Key Points
Question
Is relugolix, an oral androgen deprivation therapy, safe and effective in patients receiving radiotherapy for prostate cancer?
Findings
In these 2 randomized clinical trials of 260 men with prostate cancer who were receiving treatment with radiotherapy and androgen deprivation therapy, relugolix achieved high castration rates in 95% or more of patients across localized, locally advanced, recurrent, and/or metastatic disease. Rapid testosterone recovery was achieved with relugolix, and no new safety concerns were identified in the radiotherapy setting.
Meaning
The results of these randomized clinical trials suggest that relugolix is safe and effective in patients receiving radiotherapy for prostate cancer.
Abstract
Importance
Combination androgen deprivation therapy (ADT) with radiotherapy is commonly used for patients with localized and advanced prostate cancer.
Objective
To assess the efficacy and safety of the oral gonadotropin-releasing hormone antagonist relugolix with radiotherapy for treating prostate cancer.
Design, Setting, and Participants
This multicenter post hoc analysis of patients with localized and advanced prostate cancer receiving radiotherapy in 2 randomized clinical trials (a phase 2 trial of relugolix vs degarelix, and a subset of the phase 3 HERO trial of relugolix vs leuprolide acetate) included men who were receiving radiotherapy and short-term (24 weeks) ADT (n = 103) from 2014 to 2015 and men receiving radiotherapy and longer-term (48 weeks) ADT (n = 157) from 2017 to 2019. The data were analyzed in November 2022.
Interventions
Patients receiving short-term ADT received relugolix, 120 mg, orally once daily (320-mg loading dose) or degarelix, 80 mg, 4-week depot (240-mg loading dose) for 24 weeks with 12 weeks of follow-up. Patients receiving longer-term ADT received relugolix, 120 mg, orally once daily (360-mg loading dose) or leuprolide acetate injections every 12 weeks for 48 weeks, with up to 90 days of follow-up.
Main Outcomes and Measures
Castration rate (testosterone level <50 ng/dL [to convert to nmol/L, multiply by 0.0347) at all scheduled visits between weeks 5 and 25 for patients receiving short-term ADT and weeks 5 and 49 for patients receiving longer-term ADT.
Results
Of 260 patients (38 Asian [14.6%], 23 Black or African American [8.8%], 21 Hispanic [8.1%], and 188 White [72.3%] individuals), 164 (63.1%) received relugolix. Relugolix achieved castration rates of 95% (95% CI, 87.1%-99.0%) and 97% (95% CI, 90.6%-99.0%) among patients receiving short-term and longer-term ADT, respectively. Twelve weeks post–short-term relugolix, 34 (52%) achieved testosterone levels to baseline or more than 280 ng/dL. Ninety days post longer-term ADT, mean (SD) testosterone levels were 310.5 (122.4) (106.7) ng/dL (relugolix; n = 15) vs 53.0 ng/dL (leuprolide acetate; n = 8) among the subset assessed for testosterone recovery. Castration resistance-free survival was not statistically different between the relugolix and leuprolide acetate cohorts (hazard ratio, 0.97; 95% CI, 0.35-2.72; P = .62). Adverse events grade 3 or greater for short-term or longer-term relugolix (headache, hypertension, and atrial fibrillation) were uncommon (less than 5%).
Conclusions and Relevance
The results of these 2 randomized clinical trials suggest that relugolix rapidly achieves sustained castration in patients with localized and advanced prostate cancer receiving radiotherapy. No new safety concerns were identified when relugolix was used with radiotherapy.
This study examines 2 randomized clinical trials to assess the efficacy and safety of the oral gonadotropin-releasing hormone antagonist relugolix with radiotherapy for treating prostate cancer.
Introduction
Androgen deprivation therapy (ADT), a cornerstone treatment used across the spectrum of prostate cancer, is often administered in combination with radiotherapy in patients with localized, locally advanced, recurrent, and metastatic disease.1,2,3 In randomized clinical trials, combinations of short-term and long-term ADT with radiotherapy have substantially improved overall and cancer-specific survival compared with radiotherapy alone, from localized to low-volume metastatic disease.4,5
Gonadotropin-releasing hormone (GnRH) receptor agonists are historically the most commonly used modalities to induce androgen deprivation.6 However, these agents are associated with initial testosterone surges, subsequent micro surges, and delayed castration for approximately 3 weeks, often necessitating the concurrent use of an antiandrogen.7,8,9,10 GnRH receptor agonists have been associated with an increased risk of cardiovascular events, especially in patients with a history of cardiovascular disease.11 While testosterone suppression is the goal of ADT, low testosterone levels may persist for years after treatment cessation, which can negatively affect patient overall health and quality of life.12,13 This is especially relevant for patients who receive radiotherapy, given that prolonged or lifelong castration is usually not the intention.
In contrast to GnRH receptor agonists, GnRH receptor antagonists rapidly suppress testosterone and prevent surges and subsequent clinical flares by directly inhibiting GnRH receptors.3,9,14 The limitations of prior injectable GnRH receptor antagonists led to the development of relugolix, a first-in-class, oral, highly selective nonpeptide GnRH receptor antagonist, taken once daily.3,14 Relugolix received US Food and Drug Administration approval in 2020 based on the primary results of the global randomized phase 3 HERO trial, in which relugolix demonstrated rapid and sustained suppression of testosterone levels superior to that of leuprolide acetate (96.7% vs 88.8%; P < .001), and a greater proportion of patients with testosterone recovered 3 months post ADT cessation (54% vs 3%).15,16 Given that ADT is commonly used for a finite duration when combined with radiotherapy, there is great interest in the use of relugolix with radiotherapy. In this article, we present results of the safety and efficacy of relugolix from a post hoc analysis of a subset of patients who received short-term or longer-term courses of ADT with radiotherapy from 2 randomized clinical trials.
