharyngeal sensation.3 Laryngopharyngeal sensory neuropathy increases the risk of aspiration and inability to clear the airway of aspirate.3 Motor deficits are related to fibrotic changes that restrict movement of the swallowing apparatus, thereby impairing swallowing safety and efficiency.4
Pulmonary manifestations from chronic aspiration include pneumonia, bronchiectasis, interstitial lung disease, bronchiolitis, lung abscess, and empyema.5 Rates of aspiration pneumonia after radiation therapy increase over time, with a 23.8% incidence of aspiration pneumonia at 5-year posttreatment in comparison with 15.8% at 1-year posttreatment.6 Recurrent aspiration pneumonia in the setting of a dysfunctional larynx is not uncommon. In addition to morbidity associated with late radiation dysphagia, the 30-day mortality rate of intractable aspiration and recurrent pneumonias is 21%.7
Profound dysphagia has been associated with poor oral intake, which may lead to weight loss, dehydration, malnourishment, sarcopenia, and cachexia.8 Malnourishment after HNC treatment has been well-documented. Chemotherapy was the strongest predictor of malnutrition in patients treated for HNC, increasing the risk nearly 6-fold.9 In comparison with oral cavity cancers, patients treated for pharyngeal cancers had a higher likelihood of malnutrition.9 Long-term enteral nutrition may be needed in patients with late radiation-induced dysphagia who are unable to meet nutritional or hydration needs through oral intake.9
Progressive swallowing dysfunction associated with curative HNC treatment can substantially affect quality of life (QoL). Reduced QoL associated with radiation-induced dysphagia has been well-established.10 Difficulty swallowing has been cited as the strongest driver of decisional regret in long-term oropharyngeal cancer survivors.11 Moderate to severe swallowing dysfunction has been associated with poor QoL after HNC treatment, regardless of treatment modality.12 Furthermore, prolonged feeding tube−dependence has been shown to induce severe psychological distress associated with physical discomfort and altered self-image.13
The profound swallowing impairment associated with a dysfunctional larynx is often refractory to swallowing therapy or conservative surgical interventions.1,14 In severe cases, patients may pursue a total laryngectomy, referred to as functional laryngectomy (FL), performed in the setting of a dysfunctional larynx given that complete removal of the larynx achieves permanent separation of the airway from the alimentary tract, which eliminates aspiration risk.15 Although the natural voice is lost during total laryngectomy, alaryngeal voice rehabilitation can restore communicative techniques. Wu et al16 reported that 100% of feeding tube−dependent patients who underwent FL were able to resume oral intake with or without feeding tube supplementation. Hutcheson et al17 found that aspiration pneumonia rates, feeding tube−dependence, and oral intake improved after FL in patients with laryngopharyngeal dysfunction.
Although FL has been shown to improve oral intake and decrease rates of pneumonia, QoL in patients after elective FL has scarcely been investigated.16,17 This study aims to evaluate both clinician-rated and patient-reported changes in voice, swallowing, functional oral intake, QoL, and mood state after FL.
Methods
This cohort study was approved by the institutional review board of the University of California, Davis. Written informed consent was obtained for prospective data collection, and only patients who consented were enrolled.
Participants and Design
The study sample comprised consecutively enrolled patients electing to undergo FL due to a history of radiation-associated swallowing dysfunction and intractable aspiration from July 2016 to March 2022. Severe swallowing dysfunction was objectively confirmed on instrumental swallowing evaluation performed either in the otolaryngology clinic or fluoroscopy suite.
Collected data included patient demographic characteristics, oncologic medical history, clinician-rated metrics of swallowing function, and self-reported measures of swallowing function, voice quality, QoL, and mood states. Both clinician- and patient-rated metrics were evaluated at routine clinic visits.
Baseline evaluation occurred before the FL procedure. Twenty-two disease-free HNC survivors with long-standing profound dysphagia were enrolled; however, 2 patients (15%) died from aspiration pneumonia before receiving FL. Follow-up time points were 1, 3, and 6 months after FL, except for mood state, which was evaluated at baseline and 6 months after FL.
