Key Points
Question
What are the survival outcomes and rates of toxic effects in the delivery of stereotactic ablative radiotherapy (SABR) to patients with early-stage non–small cell lung cancer and interstitial lung disease?
Findings
This phase 2 nonrandomized clinical trial enrolled 39 patients who were treated with SABR. Median overall survival was 25 months, significantly exceeding the goal of 12 months, and the risk of treatment-related mortality was 7.7%.
Conclusion
This clinical trial met its prespecified acceptability thresholds for toxicity and efficacy, supporting the use of SABR for curative-intent treatment after a careful discussion of risks and benefits.
Abstract
Importance
Patients with interstitial lung disease (ILD) and early-stage non–small cell lung cancer (NSCLC) have been reported to be at high risk of toxic effects after stereotactic ablative radiotherapy (SABR), but for many patients, there are limited alternative treatment options.
Objective
To prospectively assess the benefits and toxic effects of SABR in this patient population.
Design, Setting, and Participants
This prospective cohort study was conducted at 6 academic radiation oncology institutions, 5 in Canada and 1 in Scotland, with accrual between March 7, 2019, and January 12, 2022. Patients aged 18 years or older with fibrotic ILD and a diagnosis of T1-2N0 NSCLC who were not candidates for surgical resection were enrolled.
Intervention
Patients were treated with SABR to a dose of 50 Gy in 5 fractions every other day.
Main Outcomes and Measures
The study prespecified that SABR would be considered worthwhile if median overall survival—the primary end point—was longer than 1 year, with a grade 3 to 4 risk of toxic effects less than 35% and a grade 5 risk of toxic effects less than 15%. Secondary end points included toxic effects, progression-free survival (PFS), local control (LC), quality-of-life outcomes, and changes in pulmonary function. Intention-to-treat analysis was conducted.
Results
Thirty-nine patients enrolled and received SABR. Median age was 78 (IQR, 67-83) years and 59% (n = 23) were male. At baseline, 70% (26 of 37) of patients reported dyspnea, median forced expiratory volume in first second of expiration was 80% (IQR, 66%-90%) predicted, median forced vital capacity was 84% (IQR, 69%-94%) predicted, and median diffusion capacity of the lung for carbon monoxide was 49% (IQR, 38%-61%) predicted. Median follow-up was 19 (IQR, 14-25) months. Overall survival at 1 year was 79% (95%, CI 62%-89%; P < .001 vs the unacceptable rate), and median overall survival was 25 months (95% CI, 14 months to not reached). Median PFS was 19 months (95% CI, 13-28 months), and 2-year LC was 92% (95% CI, 69%-98%). Adverse event rates (highest grade per patient) were grade 1 to 2: n = 12 (31%), grade 3: n = 4 (10%), grade 4: n = 0, and grade 5: n = 3 (7.7%, all due to respiratory deterioration).
Conclusions and Relevance
In this trial, use of SABR in patients with fibrotic ILD met the prespecified acceptability thresholds for both toxicity and efficacy, supporting the use of SABR for curative-intent treatment after a careful discussion of risks and benefits.
Trial Registration
ClinicalTrials.gov Identifier: NCT03485378
This nonrandomized clinical trial examines the toxicity and efficacy of stereotactic ablative radiotherapy in patients with interstitial lung disease and early-stage non–small cell lung cancer.
Introduction
Stereotactic ablative radiotherapy (SABR) is the preferred treatment option for patients with early-stage non–small cell lung cancer (NSCLC) who are medically inoperable or decline surgery.1 In the general population of patients who are not candidates for surgery, SABR achieves local control (LC) rates above 90%, with low risks of grade 3 to 4 toxic effects (<5%), and a very low risk of treatment-related mortality (<1%).2,3 This favorable toxicity profile has allowed for the use of SABR in frail populations, including older patients and patients with poor lung function.2,4
Patients with fibrotic interstitial lung disease (ILD) have been recognized as a subgroup of patients who may be at higher risk of severe radiation pneumonitis than the general population.5 Fibrotic ILDs include several diseases that affect the pulmonary interstitium, including idiopathic pulmonary fibrosis (IPF), connective tissue disease–associated ILD (CTD-ILD) (eg, associated with scleroderma), idiopathic nonspecific interstitial pneumonia, hypersensitivity pneumonitis (eg, associated with exposure to birds), and unclassifiable ILD.6 Fibrotic ILDs have typical high-resolution computed tomography (HRCT) findings, including subpleural reticulation, traction bronchiectasis, and honeycombing. Although the classification of ILD is complex, a previous review article provides an overview of ILD specifically for thoracic oncologists.6 Interstitial lung disease and radiotherapy-associated lung damage share common pathways of lung injury, which may explain the elevated risks of radiation in this patient population.7
A 2017 systematic review attempted to quantify the risk of toxic effects in patients with ILD and early-stage NSCLC who underwent curative-intent treatment.8 In that systematic review, the use of SABR was associated with a 25% risk of grade 3 or higher pneumonitis and a 15% risk of grade 5 toxic effects (ie, treatment-related death). However, grade 5 toxic effect rates varied widely across studies (from 0% to 60%), differing doses and fractionations were used, and no studies prospectively enrolled patients with fibrotic ILD, leading to uncertainty about the true risks. A subsequent 2022 systematic review examined the use of radiotherapy across all stages of lung cancer and reported similar risks of toxic effects and mortality in patients with ILD.9
The Assessment of Precision Irradiation in Early Non–Small Cell Lung Cancer and Interstitial Lung Disease (ASPIRE-ILD) trial is the first study, to our knowledge, to enroll patients with fibrotic ILD and early-stage NSCLC to test a curative-intent treatment option. The goals of ASPIRE-ILD were to confirm ILD status based on central review before treatment, to deliver a standardized SABR dose with careful planning constraints, and to assess toxic effects and survival outcomes to inform clinical decision-making in this underserved patient population.