Methods
This present study is a post hoc analysis of individual patient-level data from 2 randomized clinical trials evaluating relugolix and includes the subset of patients who received radiotherapy (Figure 1; Supplement 1 and Supplement 2). Both trials were approved by the institutional review board or independent ethics committee at each study site (Advarra [Columbia, Maryland], Copernicus Group [Durham, North Carolina], and the NRES Committee London [England]) and were conducted according to the requirements of the regulatory authorities of each country as well as the provisions of the Declaration of Helsinki 2013 and the Good Clinical Practice guidelines of the International Council for Harmonisation E6. All patients in both studies provided written informed consent.
Figure 1. Patient Identification.
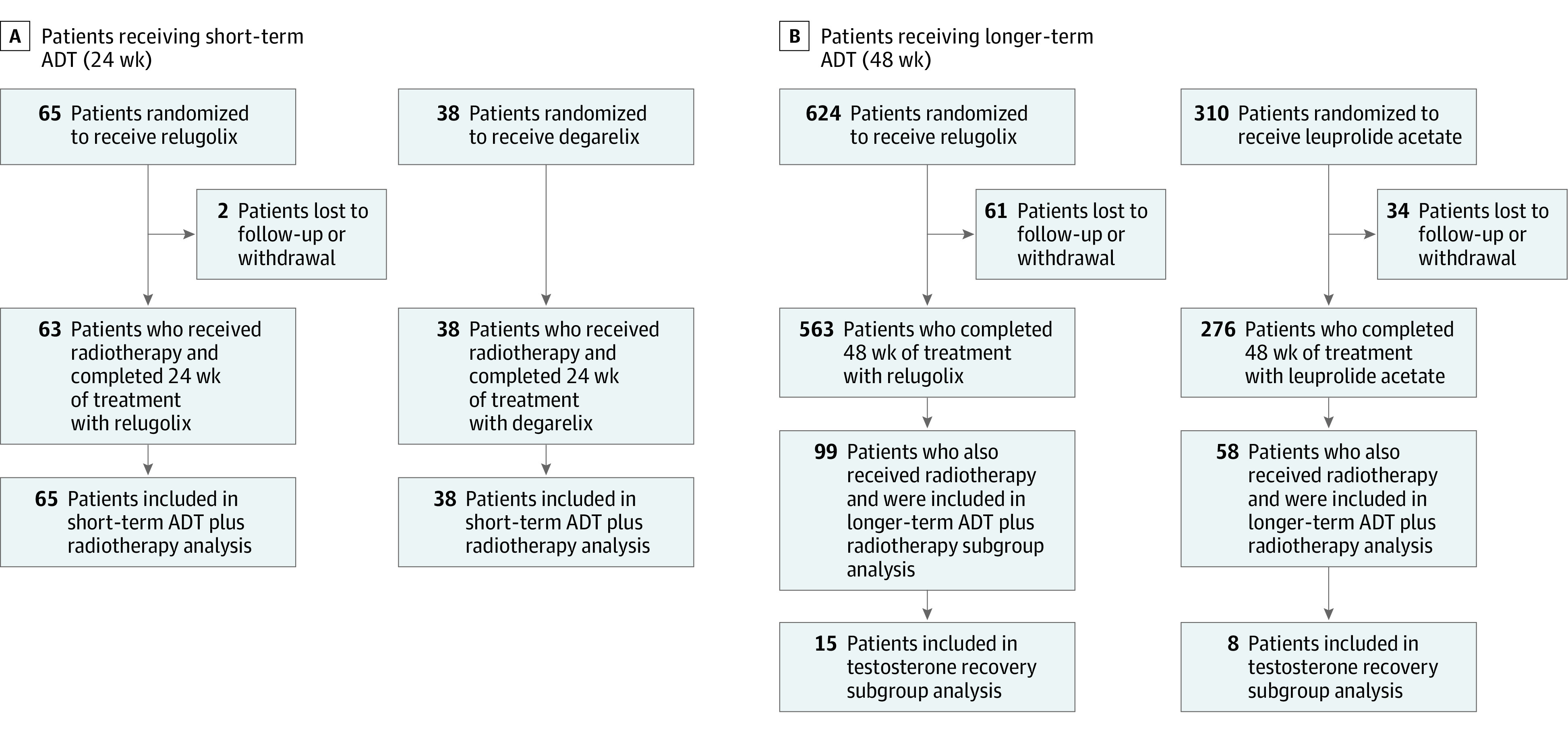
Identification of patients included in this post hoc analysis from 2 randomized clinical trials: patients receiving short-term (24 weeks) androgen deprivation therapy (ADT) plus radiotherapy who received relugolix or degarelix (A) and longer-term (48 weeks) ADT plus radiotherapy who received relugolix or leuprolide acetate (B).
The phase 2 C27003 study (NCT02135445) was conducted in patients with a new diagnosis of localized and locally advanced prostate cancer who required treatment with short-term ADT with primary external beam radiotherapy. The second trial was a subset analysis of the phase 3 HERO trial (NCT03085095) in patients with locally advanced, recurrent, or metastatic prostate cancer who required longer-term ADT (planned for 1 year or more of continuous ADT). Comprehensive inclusion and exclusion criteria for both studies can be found in eTable 1 in Supplement 3.