Measures and Instruments
Swallowing Evaluation
Swallowing function was evaluated with either videofluoroscopic swallow study or fiberoptic endoscopic evaluation of swallowing to confirm the severity of swallowing dysfunction. The Penetration-Aspiration Scale (PAS) was used to denote airway invasion.18
Oral Intake Metrics
The Functional Oral Intake Scale (FOIS), a 7-level clinician-rated ordinal scale of a patient’s level of oral intake, was used to document functional intake of liquids and solids. Levels 1 to 3 represent feeding tube dependence to various degrees ranging from nothing by mouth to daily oral intake with feeding tube supplementation. Levels 4 to 7 indicate complete oral intake with or without modifications, including diets of a single consistency to full oral intake without restriction.19 A change in scores by at least 1.0 was determined to be a minimal clinically important difference (MCID).20
The Eating Assessment Tool (EAT-10), a symptom-specific metric of dysphagia, was used to measure the patient’s perception of their swallowing function. The EAT-10 is a 10-item questionnaire, and each item is rated 0 to 4. Normative data suggest that a score of 3 or higher is abnormal and suggestive of dysphagia. Higher summed scores indicate worse perceived swallowing dysfunction.21
Voice Evaluation
The Voice Handicap Index (VHI), a patient assessment of voice quality was administered. The 30-item VHI questionnaire captures functional, physical, and emotional aspects of the patient’s voice quality. Higher composite scores represent worse perceived voice quality.22 The MCID between scores is a decrease of 4.23
Quality-of-Life Instrument
The Functional Assessment of Cancer Therapy−Head & Neck (FACT-H&N) is a reliable and valid patient-reported instrument consisting of 27 general QoL questions and 12 HNC site-specific items. Each item is rated on a 5-point Likert scale ranging from 0 to 4. As a multidimensional metric of QoL, the FACT-H&N includes the following subscales: physical, social and familial, emotional, functional well-being, and HNC-specific symptoms. Subscale scores were summed to create the global FACT-H&N score, which reflects overall QoL. The highest score possible is 148, with higher scores representing better QoL.24 The MCID between scores is from 6 to 12 units.25
Mood Instrument
The Profile of Mood States, second edition (POMS), is a validated psychological evaluation used to assess mood states and has been a reliable measure used in studies of various cancers.26 The POMS contains 37 items, and each item is rated on a 5-point Likert scale. The POMS includes 6 subscales to evaluate depression, vigor, confusion, esteem-related affect, tension, anger, and fatigue. All subscales were calculated to derive the total mood disturbance score which was used to represent general distress.26
Statistical Analysis
Continuous variables were compared using repeated measures ANOVA (analysis of variance) with post hoc tests to compare between the different time points. A Bonferroni adjustment was used to account for multiple comparisons. The POMS values for baseline and 6 months post-FL were compared using a paired sample t test. A P value < .05 was considered statistically significant for all statistical tests. Effect size measures with 95% CIs (partial η2 values for the measures EAT-10, FOIS, VHI, and FACT-H&N, and Cohen d values for POMS) were calculated. Statistical analyses were performed from July 2016 through March 2022 using SPSS, version 21.0 for the Macintosh (SPSS Inc).27
Results
The study analysis included 20 patients (mean [SD] age, 72.4 [7.0] years; 19 [95%] males and 1 [5%] female) who underwent FL and had complete data across all time points were included in the study analyses. Demographic information and clinical data are presented in Table 1; 12 patients (60%) were treated for oropharyngeal cancers, and all patients received definitive chemoradiation therapy. The mean (SD) time from completion of chemoradiation therapy to FL was 15.5 (5.5) years. Indications for FL are summarized in Table 2.
Table 1. Demographic and Clinical Characteristics of 20 Participants Who Underwent Functional Laryngectomy (FL).
| Characteristic | Participants, No. (%) |
|---|---|
| Sex | |
| Male | 19 (95) |
| Female | 1 (5) |
| Age, mean (SD), y | 72.4 (7.0) |
| Time from HNC treatment to FL, mean (SD), y | 15.5 (5.5) |
| Tumor site | |
| Oropharynx | 12 (60) |
| Larynx | 7 (35) |
| Nasopharynx | 1 (5) |
| Radiation therapy | |
| Definitive | 20 (100) |
| Adjuvant | 0 |
| Chemotherapy | 20 (100) |
| Postoperative complication | 1 (5) |
Abbreviation: HNC, head and neck cancer.