Methods
Study Design
The ASPIRE-ILD trial was an investigator-initiated, multicenter, open-label, single-arm phase 2 study.10 We enrolled patients with early-stage (T1-2N0) NSCLC who were not candidates for surgery. Patients were treated with SABR to a dose of 50 Gy in 5 fractions every other day, with a built-in dose deescalation protocol in case of unacceptable toxic effects. The trial protocol was previously published10 and is also available in Supplement 1. Patients were enrolled at 5 hospital centers located in Canada and 1 in Scotland. Research ethics approvals and other required regulatory approvals were obtained in all jurisdictions, with written informed consent obtained from all patients. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Participants
Patients were required to be aged 18 years or older, with a diagnosis of T1-2N0 NSCLC in the setting of fibrotic ILD, and not candidates for surgery. Performance status requirements were permissive, with Eastern Cooperative Oncology Group scores of 0 to 3 allowed. Pathologic diagnosis of NSCLC was strongly recommended, but if the risk of biopsy was unacceptable, growth on serial computed tomography (CT) imaging and/or positron emission tomography (PET) avidity strongly suggestive of primary NSCLC were required. Exclusion criteria included prior invasive malignant growth unless disease-free for at least 2 years, prior thoracic radiotherapy, plans for additional local or systemic anticancer therapy before progression, pregnancy, or concurrent administration of known radiosensitizers (eg, methotrexate). Interstitial lung disease treatments, such as nintedanib, pirfenidone, or steroids were allowed, but other immunosuppressive therapies (eg, mycophenolate) were held for 2 weeks before and after SABR.
Preenrollment investigation requirements included HRCT of the thorax and fluorodeoxyglucose PET/CT, and assessment by a respirologist with pulmonary function tests. Mediastinal nodal sampling was allowed but not required, as long as all regional nodes were less than 1 cm and negative on PET. The diagnosis of ILD was confirmed on central review by a dedicated ILD team. Patients who were candidates for the study but did not wish to proceed with SABR were recruited for collection of ongoing follow-up data as part of a separate observation cohort. The trial closed to accrual on January 12, 2022, and after 1-year of follow-up, the dataset was locked for outcomes on January 24, 2023.
Procedures
Following central review, an ILD-GAP Index11 was assigned to document the severity of ILD. The ILD-GAP Index is based on type of ILD, gender (patient sex), age, and physiologic factors (forced vital capacity [FVC] and diffusion capacity of the lung for carbon monoxide [DLCO]). Indices range from 0 (best prognosis) to 8 (worst prognosis) and were used to divide patients into 3 groups: cohort I: ILD-GAP 0 to 2, cohort II: ILD-GAP 3 to 5, and cohort III: ILD-GAP greater than or equal to 6. This classification would allow for differential deescalation of doses based on ILD severity if excessive toxic effects occurred in any one group.
Full details of radiotherapy planning and delivery are described in the protocol (Supplement 1). In brief, SABR was delivered using a dose of 50 Gy in 5 fractions, every second day. This dose was chosen because it is the minimum dose that delivers a biological effective dose of at least 100 Gy10 (which is a key determinant of SABR LC12), and is safe to use at all intrathoracic locations, including for tumors that abut critical hilar or mediastinal structures.13 Full details of normal tissue constraints and quality assurance are in the protocol (Supplement 1).
Outcomes
The primary end point was overall survival (OS), defined as time from enrollment to death from any cause or last follow-up, whichever occurred first. Secondary outcomes included toxic effects (Common Terminology Criteria for Adverse Events, version 4); progression-free survival (PFS), defined as time from enrollment to death from any cause, any progression of disease (local, regional, or distant), or last follow-up, whichever occurred first; LC, regional, and distant control (death as censoring event); changes in cough severity, using a 10-cm visual analog scale; quality of life (using the Functional Assessment of Cancer Therapy [FACT]-General or FACT-Lung [FACT-L] and EuroQol 5-Dimension 5-Level); and changes in pulmonary function tests, including DLCO, forced expiratory volume in first second of expiration (FEV1), and forced vital capacity (FVC).