Detailed study designs of both trials have been published.15,17 Briefly, in the phase 2 study, patients were randomized 3:2 to receive relugolix, 120 mg, orally once daily (after a single loading dose of 320 mg), or degarelix, 80 mg, 4-week depot (after a single loading dose of 240 mg), for 24 weeks with 12 weeks of follow-up. Patients received neoadjuvant ADT for 12 to 16 weeks before initiating radiotherapy and adjuvant ADT for the remainder of the 24-week period. In the phase 3 HERO study, patients were randomized 2:1 to receive relugolix, 120 mg, orally once daily (after a single loading dose of 360 mg) or leuprolide acetate injections every 12 weeks for 48 weeks. Concomitant radiotherapy was permitted after 2 months of initiating ADT. After treatment cessation, all patients were followed for an additional 30 days; to assess testosterone recovery, a subset of patients was followed for 90 days or less.
Trial Assessments
Primary end points assessed for the 2 populations were similar, with the main difference being the length of time assessed. The primary end point for patients receiving short-term ADT with radiotherapy was effective castration rate, defined as the estimated proportion of patients who had a testosterone concentration below castrate levels (<50 ng/dL; to convert to nmol/L, multiply by 0.0347) at all scheduled visits from week 5 through week 24. The primary end point assessed for patients receiving longer-term ADT was the sustained castration rate, which was defined as the cumulative probability of testosterone suppression below castrate levels (<50 ng/dL) from week 5 through week 48.
Secondary efficacy end points reported for patients receiving short-term ADT with radiotherapy included time to achieve castration and profound castration (<20 ng/dL), prostate-specific antigen (PSA) nadir and the percentage change by the end of weeks 12 and 24, mean follicle-stimulating hormone (FSH) level by the end of week 24, and estimated time to testosterone recovery among patients who had recovered to their baseline value or more than 280 ng/dL during the 12 weeks following treatment discontinuation. Secondary end points reported for patients receiving longer-term ADT with radiotherapy included the cumulative probability of testosterone suppression of less than 50 ng/dL on day 4 and day 15, cumulative probability of profound testosterone suppression (<20 ng/dL) on day 15, time to castration resistant-free survival, the proportion of patients with a PSA response on day 15 followed with confirmation on day 29, mean FSH level at the end of week 24, mean time to testosterone suppression, and mean testosterone levels 90 days after treatment cessation among the patients assessed for testosterone recovery.
Safety according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, was monitored in both study populations during follow-up and included vital signs and a symptom-based physical examination. The onset and duration of the most common and grade 3 adverse events (AEs) are reported. The onset of an AE was defined as the time from the date of the first dose to the initial event. The duration of an AE was defined as the end date of the event minus the start date of the event, plus 1.
Statistical Analysis
For patients receiving short-term ADT with radiotherapy, the primary end point of effective castration rate, defined as the estimated proportion of patients who had testosterone concentrations of less than 50 ng/dL from week 5 through week 24, was calculated using a 2-sided 95% CI by the normal approximation method. Secondary end points, including time to castration (≤50 ng/dL), time to profound castration (<20 ng/dL), and time to testosterone recovery, were analyzed using the Kaplan-Meier method. PSA and FSH were summarized over time using descriptive statistics. The data cutoff date was December 14, 2015.
For patients receiving longer-term ADT with radiotherapy, the primary end point of sustained castration rate, defined as the cumulative probability of testosterone suppression to less than 50 ng/dL from week 5 through week 48, was calculated using a 2-sided 95% CI with a log-log transformation of survival function in each treatment cohort. Time to castration resistant-free survival was analyzed using the Kaplan-Meier method. Testosterone levels were summarized over time with descriptive statistics. A 95% exact CI was calculated for PSA response using a stratified Cochran-Mantel-Haenszel test. A 2-sample t test was used for the comparison between groups for FSH levels. The data cutoff date was October 25, 2019. Statistical analyses were performed using SAS, version 9.2 or higher (SAS Institute Inc), and statistical significance was set at P < .05.
Results
Patients
Of the 260 men included in this analysis, 164 (63.1%) received relugolix (Table 118). Short-term ADT (24 weeks) and radiotherapy were administered to 103 patients (39.6%), of whom 65 patients (63.1%) received relugolix. Longer-term ADT (48 weeks) and radiotherapy were administered to 157 patients (60.4%), of whom 99 patients (63.1%) received relugolix. The mean (SD) age was similar for patients receiving short-term (70.2 [5.7] years) and longer-term (69.9 [7.6] years) relugolix and radiotherapy. Patients receiving short-term ADT began enrollment in June 2014 and completed treatment in December 2015. Patients receiving longer-term ADT began enrollment in April 2017 and completed treatment in October 2019.