Table 2. Indications for Functional Laryngectomy Due to Adverse Functional Outcomes of HNC Treatment in 20 Study Participants.
| Outcome | Participants, No. (%) |
|---|---|
| Dysphagia | |
| Chronic aspiration | 20 (100) |
| Feeding tube dependence | 16 (80) |
| Chondroradionecrosis | 1 (5) |
| Aspiration pneumonia | |
| None | 1 (5) |
| Single episode | 1 (5) |
| Recurrent | 18 (90) |
Abbreviation: HNC, head and neck cancer.
Laryngectomy Procedure
Eighteen patients (90%) required complex reconstruction of the pharyngeal defect during FL. Reconstruction approaches included 9 anterolateral thigh free flaps, 7 pectoralis myocutaneous flap overlays, 1 overlay pharyngoplasty with de-epithelialized fascial paddle, and 1 strap muscle overlay. Two patients (10%) underwent simultaneous segmental mandibulectomy for either osteoradionecrosis of the mandible or a malignant mandibular lesion. One patient (5%) developed a pharyngocutaneous fistula in the early postoperative setting, which was treated conservatively with local wound care and nothing by mouth for 1 week.
Swallowing Function
All patients had a PAS score of 7 (material enters the airway, passes below the true vocal folds, and is not ejected from the trachea despite effort) or a PAS score of 8 (material enters the airway, passes below the true vocal folds, and no effort is made to eject) confirmed on instrumental swallowing evaluation. Observed aspiration was consistent on repeated trials and did not improve with compensatory strategies or maneuvers. Nineteen patients (91%) had a history of recurrent aspiration pneumonia.
Consistent improvement in patient-reported and clinician-rated swallowing function were observed across all 4 time points. Swallowing outcomes before and after FL are depicted in Figure 1. Baseline EAT-10 scores gradually improved from a mean (SD) of 32.2 (7.4) to 23.1 (10.8) at 1 month post-FL; 12.1 (9.1) at 3 months post-FL; and 8.3 (7.4) at 6 months post-FL, with a large effect size (η2 = 0.72; 95% CI, 0.54-0.80).
Figure 1. Swallowing Metrics per Mean Scores on the Eating Assessment Tool-10 (EAT-10) and the Functional Oral Intake Scale (FOIS).
Error bars represent standard error. EAT-10 scores improved, with a large effect size (η2 = 0.72; 95% CI, 0.54-0.80), and FOIS scores demonstrated a large effect size (η2 = 0.6; 95% CI, 0.38-0.71) from baseline to 6 months after functional laryngectomy.
Sixteen patients (80%) were feeding tube dependent with a mean (SD) FOIS of 2.0 (1.5) at baseline. FOIS gradually improved across all time points as FOIS increased to 2.9 (1.7) at 1-month post-FL, 4.8 (2.5) at 3 months post-FL, and 5.2 (1.7) at 6 months post-FL, with a large effect size (η2 = 0.6; 95% CI, 0.38-0.71). Improvement in oral intake was achieved in 19 patients (95%) with increasing FOIS scores, and feeding tubes were removed from 10 of 16 patients (63%) who were feeding tube dependent. Six patients (37%) remained feeding tube dependent at 6 months post-FL. Two patients (33%) who received simultaneous segmental mandibulectomy at the time of FL remained feeding tube−dependent to some degree due to impairments in oral manipulation and posterior propulsion of consistencies other than liquids. Four patients remained feeding tube dependent due to medical comorbidities present before FL. One patient (17%) was quadriplegic following a cerebrovascular injury; 2 patients (33%) had severe esophageal stricture; and 1 patient (17%) had severe bronchiectasis, necessitating ongoing enteral supplementation.
Voice Quality
The mean (SD) baseline VHI score was 63.6 (34.0) and after FL, mean (SD) VHI scores worsened to 86.9 (33.7) at 1 month post-FL (Figure 2). Improvement of perceived voice quality was noted at 3 months post-FL with a mean (SD) VHI score of 71.3 (36.1). At 6 months post-FL, the mean (SD) VHI score was 39.7 (26.9), which was markedly improved in comparison with baseline VHI scores (η2 = 0.42; 95% CI, 0.19-0.56). At 6 months post-FL, 16 patients (80%) demonstrated an MCID with VHI scores decreasing by 4 or greater in comparison with baseline scores. For alaryngeal voice restoration, 17 patients (85%) underwent secondary tracheoesophageal puncture and tracheoesophageal voice prosthesis placement. One patient (5%) used the electrolarynx and 2 patients (10%) used other alternative methods of communication, such as a text-to-speech app on a smart tablet, for alaryngeal communication. Improved self-perceptions of voice quality at 3 months and 6 months post-FL may reflect increasing proficiency with alaryngeal voice options over time.