Statistical Analysis
The primary end point of this study was OS, with a comparison with historical controls of untreated patients with stage I NSCLC who were not candidates for surgery due to their medical status (not specifically patients with ILD), who consistently have median survival of less than 1 year, as described in several studies.14,15,16,17 This 1-year threshold is consistent with European radiotherapy guidelines, which recommend SABR in patients with early-stage NSCLC who are not surgical candidates due to medical conditions, if their life expectancy exceeds 1 year.18 Therefore, the goal was to statistically examine whether OS at 1 year exceeds 50%. A sample size of 39 patients provided greater than 80% power to detect an OS improvement of greater than 20% at 1 year, compared with a historical control of 50% (ie, 71% vs 50%), using a 1-sample binomial test at the .05 significance level, assuming 10% dropout or loss to follow-up before 1 year.
It was prespecified that SABR would be considered worthwhile if median OS was longer than 1 year, with a risk of grade 3 to 4 pulmonary toxic effects greater than 35%, and a risk of treatment-related mortality less than 15%. The mortality rate target was derived from the prior systematic review of SABR in patients with ILD,8 whereas the grade 3 to 4 toxic effect rate was derived from acceptable pneumonitis rates (30%) in patients with local advanced NSCLC, also from a prior systematic review.19 To minimize the risk of underattributing toxic effects to radiation, the protocol listed several grade 3 to 5 toxic effects (eg, dyspnea, ILD exacerbations; see protocol for full list [Supplement 1]) that would automatically be considered as related to treatment, unless there was clear evidence that the adverse event was unrelated. These were adjudicated independently by the data safety monitoring committee. Due to difficulty attributing dyspnea to radiation vs ILD, the trial did not distinguish radiation pneumonitis from ILD exacerbation, and both were considered to be radiation-related dyspnea for any grade of toxic effect, including death. A built-in protocol for deescalation was included if unacceptable toxic effects were noted in any ILD subtype or ILD-GAP cohort.
Intention-to-treat analysis was conducted. Full details of statistical analyses are provided in the eMethods in Supplement 2, and all testing was 2-sided at the .05 significance level. Data analysis was performed using SAS, version 9.4 software (SAS Institute Inc).
Results
Baseline Characteristics
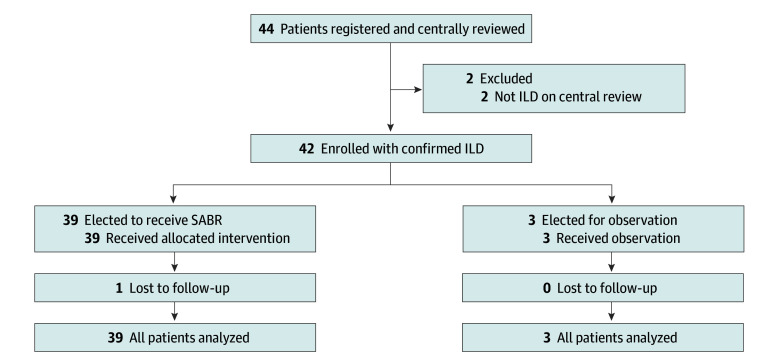
A total of 42 patients were enrolled between March 7, 2019, and January 12, 2022. Thirty-nine patients were treated with SABR, and 3 elected for observation (Figure 1). Baseline characteristics are reported in Table 1. Given the small number of patients in the observation group, the characteristics and outcomes of these 3 patients are discussed separately herein; all other results within this section pertain to the 39 patients treated with SABR, all of whom received a dose of 50 Gy in 5 fractions.
Figure 1. Patient Flowchart.
ILD indicates interstitial lung disease; SABR, stereotactic ablative radiotherapy.
Table 1. Baseline Characteristics for Enrolled Patients.