Table 1. Patient Demographic and Clinical Characteristics at Baseline.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Short-term (24 wk) ADT with radiotherapy | Longer-term (48 wk) ADT with radiotherapy | |||
| Relugolix (n = 65) | Degarelix (n = 38) | Relugolix (n = 99) | Leuprolide acetate (n = 58) | |
| Age, mean (SD), y | 70.2 (5.7) | 70.3 (7.0) | 69.9 (7.6) | 68.2 (7.5) |
| Ethnicity | ||||
| Hispanic or Latino | 2 (3) | 3 (8) | 10 (10) | 6 (10) |
| Not Hispanic or Latino | 62 (95) | 35 (92) | 83 (84) | 51 (88) |
| Not reported | 1 (2) | 0 | 6 (6) | 1 (2) |
| Race | ||||
| Asian | 0 | 0 | 26 (26) | 12 (21) |
| Black or African American | 7 (11) | 7 (18) | 5 (5) | 4 (7) |
| White | 58 (89) | 31 (82) | 60 (61) | 39 (67) |
| Other/multiraciala | 0 | 0 | 6 (6) | 2 (3) |
| Not reported | 0 | 0 | 2 (2) | 1 (2) |
| Disease state presentation | ||||
| Evidence of biochemical (PSA) or clinical relapse following local primary intervention with curative intent | NA | NA | 23 (23) | 20 (35) |
| Newly diagnosed androgen-sensitive metastatic disease | NA | NA | 16 (16) | 6 (10) |
| Advanced localized disease not suitable for primary surgical intervention with curative intent | NA | NA | 60 (61) | 32 (55) |
| Disease stage at study entryb | ||||
| Localized | 39 (60) | 19 (50) | 44 (44) | 21 (36) |
| Locally advanced | 13 (20) | 10 (26) | 33 (33) | 25 (43) |
| Metastatic | 0 | 0 | 22 (22) | 9 (16) |
| Not classifiablec | NAc | NAc | 0 | 3 (5) |
| Had prior ADTd | NA | NA | 5 (5) | 0 |
| Had prior radiotherapyd | NA | NA | 5 (5) | 3 (5) |
| Radiotherapy treatment intentd | ||||
| Primary | NA | NA | 60 (61) | 37 (64) |
| Salvage | NA | NA | 15 (15) | 10 (17) |
| Palliative | NA | NA | 24 (24) | 10 (17) |
| Unknown | NA | NA | 1 (1) | 1 (2) |
| Total Gleason scoree | ||||
| ≤6 | 5 (8) | 2 (5) | 16 (16) | 6 (10) |
| 7 | 40 (62) | 26 (68) | 35 (35) | 28 (48) |
| 8-10 | 7 (11) | 5 (13) | 47 (48) | 23 (40) |
| Unknown | 13 (20) | 5 (13) | 1 (1) | 1 (2) |
| ECOG performance statusf | ||||
| 0 | 60 (92) | 33 (87) | 87 (88) | 51 (88) |
| 1 | 4 (6) | 4 (11) | 12 (12) | 7 (12) |
| Unknown | 1 (2) | 1 (3) | 0 | 0 |
| PSA level, mean (SD), ng/mL | 9.4 (6.0) | 14.6 (21.0) | 100.4 (436.2) | 66.6 (227.0) |
| Testosterone level, mean (SD), ng/dL | 401.9 (191.0) | 416.1 (159.6) | 436.1 (147.3) | 400.1 (123.2) |
| FSH level, mean (SD), mIU/mLg | 11.8 (17.4) | 11.7 (14.8) | 12.9 (8.8) | 12.4 (12.5) |
Abbreviations: ADT, androgen deprivation therapy; ECOG, Eastern Cooperative Oncology Group; FSH, follicle-stimulating hormone; NA, not applicable; PSA, prostate-specific antigen.
SI conversion factors: to convert FSH to IU/L, multiply by 1; for PSA to μg/L, multiply by 1; for testosterone to nmol/L, multiply by 0.0347.
Other included Pacific Islander, American Indian, Alaska Native, Native Hawaiian, or self described other.
Disease stage at study entry was defined based on TNM stage at study entry;18 M1 as metastatic, T3/4 NX M0 or N1 M0 and any T N1 M0 as locally advanced, and T1 or T2 N0 M0 as localized.
For patients receiving short-term ADT with radiotherapy, baseline characteristics on tumors in distant metastasis were missing or unavailable for 14 patients in the relugolix cohort and 10 patients in the degarelix cohort; baseline characteristics on tumors in regional lymph nodes were missing or not available for 13 patients in the relugolix cohort and 9 patients in the degarelix cohort.
Almost all patients in this study were receiving radiotherapy with neoadjuvant/adjuvant ADT as the primary (first-line) therapy for their prostate disease. Thus, there were very limited data regarding prior therapies relevant to this study.
Gleason score was determined by adding primary and secondary Gleason scores together.
ECOG performance status score ranged from 0 to 5, with higher scores reflecting greater disability.
FSH normal range for adults: 1.5-12.4 IU/L.
There was some overlap in the disease stage of the patients receiving short-term and longer-term ADT when they entered the study (Table 1). Fifty-two of the 65 patients receiving short-term relugolix with radiotherapy had known Gleason scores. Forty-five (69.2%) had a Gleason score of less than 8, and 7 (10.8%) had a score of 8 or greater. During the study, all patients received external beam radiotherapy (eTable 2 in Supplement 3). Baseline demographic and clinical characteristics were similar for the degarelix cohort (Table 1).
Among the 99 patients receiving longer-term relugolix with radiotherapy, 60 (61%) presented with locally advanced disease, 23 (23%) with evidence of biochemical or clinical relapse, and 16 (16%) with newly diagnosed metastatic disease. During the study, 88 patients (89%) received external beam radiotherapy (eTable 2 in Supplement 3). Sixty (61%) received radiotherapy as primary therapy, with 15 (15%) and 24 (24%) receiving radiotherapy for salvage or palliative purposes, respectively. Baseline demographic and clinical characteristics for the leuprolide acetate cohort and overall HERO cohort are found in Table 1 and Shore et al.15 While the treatment cohorts were balanced in the total population, in this subgroup analysis, the relugolix cohort had higher PSA levels and a greater proportion of patients with metastatic disease and Gleason scores of greater than 8 than the leuprolide acetate cohort at baseline.