Figure 2. Voice Metrics per Mean Scores on the Voice Handicap Index (VHI).
Error bars represent standard error. VHI scores demonstrated a large effect size (η2 = 0.42; 95% CI, 0.19-0.56) from baseline to 6 months after functional laryngectomy.
Quality of Life
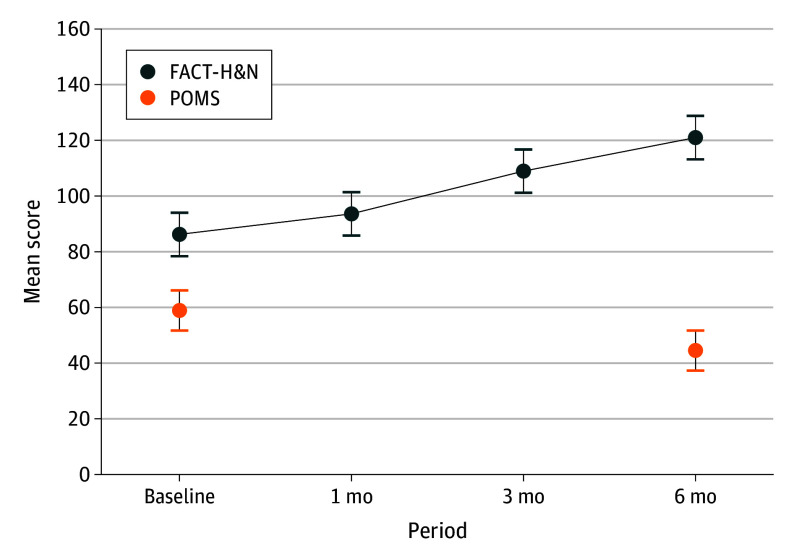
Improvements in patient-reported QoL and mood states were observed across all time points (Figure 3). FACT-H&N scores increased across social and familial, emotional, and functional well-being, and head and neck-related domains. The mean (SD) FACT-H&N score improved from 88.9 (6.5) at baseline to 91.6 (8.5) at 1 month post-FL, 114.6 (7.0). Continued improvement in self-reported QoL was observed at 3 months post-FL with a mean (SD) FACT-H&N score of 114.6 (7.0) and 6 months post-FL with a mean (SD) FACT-H&N score of 128.0 (5.4), with a large effect size (η2 = 0.64; 95% CI, 0.42-0.74). At 6 months post-FL, 17 patients (85%) demonstrated an MCID with FACT-H&N scores increasing 12 or more units in comparison with baseline scores.
Figure 3. Quality of Life Metrics per Mean Scores on the Functional Assessment of Cancer Therapy-Head & Neck (FACT-H&N) and the Profile of Mood States, Second Edition (POMS).
Error bars represent standard errors. FACT-H&N scores improved with a large effect size (η2 = 0.64; 95% CI, 0.42-0.74) and POMS scores demonstrated a large effect size (Cohen d = 1.036; 95% CI, 0.48-1.57) from baseline to 6-months after functional laryngectomy.
Mood states improved after FL as measured by mean (SD) POMS scores decreasing from 58.9 (13.2) at baseline to 44.5 (9.9) at 6 months post-FL (P < .01), which indicates an overall reduction in general distress.
Discussion
This is a novel prospective report of perceived swallowing function, voice quality, QoL, and mood states in HNC survivors after FL for profound dysphagia and intractable aspiration. Reconstructive methods in this cohort were largely determined by surgeon preference. The variation in reconstructive techniques reflects the evolution of surgical practices over time. Historically, anterior lateral thigh free-flap reconstruction was our primary method of reconstruction; however, our contemporary practice includes consideration of the pectoralis flap, and is reflected in this cohort who underwent FL in July 2016 to March 2022. Regarding free tissue transfer, in-lay flap closure was performed if closure of the pharynx resulted in a lumen diameter less than 1 cm (equaling a circumference of mucosa of ≥3 cm) to allow for inclusion of the flap in the pharyngeal wall. This was chosen over an on-lay flap in which the mucosa is closed to itself and then covered with a flap to help reduce risk of fistula. Also, if mucosal tissue and health looked concerning intraoperatively, in-lay flap closure was favored.