| Characteristic | All enrolled patients (N = 42) [data available, No.] | All patients receiving SABR (n = 39) | Enrolled patients not receiving SABR (n = 3) |
|---|---|---|---|
| Age, median (IQR), y | 77 (67-82) [42] | 78 (67-83) | 74 (70-6) |
| Sex, No. (%) | [42] | ||
| Female | 17 (40) | 16 (41) | 1 (33) |
| Male | 25 (60) | 23 (59) | 2 (67) |
| T stage, No. (%) | [42] | ||
| T1 | 34 (81) | 31 (80) | 3 (100) |
| T2 | 8 (19) | 8 (20) | 0 |
| Baseline ECOG performance status, No. (%) | [40] | ||
| 0 | 6 (15) | 6 (16) | 0 |
| 1 | 27 (68) | 25 (68) | 2 (67) |
| 2 | 6 (15) | 5 (14) | 1 (33) |
| 3 | 1 (3) | 1 (3) | 0 |
| Major symptoms, No. (%) | [40] | ||
| Dyspnea | 29 (73) | 26 (70) | 3 (100) |
| No major symptoms | 5 (12) | 5 (14) | 0 |
| Othera | 6 (15) | 6 (16) | 0 |
| Smoking history, No. (%) | 38 (93) [41] | 35 (92) | 3 (100) |
| Smoking pack-years, median (IQR) | 40 (30-50) [37] | 43 (30-50) | 20 (20-50) |
| ILD type (multidisciplinary consensus diagnosis), No. (%) | [42] | ||
| IPF | 10 (24) | 8 (21) | 2 (67) |
| NSIP | 0 | 0 | 0 |
| CT-ILD | 9 (21) | 8 (21) | 1 (33) |
| Chronic HP | 2 (5) | 2 (5) | 0 |
| Unclassifiable/other | 21 (50) | 21 (54) | 0 |
| ILD-GAP Index, No. (%) | [42] | ||
| ≤2 | 14 (33) | 14 (36) | 0 |
| 3-5 | 26 (62) | 23 (59) | 3 (100) |
| ≥6 | 2 (5) | 2 (5) | 0 |
| Baseline primary tumor size, median (IQR), cm | 2.2 (1.6-2.7) [37] | 2.2 (1.6-2.7) | 2.2 (1.5-3.1) |
| Baseline FEV1 [% predicted], median (IQR) | 79 (66-90) [41] | 80 (66-90) | 69 (55-94) |
| Baseline FVC [% predicted], median (IQR) | 84 (68-95) [40] | 84 (69-94) | 71 (46-96) |
| Baseline DLCO [% predicted], median (IQR) | 49 (38-61) [41] | 49 (38-61) | 41 (14-67) |
Abbreviations: CT, connective tissue; ECOG, Eastern Cooperative Oncology Group; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in first second of expiration; FVC, forced vital capacity; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonia; SABR, stereotactic ablative radiotherapy.
Includes: cough (n = 2), bronchitis (n = 1), chest tightness and cough (n = 1), joint pain and muscle weakness (n = 1), and weakness and reduced exercise tolerance (n = 1).
Median age was 78 (IQR, 67-83) years, 59% (n = 23) were male, 41% (n = 16) were female, and 92% (35 of 38) had a history of smoking (median, 43 [IQR, 30-50] pack-years). Most patients (31 [80%]) had T1 disease. All patients underwent staging with an HRCT of the thorax and a whole-body PET-CT scan (median standardized uptake value, 7.1 [IQR, 4.4-11]). Twenty patients (51%) had biopsy confirmation of NSCLC, and 4 patients (10%) underwent mediastinal staging due to the presence of nodes larger than 1 cm or nodes with fluorodeoxyglucose avidity. In the 19 patients without biopsy confirmation, 12 had a presumptive diagnosis based on serial CT (median standardized uptake value, 5.8 [IQR, 4.1-9.2]), and 7 based on PET/CT (median standardized uptake value, 6.8 [IQR, 6.3-8.0]).
ILD Symptoms and Severity
At baseline, 82% (32 of 39) of the patients had symptoms that were attributed to ILD, with most (70%) reporting dyspnea, and others reporting cough, reduced exercise tolerance, and/or general weakness. Median FVC was 84% (IQR, 69%-94%), median FEV1 was 80% (IQR, 66%-90%) predicted, and median DLCO was 49% (IQR, 38%-61%) predicted. Six patients (15%) were receiving home oxygen.
After central review, most patients had an unclassifiable or other subtype of ILD (21 [54%]), with IPF and CTD-ILD as the most common diagnoses in the remainder (8 [21%] for each). The high proportion of unclassifiable ILD was due to the rarity of surgical biopsy of parenchymal lung tissue for ILD diagnosis (3 patients [8%]), since most patients received their diagnosis of ILD based on symptoms, exposures, bloodwork, and imaging findings. Severity of ILD was moderate in most patients, with ILD-GAP Index of 0 to 2 in 36% (n = 14), 3 to 5 in 59% (n = 23), and greater than or equal to 6 in 5% (n = 2).