Efficacy
Short-Term ADT With Radiotherapy
The effective castration rate over 24 weeks was 95% (95% CI, 87.1%-99.0%) in the relugolix cohort and 89% (95% CI, 75.2%-97.1%) in the degarelix cohort.17 For the relugolix cohort, the secondary end points of median time to achieve testosterone levels of less than 50 ng/dL and less than 20 ng/dL were 4 days and 15 days, respectively (Figure 2A). PSA levels steadily declined through the study, with percentage changes reaching 97.4% (mean [SD] PSA nadir of 0.3 ng/mL [0.7]; to convert to μg/L, multiply by 1). FSH levels were suppressed to 1.5 mIU/mL (to convert to IU/L, multiply by 1) in the relugolix cohort through week 24. Testosterone suppression to castrate levels occurred rapidly in both cohorts, while time to testosterone recovery was more rapid for the relugolix cohort vs the degarelix cohort (Figure 3A). Thirty-four (52%) and 6 patients (16%) achieved testosterone levels to baseline or 280 ng/dL within 12 weeks of treatment discontinuation in the relugolix and degarelix cohorts, respectively.
Figure 2. Secondary End Points Associated With Testosterone, Prostate-Specific Antigen (PSA), and Follicle-Stimulating Hormone (FSH) Levels in Patients Treated With Androgen Deprivation Therapy (ADT) Plus Radiotherapy.

Secondary end points associated with testosterone (to convert to nmol/L, multiply by 0.0347), PSA, and FSH levels in patients with prostate cancer receiving short-term ADT (24 weeks) (A) and longer-term ADT (48 weeks) (B) with radiotherapy. Dark blue bars represent relugolix across both panels, the medium blue bars represent degarelix, and the light blue bars represent leuprolide acetate.
aFSH normal values for adults: 1.5-12.4 mIU/mL (to convert to IU/L, multiply by 1).
Figure 3. Testosterone Levels Over Time in Patients Treated With Androgen Deprivation Therapy (ADT) Plus Radiotherapy.

Time to testosterone (T) suppression (<50 ng/dL; to convert to nmol/L, multiply by 0.0347) and time to testosterone recovery (baseline or >280 ng/dL) in patients treated with relugolix or degarelix for 24 weeks plus radiotherapy (A) and time to testosterone suppression (<50 ng/dL) and time to testosterone recovery (≥50 ng/dL) in patients treated with relugolix or leuprolide acetate for 48 weeks plus radiotherapy (B). 50 ng/dL = castrate level; 280 ng/dL = lower limit of normal range. A subset of patients (relugolix [n = 15]; leuprolide acetate [n = 8]) in the HERO radiotherapy subgroup was assessed for testosterone recovery for up 90 days post treatment cessation. At the 90-day follow-up, testosterone levels were reported for 14 of 15 patients in the relugolix cohort.
aTime to castration-free survival was defined as the number of days from the first dose to the first testosterone measurement of less than 50 ng/dL. If a patient had all post–first dose testosterone measurements 50 ng/dL or greater, the patient’s time to castration was censored at the last testosterone measurement that was 50 ng/dL or greater. Censorship was defined as the patient initiated an alternate ADT without recovery at the last testosterone laboratory assessment.
bTime to testosterone recovery-free survival was defined as the time from 1 day after the last dose of relugolix or 4 weeks plus 1 day after the last dose of degarelix to testosterone recovery. Testosterone recovery was defined as testosterone levels back to baseline or more than 280 ng/dL, whichever occurred first. It was censored for patients initiating alternative ADT without recovery at the last testosterone laboratory assessment before the initiation of ADT.
Longer-Term ADT With Radiotherapy
The sustained castration rate over 48 weeks was 96.9% (95% CI, 90.6%-99.0%) in the relugolix cohort and 96.4% (95% CI, 86.4%-99.1%) in the leuprolide acetate cohort. For the relugolix cohort, the secondary end points of day 4 and day 15 testosterone suppression rates (<50 ng/dL) were 59.6% and 100%, respectively, compared with 0% (day 4) and 10.3% (day 15) in the leuprolide acetate cohort (Figure 2B). Relugolix achieved a rapid PSA response in more patients compared with leuprolide acetate by day 15 (75.8% vs 19.0%). Additionally, FSH levels at week 24 were suppressed to 1.6 mIU/mL in the relugolix cohort compared with 5.5 mIU/mL in the leuprolide acetate cohort. The eFigure in Supplement 3 presents these end points as continuous data in box plots.
Testosterone suppression to castrate levels occurred rapidly in the relugolix cohort, which achieved a mean testosterone level of 38 ng/dL on day 4 (Figure 3B). In contrast, testosterone levels briefly surged from baseline to day 4 in the leuprolide acetate cohort before decreasing to castrate levels by day 29. Mean (SD) testosterone levels assessed 90 days after treatment discontinuation for the testosterone recovery subgroup were 310.5 (122.4) ng/dL for the relugolix cohort compared with 53.0 (106.7) ng/dL for the leuprolide acetate cohort. Castration resistance-free survival was not statistically different between the cohorts (hazard ratio, 0.97; 95% CI, 0.35-2.72; P = .62).