Consistent improvement in patient-reported swallowing function was observed from pre-FL to 6 months post-FL. Patient reported improvement in swallowing function was further supported by rates of return to oral intake. An improvement in FOIS scores was noted from pre-FL to post-FL with all patients increasing their ability to consume some degree of oral intake, and 63% of patients undergoing feeding tube removal with return to complete oral intake. Our findings are consistent with previously published data on increased oral intake after FL, given that 100% of patients in our study were able to orally consume to some degree.4
Perceptions of voice quality varied across time points, with overall improvement at 6 months post-FL. Worsening of perceived voice quality at 1 month post-FL in comparison with perceived voice quality at baseline reflects the anticipated consequence of surgical removal of the larynx. Aphonia has been correlated with depression and anxiety in patients with total laryngectomy.28 Our institution performs secondary tracheoesophageal puncture and tracheoesophageal voice prosthesis placement. Among the 20 patients in this study, 85% underwent secondary tracheoesophageal puncture and tracheoesophageal voice prosthesis placement between 1 and 3 months after FL. Adaptation to alaryngeal communication modalities likely explains the improvement in self-perception of voice quality observed during the 3-month and 6-month post-FL time points.
Although current studies have demonstrated functional improvements in return to oral intake and reduction in aspiration pneumonia rates, it is unknown whether patients perceive benefit and satisfaction with their decision to pursue elective FL. The findings of this study contribute to the existing body of literature by highlighting changes to the multidimensional concept of QoL after FL. Across all time points, patients demonstrated consistent improvement in FACT-H&N and POMS scores, indicating improved QoL across all domains and a reduction in general distress after FL. The effect sizes of all the observed changes in this study were large, with moderately wide confidence intervals, suggesting a clinically meaningful difference between the pre-FL and post-FL statuses, warranting further investigation in larger cohorts.
Radiation therapy for HNC can result in a dysfunctional larynx due to atrophy, fibrosis, and sensory laryngeal neuropathy.1,2 Profound dysphagia with chronic aspiration has been associated with dehydration, malnutrition, pneumonia, and death.3 In patients with severe dysphagia and intractable aspiration, FL improves overall health by eliminating aspiration and allowing for return to oral intake.15
In patients with total laryngectomy, poor QoL has been reported with high rates of depression and anxiety.28 When compared with presurgical QoL, patients reported decreased levels of satisfaction with physical function, social contact, financial stability, and speech after total laryngectomy. Satisfaction in these QoL domains did not recover to presurgical levels.29 A reduction in social outings with friends and frequency of eating in public restaurants are associated with increased feelings of social isolation after total laryngectomy surgery.30
While total laryngectomy eliminates the risk of aspiration, the incidence of dysphagia is approximately 17% to 72% after surgery.31 It is important to note that surgery- and radiation-associated swallowing impairments can persist and contribute to ongoing dysphagia after total laryngectomy. Removal of the hyolaryngeal complex may produce impaired pharyngeal clearance due to impaired base of tongue retraction and traction needed for upper esophageal sphincter opening. Pharyngeal stasis may be associated with postsurgical edema and altered anatomy of the neopharynx. Furthermore, postradiation changes to oral and oropharyngeal structures may continue to affect swallowing efficiency.32
There are limited data exploring the role of FL in improving QoL in patients with dysfunctional larynges. Theunissen et al33 presented retrospectively collected data of 11 of 25 patients who underwent FL. At a mean of 5 years post-FL, patients completed the European Organization for Research and Treatment of Cancer Quality of Life C30 and Head and Neck Module 35; difficulties with speech, taste and smell sensations, mouth opening, and saliva viscosity were rated as the highest burdens. Most of the published literature on FL focuses on rates of feeding tube removal and return to oral intake. In a study of 32 HNC survivors who underwent FL, rates of feeding tube dependence decreased from 81% to 34% in a 1-year postoperative period.34 Wu et al16 found high rates of functional communication and oral intake with 89% of patients achieving adequate alaryngeal voice and 100% of patients returning to some degree of oral intake after FL. In a study evaluating oral intake across a 1-year period after FL, a statistically significant improvement in FOIS scores, frequency of aspiration pneumonia, and nothing-by-mouth status were noted across 3-month and 1-year time points post-FL.35
The death of 2 patients enrolled in the study before they received FL is of particular importance. Both patients died of recurrent aspiration pneumonia approximately 1 month after study enrollment and before the surgical date. This highlights the need for early identification of post-HNC swallowing impairment, and for high prioritization and expeditious referral of candidate patients for consideration of FL.