Survival and Oncologic Outcomes
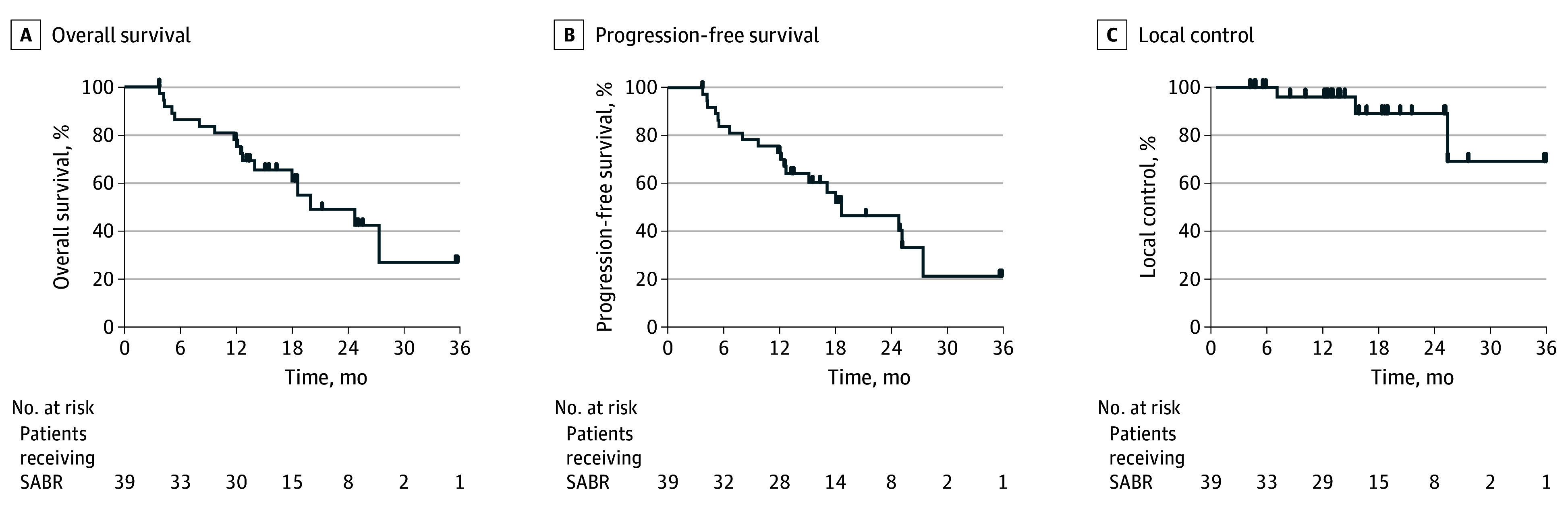
Median follow-up was 19 (IQR, 14-25) months. Kaplan-Meier estimates of OS, PFS, and LC are shown in Figure 2. There were 17 death events during the follow-up period, 1 of which was cancer-related due to distant disease progression. Overall survival at 1 year was 79% (95% CI, 62%-89%; P < .001 by binomial test vs the unacceptable rate), and median OS was 25 months (95% CI, 14 months to not reached). Overall survival between biopsy-proven vs non–biopsy-proven cohorts was not significantly different. Progression-free survival at 1 year was 74% (95% CI, 57%-85%), and median PFS was 19 (95% CI, 13-28) months. At 2 years, LC was 92% (95% CI, 69%-98%), regional control was 86% (95% CI, 60%-95%), and distant control was 91% (95% CI, 64%-98%) (eFigure 1 in Supplement 2). There were no significant differences in survival based on ILD-GAP Index; however, differences in OS and PFS were observed across ILD subtypes (eFigure 2 in Supplement 2), with IPF, CTD-ILD, and chronic hypersensitivity pneumonitis subtypes demonstrating lower OS than those with other/unclassifiable ILD. The 1-year overall survival ranged from 62.5% (95% CI, 22.9%-86.1%) to 100% (95% CI, 100%-100%) (P = .02) and progression-free survival ranged from 50.0% (95% CI, 15.2%-77.5%) to 100% (95% CI, 100%-100%) (P = .03), depending on subtype. Adjusting for competing risk of death in a post hoc analysis, at 2 years, the cumulative incidence of local failure was 6.2% (95% CI, 1.0%-19%), regional failure was 10% (95% CI, 2.4%-25%), and distant failure was 7.2% (95% CI, 1.1%-22%). Similarly, there were no significant differences in survival based on ILD-GAP Index or ILD subtype adjusting for competing risk of death.
Figure 2. Kaplan-Meier Plots for All 39 Patients Receiving Stereotactic Ablative Radiotherapy (SABR).
Adverse Event Rates
Adverse event rates are reported in Table 2. Overall adverse event rates (highest grade per patient, with some patients experiencing more than 1 toxic effect) were 30.8% for grade 1 to 2 (n = 12), 10.3% for grade 3 (n = 4), none for grade 4, and 7.7% for grade 5 (n = 3). All 3 grade 5 events were due to respiratory deterioration at 3.7, 4.2, and 13 months postenrollment, 2 in patients with CTD-ILD and 1 in a patient with IPF. The 14 deaths deemed unrelated to SABR were due to cardiac causes (heart failure, myocardial infarction, congestive heart failure n = 3); 1 each of progression of disease, head trauma due to a fall, COVID-19, failure of donor lungs after bilateral transplant, kidney failure, multiple organ failure, and pulmonary embolus; and unknown cause (without evidence of respiratory deterioration, n = 4). Overall rates did not differ significantly by ILD-GAP category or ILD subtype.
Table 2. Summary of Adverse Events Related to SABR for All 39 Patients Receiving SABR.
| Adverse event | Grade | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Back pain | 1 | 0 | 0 | 0 | 0 |
| Bronchopulmonary hemorrhage | 0 | 0 | 1 | 0 | 0 |
| Cough | 1 | 0 | 0 | 0 | 0 |
| Dyspnea | 1 | 3 | 2 | 0 | 2 |
| Esophagitis | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 4 | 1 | 0 | 0 | 0 |
| Lung infection | 0 | 0 | 1 | 0 | 0 |
| Nausea | 0 | 1 | 0 | 0 | 0 |
| Noncardiac chest pain | 0 | 1 | 0 | 0 | 0 |
| Pleural effusion | 0 | 1 | 0 | 0 | 0 |
| Pneumonitis | 0 | 1 | 2 | 0 | 0 |
| Pulmonary fibrosis | 2 | 0 | 0 | 0 | 0 |
| Respiratory failure | 0 | 0 | 0 | 0 | 1 |
Abbreviation: SABR, stereotactic ablative radiotherapy.