Safety
Short-Term ADT With Radiotherapy
Hot flashes, the most common AE of any grade in both groups, was experienced by 37 of 65 patients (57%) and 23 of 38 patients (61%) in the relugolix and degarelix cohorts, respectively (Table 2). Onset of symptoms was experienced at a mean (SD) of 19.3 (16.3) days in the relugolix cohort and 28.9 (27.3) days in the degarelix cohort for a mean (SD) duration of 186.0 (75.6) and 201.2 (73.5) days, respectively. Other AEs experienced by more than 10% of the population in both cohorts included fatigue, diarrhea, cataract, nocturia, and pollakiuria. Symptom onset and the duration of each are reported in Table 2. Grade 3 or higher AEs experienced by more than 2% of the population included headache and hypertension in the relugolix cohort (n = 1 each).
Table 2. Onset and Duration of Grade 3 or Higher (≥2% of Population) and Most Common (≥10% of Population) AEsa,b.
| AE | No. (%) | |
|---|---|---|
| Relugolix (n = 65) | Degarelix (n = 38) | |
| For patients receiving short-term ADT (24 wk) with radiotherapy | ||
| Grade 3 or higher in ≥2% of population | ||
| Headache | 1 (2) | 0 |
| Time to AE, dc | 173.0 (NE) | NE |
| Duration of AE, dc | 43.0 (NE) | NE |
| Hypertension | 1 (2) | 0 |
| Time to AE, d | 173.0 (NE) | NE |
| Duration of AE, d | 13.0 (NE) | NE |
| Any grade in ≥10% of population | ||
| Hot flash | 37 (57) | 23 (61) |
| Time to AE, d | 19.3 (16.3) | 28.9 (27.3) |
| Duration of AE, d | 186.0 (75.6) | 201.2 (73.5) |
| Fatigue | 17 (26) | 6 (16) |
| Time to AE, d | 48.6 (45.9) | 51.7 (35.2) |
| Duration of AE, d | 170.4 (82.4) | 166.8 (48.4) |
| Diarrhea | 12 (19) | 5 (13) |
| Time to AE, d | 100.1 (66.7) | 131.0 (25.0) |
| Duration of AE, d | 61.4 (91.9) | 24.2 (23.8) |
| Cataract | 10 (15) | 7 (18) |
| Time to AE, d | 119.0 (81.5) | 73.7 (90.7) |
| Duration of AE, d | 213.5 (170.8) | 181.1 (102.3) |
| Nocturia | 9 (14) | 5 (13) |
| Time to AE, d | 90.1 (65.9) | 107.8 (378.0) |
| Duration of AE, d | 121.3 (84.9) | 148.4 (39.6) |
| Pollakiuria | 8 (12) | 5 (13) |
| Time to AE, d | 60.5 (57.0) | 109.4 (29.2) |
| Duration of AE, d | 170.6 (63.8) | 122.8 (62.5) |
| Relugolix (n = 99) | Leuprolide acetate (n = 58) | |
| For patients receiving longer-term ADT (48 wk) with radiotherapy | ||
| Grade 3 or higher in ≥2% of population | ||
| Atrial fibrillation | 2 (2) | 0 |
| Time to AE, d | 203.5 (89.8) | NE |
| Duration of AE, d | 9.0 (1.4) | NE |
| Hypertension | 2 (2) | 1 (2) |
| Time to AE, d | 58.0 (50.9) | 89.0 (NE) |
| Duration of AE, d | 165.0 (230.5) | 51.0 (NE) |
| Any grade in ≥10% of population | ||
| Hot flash | 54 (55) | 25 (43) |
| Time to AE, d | 30.7 (42.2) | 34.2 (26.0) |
| Duration of AE, d | 306.0 (102.2) | 312.9 (94.7) |
| Fatigue | 29 (29) | 10 (17) |
| Time to AE, d | 73.1 (80.7) | 96.6 (105.2) |
| Duration of AE, d | 272.4 (102.5) | 234.7 (142.7) |
| Diarrhea | 25 (25) | 9 (16) |
| Time to AE, d | 150.1 (103.1) | 149.3 (80.4) |
| Duration of AE, d | 68.1 (80.6) | 56.9 (84.6) |
| Constipation | 22 (22) | 7 (12) |
| Time to AE, d | 108.0 (88.7) | 56.3 (64.0) |
| Duration of AE, d | 116.0 (130.5) | 132.6 (147.1) |
| Arthralgia | 14 (14) | 6 (10) |
| Time to AE, d | 143.9 (119.0) | 179.2 (141.4) |
| Duration of AE, d | 158.0 (109.7) | 161.0 (159.9) |
| Back pain | 14 (14) | 8 (14) |
| Time to AE, d | 135.6 (107.4) | 144.4 (117.5) |
| Duration of AE, d | 121.7 (116.7) | 140.5 (149.6) |
| Dysuria | 12 (12) | 4 (7) |
| Time to AE, d | 126.2 (83.9) | 130.5 (119.7) |
| Duration of AE, d | 91.9 (77.4) | 74.3 (45.4) |
| Nocturia | 11 (11) | 5 (9) |
| Time to AE, d | 79.5 (66.4) | 79.0 (27.9) |
| Duration of AE, d | 153.1 (124.0) | 197.0 (107.0) |
| Weight increase | 10 (10) | 3 (5) |
| Time to AE, d | 267.0 (102.2) | 169.0 (145.5) |
| Duration of AE, d | 82.4 (65.7) | 200.7 (146.9) |
Abbreviations: ADT, androgen deprivation therapy; AE, adverse event; NE, not estimable.
Onset or time to AE is defined as the time from the date of the first dose to the initial adverse event.
Duration of AE is defined as the end date of the AE – start date of the AE + 1.
Time to AE and duration of AE measured as mean (SD).
Longer-Term ADT With Radiotherapy
AEs that were experienced by more than 10% of the population in the relugolix cohort included hot flash, fatigue, diarrhea, constipation, arthralgia, back pain, dysuria, nocturia, and weight increase (Table 2). Select AEs were seen with numerically higher frequency in the relugolix vs the leuprolide acetate cohort; however, time of event onset and duration were similar between cohorts, with large intracohort variation. Grade 3 or higher AEs were uncommon; 2 patients each experienced grade 3 or higher atrial fibrillation and hypertension in the relugolix cohort. In the leuprolide acetate cohort, 1 patient experienced grade 3 or higher hypertension.
Discussion
Results from patients receiving relugolix and radiotherapy in the phase 2 C27003 and phase 3 HERO trials expand our growing knowledge base on the efficacy and safety of the oral GnRH receptor antagonist relugolix in combination with radiotherapy. Relugolix appears safe, with no new safety concerns reported in patients who received this combination. Short-term (24 weeks) treatment with relugolix had rapid testosterone suppression, rapid PSA suppression, and faster testosterone recovery than the degarelix cohort. Similar results were shown with longer-term (48 weeks) relugolix treatment, with results being comparable between this radiotherapy subgroup analysis and the HERO primary analysis.15 Additionally, there appeared to be no difference in time to castration resistant-free survival between the relugolix and leuprolide acetate cohorts in this radiotherapy subgroup analysis. These results provide evidence of the clinical use of this therapy for patients receiving radiotherapy plus ADT.
The recent Meta-Analysis of Randomized trials in Cancer of the Prostate consortium of 12 randomized clinical trials quantified the absolute and relative benefit of short-term and long-term ADT with radiotherapy for men with intermediate-risk and high-risk prostate cancer.4 Although biomarkers are sought after to optimally select which patients require ADT, it is clear that in unselected groups of patients, there is an oncologic benefit of the combination of radiotherapy plus ADT. However, the adverse effects, logistics, and psychological challenges patients experience from receiving traditional GnRH receptor agonist treatments are nontrivial.10,19 The therapeutic burden of these forms of ADT frequently lead patients to either decline to receive ADT or not meet guideline-concordant durations of ADT. The challenges include requirements to take time off work to travel to a hospital for an injection, fear of needles or potential injection site reactions, use of concurrent antiandrogen therapy to block the testosterone flare, prolonged recovery to normal testosterone, and the inability to rapidly discontinue ADT if they do not tolerate the adverse effects.10
Relugolix, like all prior US Food and Drug Administration–approved forms of ADT, reached the therapeutic bar of sustained castration. There was superior sustained castration compared with leuprolide acetate in the HERO phase 3 trial. The kinetics of testosterone recovery and delivery route of relugolix are favorable, especially for patients receiving finite durations of ADT. The ability of relugolix to suppress testosterone within hours and achieve deep levels of sustained castration and very rapid testosterone recovery make it highly desirable as a form of ADT to be used in combination with radiotherapy. Treatment with GnRH receptor agonists for even just 6 months has been shown to have a 19-month time to testosterone recovery, with approximately 30% of men not achieving normal testosterone levels within 18 months.20 In men receiving long-term ADT (median duration of 24 months), it takes multiple years to recover normal T levels, with 47% of men not recovering normal testosterone levels within 2 years.21 In contrast, even after 48 weeks of relugolix treatment, most men recovered to normal testosterone levels (≥280 ng/dL) within 90 days after discontinuing ADT (vs <5% with leuprolide acetate).15
Prior trials and meta-analyses, including the HERO trial, demonstrated the potential for fewer major adverse cardiovascular events with GnRH receptor antagonists compared with agonists,15,22,23 although to our knowledge other studies have not shown this benefit.24,25,26 Other AEs were generally similar for patients in the HERO trial who did or did not receive radiotherapy. While rates of diarrhea were higher in patients who received radiotherapy than in the total HERO population (22% vs 10%), they were mostly considered mild (grades 1 and 2) and presented for a duration of less than 70 days. Similarly, these rates were increased in patients who received relugolix compared with leuprolide acetate, as shown in the overall study population.
Limitations
There were limitations to this study. Neither of the 2 randomized clinical trials included were designed to collect long-term oncologic outcomes, such as thromboembolic events or PSA failure; however, an ongoing study (OPTYX [NCT05467176]) has been designed to assess longer-term safety and effectiveness outcomes. The radiotherapy subset analysis of the HERO phase 3 trial was unplanned, and complete details of radiotherapy dose and volume irradiated were not collected. Similarly, clinicopathologic prognostic features to better define patients’ disease state were not collected (eg, percentage of positive cores, presalvage radiotherapy PSA) given the international nature and the trial’s primary end point of sustained castration. Lastly, the HERO study included patients who received palliative and definitive/postoperative radiotherapy.
Conclusions
In this post hoc analysis of 2 randomized clinical trials, relugolix appeared safe and effective at inducing rapid and sustained testosterone suppression across patients treated with short-term and longer-term ADT in combination with radiotherapy. Relugolix has more rapid testosterone recovery compared with degarelix, when used as short-term ADT, or leuprolide acetate, when used as longer-term ADT. The ability for clinicians and patients to reliably control castration for desired durations makes this an optimal treatment option for many patients receiving radiotherapy and ADT.
C27003 Study Trial Protocol
HERO Study Trial Protocol
eTable 1. Eligibility Criteria for Patients Receiving Short- and Longer-term ADT
eTable 2. Radiation Therapy Received During Study
eFigure. Box Plots of Secondary Endpoints Related to Testosterone, PSA, and FSH levels in Patients Receiving ADT Plus Radiotherapy
Data sharing statement
References
- 1.Merseburger AS, Alcaraz A, von Klot CA. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016;9:7263-7274. doi: 10.2147/OTT.S117176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network . Clinical practice guidelines in oncology: prostate cancer, version 2. Accessed October 27, 2022. https://jnccn.org/view/journals/jnccn/17/5/article-p479.xml
- 3.Shore ND, Abrahamsson PA, Anderson J, Crawford ED, Lange P. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer Prostatic Dis. 2013;16(1):7-15. doi: 10.1038/pcan.2012.25 [DOI] [PubMed] [Google Scholar]
- 4.Kishan AU, Sun Y, Hartman H, et al. ; MARCAP Consortium Group . Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol. 2022;23(2):304-316. doi: 10.1016/S1470-2045(21)00705-1 [DOI] [PubMed] [Google Scholar]
- 5.Parker CC, James ND, Brawley CD, et al. ; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy Investigators . Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366. doi: 10.1016/S0140-6736(18)32486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta E, Guthrie T, Tan W. Changing paradigms in management of metastatic castration resistant prostate cancer (mCRPC). BMC Urol. 2014;14:55. doi: 10.1186/1471-2490-14-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71(6):1001-1006. doi: 10.1016/j.urology.2007.12.070 [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;(suppl 3):S10-S14. [PMC free article] [PubMed] [Google Scholar]
- 9.Van Poppel H, Klotz L. Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol. 2012;19(7):594-601. doi: 10.1111/j.1442-2042.2012.02997.x [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Zaorsky NG, Bagshaw HP, et al. An expert review on the combination of relugolix with definitive radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2022;113(2):278-289. doi: 10.1016/j.ijrobp.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 11.O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243-1251. doi: 10.1200/JCO.2014.59.1792 [DOI] [PubMed] [Google Scholar]
- 12.Mearini L, Zucchi A, Costantini E, Bini V, Porena M. Intermittent androgen suppression in prostate cancer: testosterone levels and its implication. J Sex Med. 2011;8(4):1218-1227. doi: 10.1111/j.1743-6109.2010.02169.x [DOI] [PubMed] [Google Scholar]
- 13.Nascimento B, Miranda EP, Jenkins LC, Benfante N, Schofield EA, Mulhall JP. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J Sex Med. 2019;16(6):872-879. doi: 10.1016/j.jsxm.2019.03.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean DB, Shi H, Faessel HM, Saad F. Medical castration using the investigational oral GnRH antagonist TAK-385 (relugolix): phase 1 study in healthy males. J Clin Endocrinol Metab. 2015;100(12):4579-4587. doi: 10.1210/jc.2015-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shore ND, Saad F, Cookson MS, et al. ; HERO Study Investigators . Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196. doi: 10.1056/NEJMoa2004325 [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration . ORGOVYX (relugolix) prescribing information. Accessed December 22, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214621s000lbl.pdf
- 17.Dearnaley DP, Saltzstein DR, Sylvester JE, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78(2):184-192. doi: 10.1016/j.eururo.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer . Prostate. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. [Google Scholar]
- 19.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531-1538. doi: 10.1111/j.1464-410X.2008.08183.x [DOI] [PubMed] [Google Scholar]
- 20.Roy S, Grimes S, Eapen L, et al. Impact of sequencing of androgen suppression and radiation therapy on testosterone recovery in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2020;108(5):1179-1188. doi: 10.1016/j.ijrobp.2020.06.017 [DOI] [PubMed] [Google Scholar]
- 21.Spiegel DY, Hong JC, Oyekunle T, et al. A nomogram for testosterone recovery after combined androgen deprivation and radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2019;103(4):834-842. doi: 10.1016/j.ijrobp.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 22.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565-573. doi: 10.1016/j.eururo.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 23.Abufaraj M, Iwata T, Kimura S, et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44-53. doi: 10.1016/j.eururo.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Crawford ED, Atkinson S, Boldt-Houle D, Gordan LN. Real-world analyses of major adverse cardiovascular event risk by drug class after initiation of androgen deprivation therapy. J Clin Oncol. 2022;40(6)(suppl):46. doi: 10.1200/JCO.2022.40.6_suppl.046 [DOI] [Google Scholar]
- 25.George G, Garmo H, Scailteux LM, et al. Risk of cardiovascular disease following gonadotropin-releasing hormone agonists vs antagonists in prostate cancer: real-world evidence from five databases. Int J Cancer. 2021;148(9):2203-2211. doi: 10.1002/ijc.33397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes RD, Higano CS, Slovin SF, et al. ; PRONOUNCE Study Investigators . Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295-1307. doi: 10.1161/CIRCULATIONAHA.121.056810 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C27003 Study Trial Protocol
HERO Study Trial Protocol
eTable 1. Eligibility Criteria for Patients Receiving Short- and Longer-term ADT
eTable 2. Radiation Therapy Received During Study
eFigure. Box Plots of Secondary Endpoints Related to Testosterone, PSA, and FSH levels in Patients Receiving ADT Plus Radiotherapy
Data sharing statement