These study findings are valuable not only for patients considering FL, but also for clinicians involved in their care. The decision to undergo elective FL can be daunting for patients and necessitates serious consideration of its potential risks and benefits. These data highlight that while functional oral intake, rates of feeding tube dependence and voice quality improve after FL, so do overall QoL and mood states. These findings can help inform shared patient-clinician decision-making in the preoperative period and provide a possible timeline for expected changes to functional and psychosocial well-being after FL.
Limitations
Limitations inherent to this study include a small and homogenous cohort. Only 1 patient was female, which limited our ability to extrapolate QoL changes in women. Furthermore, the global FACT-H&N score was used as a metric of overall QoL. Analysis of the FACT-H&N subscales would provide more granular information on the specific domains of physical, emotional, functional, and social and familial well-being.
Conclusion
The findings of this prospective cohort study indicate that FL for dysfunctional larynges in HNC survivors was associated with a clinically meaningful improvement in the self-perceptions of swallowing function and voice quality, and functional swallowing outcomes with return to oral intake and feeding tube removal. Furthermore, FL was associated with an improved global quality of life and mood states across a 6-month period.
Data Sharing Statement
References
- 1.King SN, Dunlap NE, Tennant PA, Pitts T. Pathophysiology of radiation-induced dysphagia in head and neck cancer. Dysphagia. 2016;31(3):339-351. doi: 10.1007/s00455-016-9710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubblefield MD. Radiation fibrosis syndrome: Neuromuscular and musculoskeletal complications in cancer survivors. PM R. 2011;3(11):1041-1054. doi: 10.1016/j.pmrj.2011.08.535 [DOI] [PubMed] [Google Scholar]
- 3.Mehdizadeh OB, Dhar SI, Evangelista L, Nativ-Zeltzer N, Bewley AF, Belafsky PC. Prevalence of profound laryngeal sensory neuropathy in head and neck cancer survivors with feeding tube-dependent oropharyngeal dysphagia. Head Neck. 2020;42(5):898-904. doi: 10.1002/hed.26059 [DOI] [PubMed] [Google Scholar]
- 4.De Felice F, de Vincentiis M, Luzzi V, et al. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research, and management. Oral Oncol. 2018;77:125-130. doi: 10.1016/j.oraloncology.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 5.Lee AS, Ryu JH. Aspiration pneumonia and related syndromes. Mayo Clin Proc. 2018;93(6):752-762. doi: 10.1016/j.mayocp.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Boero IJ, Hwang L, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer. 2015;121(8):1303-1311. doi: 10.1002/cncr.29207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorenc M, Kozjek NR, Strojan P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep Pract Oncol Radiother. 2015;20(4):249-258. doi: 10.1016/j.rpor.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19(1):35-42. doi: 10.1016/j.semradonc.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114(8):1362-1367. doi: 10.1097/00005537-200408000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Goepfert RP, Fuller CD, Gunn GB, et al. Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck. 2017;39(11):2151-2158. doi: 10.1002/hed.24879 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi: 10.1016/j.ijrobp.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Ojo O, Keaveney E, Wang XH, Feng P. The effect of enteral tube feeding on patients’ health-related quality of life: A systematic review. Nutrients. 2019;11(5):1046. doi: 10.3390/nu11051046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118(23):5793-5799. doi: 10.1002/cncr.27631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olinde L, Evangelista L, Bewley AF. Functional laryngectomy for the dysfunctional larynx: indications and outcomes in setting of prior chemoradiotherapy. Curr Opin Otolaryngol Head Neck Surg. 2021;29(6):473-478. doi: 10.1097/MOO.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 16.Wu MP, Goldsmith T, Holman A, et al. Risk factors for laryngectomy for dysfunctional larynx after organ preservation protocols: a case-control analysis. Otolaryngol Head Neck Surg. 2021;164(3):608-615. doi: 10.1177/0194599820947702 [DOI] [PubMed] [Google Scholar]