Quality of Life and Respiratory Status
Quality of life scores over time for the FACT-L total, lung cancer subscale, and cough severity scores are shown in Figure 3, with the physical, social, emotional, and functional well-being subscales and other metrics provided in eFigure 3 and the eTable in Supplement 2. FACT-L scores decreased over time (estimate: −1.31; 95% CI, −2.71 to 0.10; P = .07), with significant decreases in the social (estimate: −0.41; 95% CI, −0.78 to −0.04; P = .03) and functional (estimate: −0.52; 95% CI, −0.99 to −0.04; P = .03) well-being subdomains, and also in the FACT-L Trial Outcome Index, which combines the physical, functional, and lung cancer subscale subdomains (estimate: −0.96; 95% CI, −1.80 to −0.13; P = .03). Cough severity scale scores worsened over time ((estimate: 2.70; 95% CI, 0.40-5.01; P = .02). Pulmonary function test results over time are shown in eFigure 4 in Supplement 2. Comparing last-available pulmonary function test results with baseline, DLCO decreased (median, −4% [IQR, −12% to 4%]; P = .046), FVC decreased (median, −2.5% [IQR, −7% to 1%]; P = .11), and FEV1 remained stable (median change, 0% [IQR, −3% to 2%]). This corresponded with median times from enrollment of 13 (IQR, 7.4-19) months for DLCO, 13 (IQR, 7.3-18) months for FVC, and 12 (IQR, 6.8-18) months for FEV1.
Figure 3. Changes in Quality of Life Over Time.
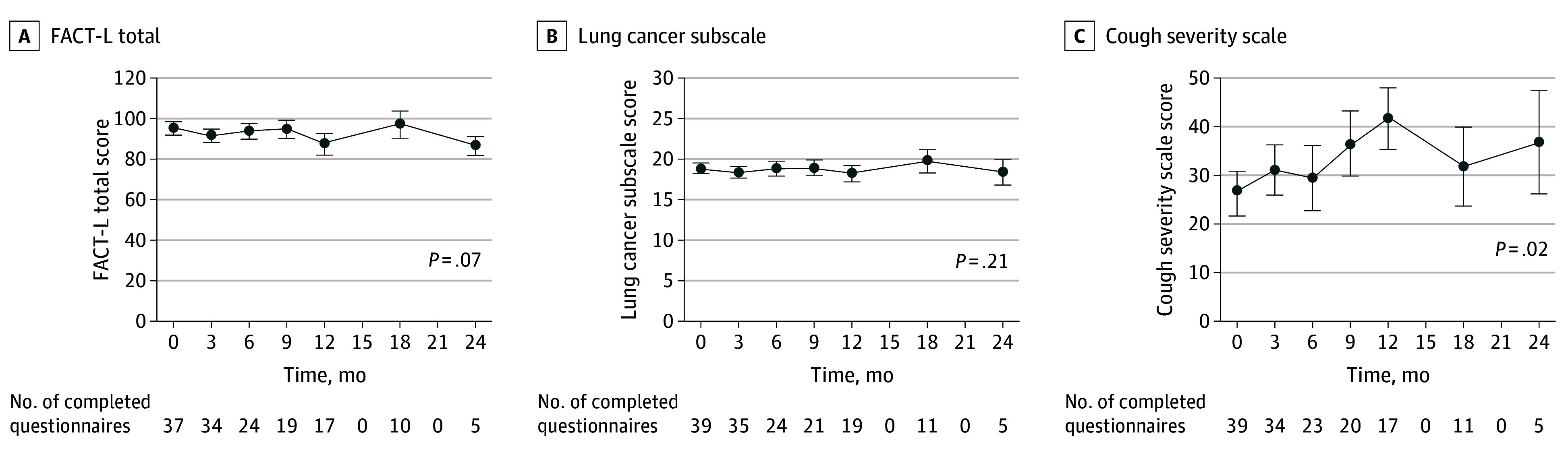
FACT-L indicates Functional Assessment of Cancer Therapy: Lung. Error bars represent SEs.
Untreated Patients
Three patients elected to proceed with observation alone, without SABR, and their baseline characteristics are reported in Table 1. All had ILD-GAP Index scores of 3 to 5, 2 had a diagnosis of IPF, and 1 had a diagnosis of CTD-ILD. Two of these patients died within the follow-up period (at 3.7 months from a medically assisted death and at 13 months from an unknown cause), with 1 patient alive at 25 months of follow-up, and the median survival of these 3 patients was 13 (IQR, 8.4-19) months.
Discussion
In this population of patients with ILD and early-stage NSCLC, SABR was associated with a median survival more than double the expected median survival in untreated patients, meeting the primary end point of the trial. The observed risk of grade 3 to 5 toxic effects was approximately half of the risk reported in a prior systematic review.8 These differing results could be due to some of the features of the trial: the careful planning of SABR, the use of a minimum SABR dose (eg, 50 Gy in 5 fractions), or the careful assessment and follow-up by respirology during this study. Conversely, systematic reviews8,9 may have overestimated toxicity if institutions observing treatment-related fatalities in patients with ILD then proceed to publish their results but those without toxicity do not. Regardless of the mechanism, these data help to inform the discussion with patients regarding the risks and benefits of SABR for early-stage NSCLC in the setting of ILD.
The toxic effect rates observed in this trial are within the range accepted in oncology in the delivery of curative-intent treatment. In the setting of oncologic surgery, several interventions are associated with 90-day mortality risks in the range of 4% to 8%, including pneumonectomy,20 esophagectomy,21 and pancreatectomy.22 In the context of radiotherapy, accepted treatment-related mortality risks have generally been lower than with surgery, although high-risk situations exist. For example, in recurrent head and neck cancers, curative-intent reirradiation with chemotherapy is offered to selected patients, with associated grade 5 toxic effect rates of 7% to 8% and grade 3 to 4 toxic effect rates of 39% to 55% in prospective trials.23,24
In any patient with ILD and early-stage NSCLC, alternate curative-intent treatment options besides SABR should also be considered. Some patients with ILD may be amenable to surgery, although they are at higher risk of complications than patients without ILD.25 Thermal ablative techniques, such as radiofrequency ablation, may also be considered; however, their relative safety benefits compared with SABR are unclear. Observation is also an option worthy of consideration, particularly in patients with documented slow-growing lesions (eg, adenocarcinomas arising from ground-glass lesions), but most patients with ILD have life expectancies that exceed the expected survival duration with untreated NSCLC.11
Limitations
The findings of this trial should be considered in the context of its limitations. Most patients had ILD-GAP indices of 0 to 5, and therefore the conclusions may not be generalizable to patients with higher scores. Patients with ILD-GAP indices of 6 or higher are also expected to have a shorter ILD-related life expectancy, altering the risk-benefit ratio when treatment is being considered. The sample size of 39, although adequately powered for the primary end point, does not allow for robust conclusions within any subgroups. Although all patients had ILD, the subtype of ILD was often not classifiable, since most patients did not have a surgical lung biopsy (ie, a biopsy of lung parenchyma) for an ILD diagnosis. Although we were careful in estimating toxic effect risks by automatically attributing pulmonary toxic effects to radiation, there remains the risk that toxic effect rates are underestimated, and future studies with a larger sample size would be helpful in this regard. In assessing toxic effect risks in comparison with the prespecified hypotheses, we relied only on point estimates and did not power the trial to definitely exclude the acceptability thresholds from the 95% CIs of our toxic effect rates. In addition, very few patients elected to undergo observation only, precluding any conclusions pertaining to that approach.
Conclusions
In this nonrandomized clinical trial, the use of SABR in patients with ILD met prespecified acceptability thresholds for both toxicity and efficacy, supporting the use of SABR for curative-intent treatment after a careful discussion of risks and benefits. Further studies exploring pharmacologic options to reduce radiotherapy-induced toxic effects may be beneficial in this population.
Trial Protocol
eMethods. Supplemental Statistical Methods
eFigure 1. Regional Control and Distant Control Estimates for Patients Receiving SABR
eFigure 2. Kaplan-Meier Plots for All Patients Receiving SABR (n = 39) for Overall Survival
eTable. Changes in Quality of Life Over Time for All Patients Receiving SABR
eFigure 3. Changes in Quality of Life Over Time for Physical Well-Being, Social Well-Being, Emotional Well-Being, Functional Well-Being, FACT-G Total, Trial Outcome Index, EQ-5D-5L Health State, and EQ-5D-5L Index
eFigure 4. Changes in Pulmonary Function Over Time for Percent Predicted for FEV1, FVC, and DLCO
Data Sharing Statement
References
- 1.NCCN Clinical practice guidelines in oncology - non-small cell lung cancer version 1. 2023, December 22, 2022, https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 2.Boily G, Filion É, Rakovich G, et al. ; Comité de l’évolution des pratiques en oncologie . Stereotactic ablative radiation therapy for the treatment of early-stage non–small-cell lung cancer: CEPO review and recommendations. J Thorac Oncol. 2015;10(6):872-882. doi: 10.1097/JTO.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Kang J, Ren S, Xing L, Xu Y. Comparison of stereotactic body radiotherapy and radiofrequency ablation for early-stage non–small cell lung cancer: a systematic review and meta-analysis. Ann Transl Med. 2022;10(2):104. doi: 10.21037/atm-21-6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82(3):1149-1156. doi: 10.1016/j.ijrobp.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Bahig H, Filion E, Vu T, et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6(5):367-374. doi: 10.1016/j.prro.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 6.Goodman CD, Nijman SFM, Senan S, et al. ; International Association for the Study of Lung Cancer Advanced Radiation Technology Committee . A primer on interstitial lung disease and thoracic radiation. J Thorac Oncol. 2020;15(6):902-913. doi: 10.1016/j.jtho.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Türkkan G, Willems Y, Hendriks LEL, et al. Idiopathic pulmonary fibrosis: current knowledge, future perspectives and its importance in radiation oncology. Radiother Oncol. 2021;155:269-277. doi: 10.1016/j.radonc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Senan S, Nossent EJ, et al. Treatment-related toxicity in patients with early-stage non–small cell lung cancer and coexisting interstitial lung disease: a systematic review. Int J Radiat Oncol Biol Phys. 2017;98(3):622-631. doi: 10.1016/j.ijrobp.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Saha A, Dickinson P, Shrimali RK, Salem A, Agarwal S. Is thoracic radiotherapy an absolute contraindication for treatment of lung cancer patients with interstitial lung disease? a systematic review. Clin Oncol (R Coll Radiol). 2022;34(12):e493-e504. doi: 10.1016/j.clon.2022.01.043 [DOI] [PubMed] [Google Scholar]
- 10.Palma DA, Chen H, Bahig H, et al. Assessment of precision irradiation in early non-small cell lung cancer and interstitial lung disease (ASPIRE-ILD): study protocol for a phase II trial. BMC Cancer. 2019;19(1):1206. doi: 10.1186/s12885-019-6392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723-728. doi: 10.1378/chest.13-1474 [DOI] [PubMed] [Google Scholar]
- 12.Onishi H, Nagata Y, Shirato H, et al. Stereotactic body radiotherapy (SBRT, BED ≥ 100 Gy) for operable stage I non–small cell lung cancer: is SBRT comparable to surgery? Int J Radiat Oncol Biol Phys. 2007;69(3)(suppl):S86-S87. doi: 10.1016/j.ijrobp.2007.07.157 [DOI] [PubMed] [Google Scholar]
- 13.Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol. 2019;37(15):1316-1325. doi: 10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non–small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153-5159. doi: 10.1200/JCO.2010.30.0731 [DOI] [PubMed] [Google Scholar]
- 15.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705-1712. doi: 10.1200/JCO.2006.08.1455 [DOI] [PubMed] [Google Scholar]
- 16.Chadha AS, Ganti AK, Sohi JS, Sahmoun AE, Mehdi SA. Survival in untreated early stage non–small cell lung cancer. Anticancer Res. 2005;25(5):3517-3520. [PubMed] [Google Scholar]
- 17.Rosen JE, Keshava HB, Yao X, Kim AW, Detterbeck FC, Boffa DJ. The natural history of operable non–small cell lung cancer in the National Cancer Database. Ann Thorac Surg. 2016;101(5):1850-1855. doi: 10.1016/j.athoracsur.2016.01.077 [DOI] [PubMed] [Google Scholar]
- 18.Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non–small cell lung cancer. Radiother Oncol. 2017;124(1):11-17. doi: 10.1016/j.radonc.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 19.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444-450. doi: 10.1016/j.ijrobp.2012.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun J, Choi YS, Hong TH, et al. Nononcologic mortality after pneumonectomy compared to lobectomy. Semin Thorac Cardiovasc Surg. 2022;34(3):1122-1131. doi: 10.1053/j.semtcvs.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 21.D’Journo XB, Boulate D, Fourdrain A, et al. ; International Esodata Study Group . Risk prediction model of 90-day mortality after esophagectomy for cancer. JAMA Surg. 2021;156(9):836-845. doi: 10.1001/jamasurg.2021.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the National Cancer Data Base. Ann Surg Oncol. 2014;21(13):4059-4067. doi: 10.1245/s10434-014-4036-4 [DOI] [PubMed] [Google Scholar]
- 23.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30(3):281-288. doi: 10.1002/hed.20697 [DOI] [PubMed] [Google Scholar]
- 24.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26(34):5518-5523. doi: 10.1200/JCO.2007.15.0102 [DOI] [PubMed] [Google Scholar]
- 25.Tao H, Onoda H, Okabe K, Matsumoto T. The impact of coexisting lung diseases on outcomes in patients with pathological stage I non–small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2018;26(6):1009-1015. doi: 10.1093/icvts/ivx441 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Supplemental Statistical Methods
eFigure 1. Regional Control and Distant Control Estimates for Patients Receiving SABR
eFigure 2. Kaplan-Meier Plots for All Patients Receiving SABR (n = 39) for Overall Survival
eTable. Changes in Quality of Life Over Time for All Patients Receiving SABR
eFigure 3. Changes in Quality of Life Over Time for Physical Well-Being, Social Well-Being, Emotional Well-Being, Functional Well-Being, FACT-G Total, Trial Outcome Index, EQ-5D-5L Health State, and EQ-5D-5L Index
eFigure 4. Changes in Pulmonary Function Over Time for Percent Predicted for FEV1, FVC, and DLCO
Data Sharing Statement