- 17.Hutcheson KA, Alvarez CP, Barringer DA, Kupferman ME, Lapine PR, Lewin JS. Outcomes of elective total laryngectomy for laryngopharyngeal dysfunction in disease-free head and neck cancer survivors. Otolaryngol Head Neck Surg. 2012;146(4):585-590. doi: 10.1177/0194599811432264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93-98. doi: 10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- 19.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516-1520. doi: 10.1016/j.apmr.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 20.Everton LF, Benfield JK, Hedstrom A, et al. Psychometric assessment and validation of the dysphagia severity rating scale in stroke patients. Sci Rep. 2020;10(1):7268. doi: 10.1038/s41598-020-64208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919-924. doi: 10.1177/000348940811701210 [DOI] [PubMed] [Google Scholar]
- 22.Jacobson BH, Johnson A, Grywalski C, et al. The voice handicap index (VHI). Am J Speech Lang Pathol. 1997;6(3):66-70. doi: 10.1044/1058-0360.0603.66 [DOI] [Google Scholar]
- 23.Young VN, Jeong K, Rothenberger SD, et al. Minimal clinically important difference of voice handicap index-10 in vocal fold paralysis. Laryngoscope. 2018;128(6):1419-1424. doi: 10.1002/lary.27001 [DOI] [PubMed] [Google Scholar]
- 24.List MA, D’Antonio LL, Cella DF, et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy head and neck scale: a study of utility and validity. Cancer. 1996;77(11):2294-2301. doi: [DOI] [PubMed] [Google Scholar]
- 25.Ringash J, Bezjak A, O’Sullivan B, Redelmeier DA. Interpreting differences in quality of life: the FACT-H&N in laryngeal cancer patients. Qual Life Res. 2004;13(4):725-733. doi: 10.1023/B:QURE.0000021703.47079.46 [DOI] [PubMed] [Google Scholar]
- 26.Shacham S. A shortened version of the profile of mood states. J Pers Assess. 1983;47(3):305-306. doi: 10.1207/s15327752jpa4703_14 [DOI] [PubMed] [Google Scholar]
- 27.IBM SPSS Inc. SPSS software. Accessed July 23, 2023. https://www.ibm.com/spss
- 28.Braz DS, Ribas MM, Dedivitis RA, Nishimoto IN, Barros AP. Quality of life and depression in patients undergoing total and partial laryngectomy. Clinics (Sao Paulo). 2005;60(2):135-142. doi: 10.1590/S1807-59322005000200010 [DOI] [PubMed] [Google Scholar]
- 29.Singer S, Danker H, Guntinas-Lichius O, et al. Quality of life before and after total laryngectomy: results of a multicenter prospective cohort study. Head Neck. 2014;36(3):359-368. doi: 10.1002/hed.23305 [DOI] [PubMed] [Google Scholar]
- 30.Perry A, Casey E, Cotton S. Quality of life after total laryngectomy: functioning, psychological well-being and self-efficacy. Int J Lang Commun Disord. 2015;50(4):467-475. doi: 10.1111/1460-6984.12148 [DOI] [PubMed] [Google Scholar]
- 31.Maclean J, Cotton S, Perry A. Post-laryngectomy: it’s hard to swallow: an Australian study of prevalence and self-reports of swallowing function after a total laryngectomy. Dysphagia. 2009;24(2):172-179. doi: 10.1007/s00455-008-9189-5 [DOI] [PubMed] [Google Scholar]
- 32.Landera MA, Lundy DS, Sullivan PA. Dysphagia after total laryngectomy. Perspectives on Swallowing and Swallowing Disorders (Dysphagia). 2010;19(2):39-44. doi: 10.1044/sasd19.2.39 [DOI] [Google Scholar]
- 33.Theunissen EA, Timmermans AJ, Zuur CL, et al. Total laryngectomy for a dysfunctional larynx after (chemo)radiotherapy. Arch Otolaryngol Head Neck Surg. 2012;138(6):548-555. doi: 10.1001/archoto.2012.862 [DOI] [PubMed] [Google Scholar]
- 34.Farlow JL, Birkeland AC, Hardenbergh A, et al. Speech and swallowing outcomes after laryngectomy for the dysfunctional irradiated larynx. Eur Arch Otorhinolaryngol. 2020;277(5):1459-1465. doi: 10.1007/s00405-020-05809-y [DOI] [PubMed] [Google Scholar]
- 35.Topf MC, Magaña LC, Salmon K, et al. Safety and efficacy of functional laryngectomy for end-stage dysphagia. Laryngoscope. 2018;128(3):597-602. doi: 10.1002/lary.26760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement