Abstract
The high burden of disease and the long-lasting sequelae following Streptococcus pyogenes (Strep A) infections make the development of an effective vaccine a global health priority. Streptolysin O (SLO), is a key toxin in the complex pathogenesis of Strep A infection. Antibodies are elicited against SLO after natural exposure and represent a key target for vaccine-induced immunity. Here we present the setup and characterization of a hemolysis assay to measure functionality of anti-SLO antibodies in human sera. Assay specificity, precision, linearity, reproducibility, and repeatability were determined. The assay was demonstrated to be highly sensitive, specific, reproducible, linear and performed well in assessing functionality of anti-SLO antibodies induced by exposed individuals. Moreover, different sources of critical reagents, in particular red- blood cells, have been compared and had minimal impact on assay performance. The assay presented here has throughput suitable for evaluating sera in vaccine clinical trials and sero-epidemiological studies to gain further insights into the functionality of infection- and vaccine-induced antibodies.
Keywords: Streptococcus pyogenes, Strep A, Vaccine, Hemolysis, Streptolysin O, Functional assay
1. Introduction
Streptococcus pyogenes (Strep A) is a Gram-positive bacterium responsible for >500 thousand deaths every year, and primarily affects those living in resource-limited settings (Antimicrobial Resistance, C, 2022). Strep A manifests along a spectrum from asymptomatic carriage, mild superficial infections (sore throat and skin sores) to severe and potentially fatal invasive disease. Furthermore, repeated exposure in susceptible individuals can lead to pathological autoimmune sequelae including rheumatic fever that may result in rheumatic heart disease RHD (Macleod et al., 2019). According to the World Health Organization (WHO), Strep A is one of the most neglected diseases relative to its burden worldwide (Palafox et al., 2017). Strep A is also a key driver of antibiotic prescriptions and resistance, responsible for >400 million antibiotic prescriptions every year (Dooling et al., 2014). Thus, developing a vaccine is a global health priority, and an effective vaccine would reduce the burden of superficial infections, invasive disease and pathological autoimmune sequelae.
There has been increasing effort over the last decade to develop a Strep A vaccine via different approaches, with promising results obtained in preclinical studies (Dale et al., 2011; van Sorge et al., 2014; Dale and Walker, 2020; Sekuloski et al., 2018). GSK Global Health Vaccines R&D (GVGH) is developing a Strep A vaccine that is fully aligned with the WHO preferred product characteristics and technology roadmap. The current candidate is an M protein-free multicomponent vaccine targeting surface and secreted virulence factors identified by reverse vaccinology (Bensi et al., 2012). It consists of three recombinant protein antigens - Streptolysin O (SLO), S. pyogenes cell envelope protease (SpyCEP) and S. pyogenes adhesion and division protein (SpyAD), as well as one glycoconjugate, the Group A carbohydrate conjugated to carrier protein CRM197 (Kabanova et al., 2010). Together these antigens have been shown to elicit protection in animal models and are highly conserved in clinical Strep A isolates (Bensi et al., 2012; Cunningham, 2000; Lancefield, 1933; Braun, 1983; Davies et al., 2019; Lacey et al., 2023), meaning that the coverage of this vaccine should be virtually 100%.
To support vaccine development and better understand the immune response after natural Strep A exposure, multiple serological-based studies have been performed. These have confirmed that antibodies against key vaccine antigen targets are usually present in convalescent individuals (Keeley et al., 2023; Salie et al., 2023; Hysmith et al., 2017; Whitcombe et al., 2022a; Whitcombe et al., 2022b). However, determining the presence of binding antibodies alone is insufficient to infer potential functional activity. Antibody function is critical to establish for vaccine antigens, especially during early clinical assessments of the new vaccine. SLO is responsible for eukaryotic cell disruption and immune evasion (Chiarot et al., 2013; Feil et al., 2014; Uchiyama et al., 2015) and has >99% carriage in clinical Strep A collections with <2% sequence divergence (Davies et al., 2019). For these reasons it is included in a number of combination vaccine candidates in addition to the GVGH Strep A vaccine (Bensi et al., 2012; Rivera-Hernandez et al., 2016; Bi et al., 2019). Vaccine induced antibodies that can bind and neutralise SLO activity represent an important target to prevent the cellular damage caused by Strep A infections. However, standardized assays that can measure functional anti-SLO antibodies in large vaccine trials are lacking.
Hemolysis inhibition assays are a well-established method for quantifying the in vitro ability of a compound to inhibit red blood cell lysis in presence of active toxin, and have been used extensively for multiple pathogens to determine functional activity of vaccine induced sera (Bensi et al., 2012; Paton et al., 1983). Here we describe the optimization and characterization of an in vitro hemolysis inhibition method for testing functional anti-SLO antibodies in human sera (Bensi et al., 2012). Precision, specificity and linearity were determined and the correlation between anti-SLO antibody titers and functionality was explored, as well as the impact of critical reagents such as the source of red blood cells.
2. Materials and methods
2.1. In vitro hemolysis inhibition assay
Red blood cells were isolated from defribrinated rabbit blood and washed four times in Dulbecco's phosphate-buffered saline (DPBS; Gibco), then suspended in the original blood volume (blood cells suspension). Eight serial two-fold dilutions of test sera (75 μL) in DPBS were prepared in 96-well plates with U-shaped bottoms (Corning). Following this, 75 μL containing 2400 units/mL (final concentration 900 units/mL) of SLO toxin (Merck) diluted in DPBS-DTT 40 mM (final concentration 15 mM) were added to the plates and incubated at room temperature (22 ±2 °C) for 30 min, before adding 50 μL of the rabbit blood cells suspension (5% in volume). Plates were incubated for an additional 30 min at 37 °C. Plates were finally centrifuged for 5 min at 1000 ×g and 100 μL of supernatant was carefully transferred to 96-well flat-bottom plates (Corning). The absorbance was read at 540 nm to quantify the hemoglobin released during the hemolysis reaction. Controls were represented by wells containing SLO and red blood cells, or red blood cells only, respectively exhibiting maximum and minimum hemolysis. The analysis of the data was performed using GraphPad software, by fitting a sigmoidal 4-parameter logistic curve to the Log-transformed serum dilution values versus the OD at 540 nm and obtaining the IC50. The IC50 was defined as the capacity of tested serum to inhibit 50% of SLO-mediated red blood cell hemolysis.
2.2. Assay specificity
To assess the specificity of the hemolysis inhibition assay, intravenous immunoglobulin (IVIg; Privigen; CSL Behring) was incubated with an equal volume of inactive mutant SLO (Bensi et al., 2012) at different concentrations (335, 100, 50, 25, 10, 1 and, 0.1 μg/mL) or DPBS only (IC50 control, no inhibition) overnight at 4 °C. Following this pre-incubation, the samples were tested for hemolysis activity as per the hemolysis assay protocol described above. Percentage homologous inhibition, defined as (IC50 Control – IC50 Inhibited sample)/IC50 Control X 100, was calculated for each concentration of inhibitor. The lowest inactive SLO concentration able to inhibit ≥70% of hemolysis compared to the IC50 control (25 μg/mL) was then used to determine the heterologous inhibition of antibodies to the other vaccine antigens SpyCEP, SpyAD and GAC polysaccharide.
2.3. Precision
The precision of the assay was determined using Privigen IVIg as a test sample. IVIg was independently titrated 11 times (starting from 1:100, 2-fold) and tested in the standard assay as described above. This was repeated on three different days and by two operators. Log10 transformed IC50 of the resulting 66 independent replicates were subsequently analyzed using a random-effects model alongside day and operator covariables, allowing calculation of the intermediate precision, repeatability and variance attributed to each factor via residual maximum likelihood method using MiniTab software (Minitab, LLC). Coefficients of variance for reliability and intermediate precision were then calculated from the Log-transformed variance components and reverted to original IC50 with the eq. CV = √(e^((sLn)2)– 1), where sLn is calculated as standard deviation of Log-transformed variance components multiplied by Ln(10).
2.4. Linearity
Assay linearity was determined by independently diluting IVIg (neat and 2-fold apart to 1:8) prior to testing in the standard assay. Correlation between observed Log10 transformed IC50 and the Log10 nominal IC50 (represented by geometric mean of 66 separate measurements obtained from the precision assays), divided by the dilution factor, was determined.
2.5. Lower limit of quantitation and Lower limit of detection
To determine the limit of quantitation (LoQ) and limit of detection (LoD) of the hemolysis assay, the IVIg sample was pre-diluted 1:40 in DPBS to generate a sample with a low but detectable IC50. This sample was assayed in eleven independent replicates, titrated as per the standard assay protocol, and the resulting IC50 values of each replicate were used to calculate LoD and LoQ according to the ICH guideline Q2(R1) (Guideline, 2005). In brief, the standard deviation (SD) of Log transformed IC50 obtained for the samples and the lowest serum concentration tested in the assay (X, in our case = 2.67) were used in the following formulas: LoD = 10^(3.3 × SD) × X and LoQ = 10^(10 × SD) × X.
2.6. Source and analysis of human sera
Ten individual anonymised sera were obtained from a study of Strep A skin and throat infections conducted in Auckland New Zealand (Health and Disability Ethics Committee approval 17/NTA/262) (Bennett et al., 2022). Three serum pools, generated by adding equal volume of at least 30 (range 30–81) individual samples from children aged 24–59 months in The Gambia (Lindsey et al., 2019), were also used (approved by the joint Gambia Government/Medical Research Council Unit, the Gambia Ethics Committee and the London School of Hygiene and Tropical Medicine Research Ethics Committee - ref. 19,163). The ten individual sera and three sera pools were serially diluted 2-fold starting at 1:25 (10 dilutions total) and tested alongside IVIg Privigen as a positive control in the standard hemolysis assay. All samples were tested across three different days as independent samples by one operator. The ten individual human sera were additionally tested in an 8-plex Luminex based assay as described (Whitcombe et al., 2022a) to quantify binding anti-SLO IgG titres. The IgG titres were compared to the hemolysis IC50 values using an orthogonal regression model in the MiniTab software.
2.7. Critical reagents
Additional sources of red blood cells were assessed using Privigen IVIg as test sample, as per the standard hemolysis assay protocol. These included commercially sourced defibrinated sheep blood (Fort Richard Laboratories NZ), whole rabbit blood collected in a sodium heparin tube from a healthy female New Zealand white rabbit (ethics AEC 25097), and human blood collected in a sodium heparin tube from a healthy volunteer who was blood group B+ (Auckland Health Research Ethics Committee approval AH24859). IC50 obtained from three replicates for each red blood cell source were analyzed in Graphpad software using a one-way ANOVA (Kruskall-Wallis multiple comparison with a Dunn's post hoc analysis).
3. Results
We developed and tested the performance of an in vitro hemolysis assay that makes use of red blood cells (RBCs) from various commercial sources to assess neutralizing activity (i.e. functionality) of anti-SLO antibodies in human sera in presence of active SLO. The readout of the assay is the absorbance measured at 540 nm of a red blood cell suspension, with high absorbance indicating haemolysis via release of hemoglobin from RBCs. Following incubation with test serum and active SLO toxin, increasing levels of serum antibodies able to inhibit the hemolytic function of SLO will result in a reduction in absorbance. Results are presented as IC50, which represents the lowest sera dilution able to inhibit 50% of the activity of SLO, and thus giving 50% of absorbance between the maximum and minimum in the assay (Fig. 1A).
Fig. 1.
Assay readout for precision assessment. A) Typical readout of the assay for a single serum sample, where individual green dots represent the sample at different dilutions. A 4 paramter sigmoidal curve is fitted and IC50 is calculated as the serum dilution giving 50% absorbance at 540 nm between the and the maximum and minumum hemolysis values for that experiment (dotted lines). B) The IC50 of each sample performed by each operator are represented in blue (for operator 1) and pink (for operator 2), repeats on different days are represented by different shapes (day 1 = circles, day 2 = triangles, day 3 = stars). Geometric mean is represented by the gray line. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1. Precision
Precision of the hemolysis inhibition assay was determined by testing the same human IVIg sample 11 times on 3 different days by two different operators (Fig. 1B). The intermediate precision, a measure of variability in the same lab by performing the analysis in different days and by different operators, was 35.4% which is in line with the expectation for functional assays (Guideline, 2005). The repeatability, which measures the precision of the method under the same working conditions (i.e. any factors other than day and operator), was 6.8%. The assay was therefore deemed to be precise. A quantified reference international standard is not available, therefore accuracy of the method could't be assessed.
3.2. Linearity
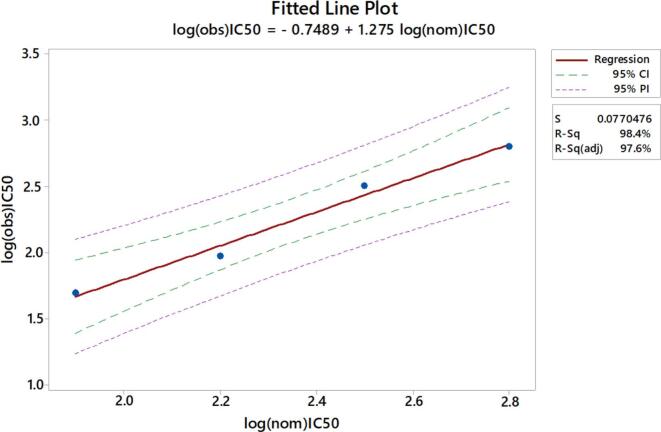
Linearity was assessed by testing an independently diluted human IVIg sample in the standard assay. The regression analysis showed a high correlation (R squared = 0.984) between observed Log10 IC50 and nominal Log10 IC50 at each specific dilution (Fig. 2). Based on this the assay was concluded to be linear.
Fig. 2.
Linearity. The same IVIg sample was tested neat and at 3 different 2-fold apart dilutions and compared to nominal values. Linear regression (red line) and 90% confidence interval (CI) (green line) are reported. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Assay specificity
To determine assay specificity, human IVIg was incubated with different concentrations of homologous antigen (inactive vaccine antigen SLO) prior to testing with the hemolysis inhibition assay. Percentage of inhibition at each concentration of homologous antigen was calculated in comparison to an uninhibited sample. The lowest concentration of inactive SLO able to inhibit the signal by at least 70% was 25 μg/mL and this concentration was selected for further assessments of specificity. Human IVIg was subsequently incubated with homologous antigen (SLO) and heterologous antigens (SpyAD, SpyCEP and group A carbohydrate polysaccharide – GAC PS)) at the same concentration prior to performing the hemolysis inhibition assay (Fig. 3). High specificity was observed with the percentage of inhibition approximately 98% with the homologous competitor (SLO) compared with <25% with the heterologous antigen competitors (23%, 17%, and 12% for SpyAD, SpyCEP, and GAC respectively).
Fig. 3.
Assay specificity. Percentage inhibition of SLO-induced hemolysis in Privigen IVIg using homologous and heterologous antigen competitors (25 mg/mL).
3.4. Limits of Detection and Quantitation (LoD and LoQ)
To determine the limits of the assay in the most variable conditions, we pre-diluted IVIg to generate a sample with a low but detectable hemolysis titre and tested this 11 times in a standard assay. The Limit of Detection (LoD), representing the lowest hemolysis titre that can be detected under the assay conditions was 3.0 IC50. The Limit of Quantitation (LoQ) of the assay, representing the lowest hemolysis titre that can be quantified with a suitable precision, was 3.8 IC50.
3.5. Determination of hemolysis inhibition titers in human sera
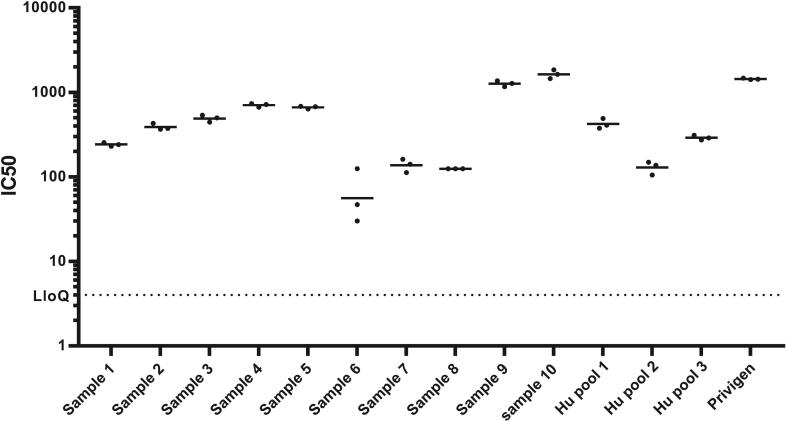
Having confirmed the performance of the method with IVIg and refined the acceptance criteria, we assessed the suitability of the assay to determine hemolysis inhibition in human sera. We tested sera from participants from different geographical settings (individual sera from New Zealand (Bennett et al., 2022) and serum pools from The Gambia (Lindsey et al., 2019)) in a standard hemolysis assay (Fig. 4). Each sample was tested independently across three days to further assess precision and the assay was shown to reproducibly determine a hemolysis inhibition titre (IC50) for sera across a range of titers (the % inter-assay CV between the three days was <20%). This data also shows that functional antibodies against SLO are generated after natural Strep A exposure and infection.
Fig. 4.
IC50 obtained testing sera from ten individual human samples and three pooled samples as well as IVIg by the same operator in three different runs, conducted in different days (solid circles). Horizontal lines represent the average of the three independent repeats. Dotted line represents the LoQ of the assay.
To explore the relationship between IC50 values of the ten individual human serum samples measured with the hemolysis assay and their respective anti-SLO antibody titres measured with a Luminex-based assay (Whitcombe et al., 2022a), an orthogonal regression model was applied. The analysis showed a strong correlation between the two datasets (Fig. 5) (Pearson r = 0.8891, with a p value of 0.0006 and a linear regression analysis with R2 = 0.7905), suggesting that increasing anti-SLO binding antibody titers results in higher functionality of the tested sera.
Fig. 5.
Correlation between Anti-SLO IgG (μg/mL) measured by Luminex and Hemolysis IC50 titre measured by hemolysis assay. Each orange circle represents one individual. Dashed line represents the linear regression.
3.6. Assessment of the impact of critical reagents
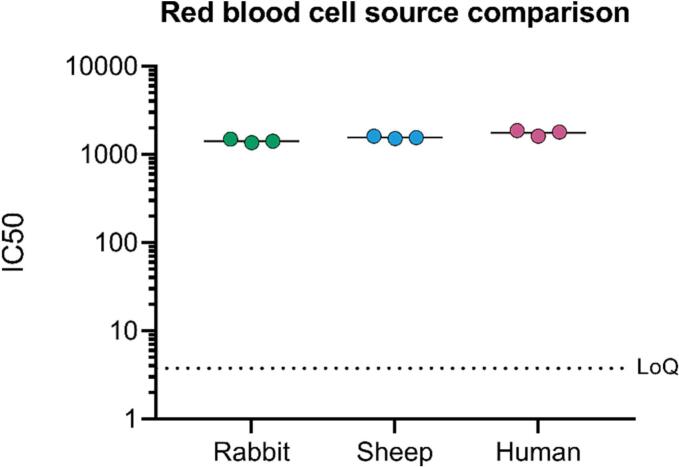
Red blood cells (RBC) are a critical reagent in the hemolysis assay but may be difficult to standardize. The compatibility of the assay with alternative sources of RBCs was therefore assessed. Washed RBCs from defibrinated sheep blood, rabbit blood and human blood were used to measure the hemolysis titre of IVIg (Fig. 6). The IC50 of the three replicates, and among the three different RBC types were overall very similar: the average IC50 was 1428 (inter-assay CV between replicates = 5%) for rabbit blood, 1561 (inter-assay CV = 6%) for sheep blood and 1762 (inter-assay CV = 7%) for human blood, and the only significant difference was between rabbit and human blood (p-value 0.022).
Fig. 6.
Comparison of results using different sources of red blood cells. IC50 of three replicates of IVIg tested independently using the three different sources of RBCs. Horizontal lines represent the mean IC50 value, for rabbit, sheep and human, respectively. Dashed line represents the LoQ of the assay. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
An effective and safe vaccine is urgently needed to reduce the burden of Strep A disease. The development of standardized immunoassays that can measure functional antibody responses to Strep A vaccine antigens is an integral part of vaccine development (Whitcombe et al., 2022a; Keeley et al., 2022). Here we present in-depth characterization of an in vitro hemolysis inhibition assay that quantifies neutralizing anti-Streptolysin O antibodies in human sera. The assay was shown to be highly specific, precise and repeatable and is able to linearly quantify the functional antibody response against SLO. All measurements of assay parameters fell within ICH guidelines, therefore the assay is fit-for-purpose to support vaccine trials (Guideline, 2005).
The method we described, compared to other methods (Uchiyama et al., 2015; Shewell et al., 2014; Limbago et al., 2000; Sumby et al., 2005; Evans et al., 2013; Rivera-Hernandez et al., 2019), have the advantage of using commercially available purified recombinant SLO instead of Streptococcus pyogenes culture supernatants, for which is difficult to determine the right amount of secreted SLO imparing the variability of the assay. We furthermore showed that different red blood sources (rabbit, sheep and human) could be substituted into the assay with minimal impact on the results obtained, illustrating flexibility for users in settings in which defibrinated rabbit blood may not be readily available. It should be noted that the use of human blood has yet to be fully explored and complexities such as anti-blood group antibodies in the test serum could impact the results (Shewell et al., 2014). Further investigation will be necessary to formally assess the impact of different blood group types in the assay. Animal blood also offers the potential advantage of less variability between batches/donations. Evaluation of functionality among samples within a clinical study is performed using the same source of critical reagents so that results can be biologically compared (CV% using the same source of RBC is <7% independently by the source of cells used).
The proposed plate layout comprises 11 individual sera tested at 8 dilutions and a standard serum represented by commercial IVIg to validate the plate. This layout, coupled with the short time needed to complete the assay (around 2 h), renders the assay attractive for large trials and studies. Indeed, up to 88 sera/day per operator can be readily assayed. Furthermore, the assay does not need automation or expensive equipment, enabling implementation in different settings, including laboratories in low resource settings. Data analysis based on direct interpolation of a 4PL logistic curve based on sera dilution facilitate a continuous readout and ensures an operator-independent and rapid calculation of results. This approach also maximizes precision by minimizing inherent variability of a single tested data point.
The hemolysis assay results were highly correlated with the titre of anti-SLO antibodies in a limited number of human sera in this study. Corroborating this finding in large scale natural infection studies and after vaccination in clinical trials will determine if this correlation occurs across age groups and in different settings. Furthermore, systematic validation of this assay alongside additional functional assays, such as those that measure inhibition of virulence factor activity for other vaccine antigens, as well as opsonophagocytosis and killing (Jones et al., 2018) will aid in the evaluation and determination of a correlate of protection for Strep A.
In conclusion the SLO assay described is a fit-for-purpose assay for Strep A vaccine clinical trials, sero-epidemiological and longitudinal cohort studies and may accelerate development of Strep A vaccines containing SLO as an antigen.
Author contributions
Conceptualization, MC, AW, LR, LM, and OR.; methodology, MC, LR, AW, LM, and OR.; software, MC, AK, LM, OR.; validation MC, AK, LM, and OR.; formal analysis, MC, AK, LM, TdS, DG, and OR; investigation MC, AK, LM, and OR.; resources MC, LM, MI, DG, and OR, JB, and NJM; data curation, MC, AW.; writing—original draft preparation, MC, AW, AK, LM and OR; writing—review and editing, MC, AW, AK, LM, MI, TdS, DG, JB, NJM and OR visualization, MC, AW, AK, LM and OR.; supervision, MC, LM, MI, TdS, DG, NJM and OR.; project administration, TdS, FBS, NJM, MI, DG, and OR.; funding acquisition, FBS, NJM, DG. All authors have read and agreed to the submitted version of the manuscript.
Funding
This work was performed with funds from CARB-X (research subaward agreement grant number 4500003786). AK is funded by a Wellcome Trust PhD Training Fellowship for Clinicians (225467/Z/22/Z). This work was also supported by funding from the New Zealand Ministry of Health as part of an initiative to accelerate Strep A Vaccine Development for Aotearoa New Zealand (370383-00).
Institutional review board statement
the study was conducted in accordance with the Declaration of Helsinki. Use of archived samples for assessing functional responses to Strep A was approved by the joint Gambia Government/Medical Research Council Unit The Gambia Ethics Committee and the London School of Hygiene and Tropical Medicine Research Ethics Committee (ref 19163). Samples from New Zealand were treated in accordance with ethics approval conditions from the Health and Disability Ethics Committee (ref (Salie et al., 2023)/NTA/262).
Informed consent statement
Informed consent was obtained from the parents of all children participating in the study (NCT02972957) from which pooled serum was derived. Parents gave consent for storage and future use usage of samples for the purposes of scientific research. Parents or guardians of children in New Zealand gave informed consent for the collection and testing of blood samples.
CRediT authorship contribution statement
Martina Carducci: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Alana Whitcombe: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Luca Rovetini: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. Luisa Massai: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. Alexander J. Keeley: Data curation, Resources, Writing – original draft, Writing – review & editing. Thushan I. de Silva: Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Julie Bennett: Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. Francesco Berlanda Scorza: Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. Miren Iturriza: Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Nicole J. Moreland: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Danilo G. Moriel: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Omar Rossi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
This work was performed with funds from CARB-X, and with internal funding form GlaxoSmithKline Biologicals SA, who is the sponsor of the study.The work was also supported by funding from the New Zealand Ministry of Health (Te Whatu Ora) as part of an initiative to accelerate Strep A vaccine development for Aotearoa New Zealand. The external funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. M.C., L.R., L.M, M.I, F.B.S., D.G.M. and O.R. are employees of the GSK group of companies. FBS, MI, DGM, MC, and OR report ownership of GSK shares/share options.
References
- Antimicrobial Resistance, C Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., et al. Risk factors for group a streptococcal pharyngitis and skin infections: a case control study. Lancet Reg. Health West Pac. 2022;26 doi: 10.1016/j.lanwpc.2022.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensi G., et al. Multi high-throughput approach for highly selective identification of vaccine candidates: the group a Streptococcus case. Mol. Cell. Proteomics. 2012;11(6) doi: 10.1074/mcp.M111.015693. M111 015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S., et al. A multicomponent vaccine provides immunity against local and systemic infections by group a Streptococcus across serotypes. mBio. 2019;10(6) doi: 10.1128/mBio.02600-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D.G. The use of streptococcal antigens to probe the mechanisms of immunity. Microbiol. Immunol. 1983;27(10):823–836. doi: 10.1111/j.1348-0421.1983.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Chiarot E., et al. Targeted amino acid substitutions impair streptolysin O toxicity and group a Streptococcus virulence. mBio. 2013;4(1) doi: 10.1128/mBio.00387-12. e00387-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.W. Pathogenesis of group a streptococcal infections. Clin. Microbiol. Rev. 2000;13(3):470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J.B., Walker M.J. Update on group a streptococcal vaccine development. Curr. Opin. Infect. Dis. 2020;33(3):244–250. doi: 10.1097/QCO.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J.B., et al. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group a streptococci. Vaccine. 2011;29(46):8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M.R., et al. Atlas of group a streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat. Genet. 2019;51(6):1035–1043. doi: 10.1038/s41588-019-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling K.L., et al. Overprescribing and inappropriate antibiotic selection for children with pharyngitis in the United States, 1997-2010. JAMA Pediatr. 2014;168(11):1073–1074. doi: 10.1001/jamapediatrics.2014.1582. [DOI] [PubMed] [Google Scholar]
- Evans B.C., et al. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013;73 doi: 10.3791/50166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil S.C., et al. Structural studies of Streptococcus pyogenes streptolysin O provide insights into the early steps of membrane penetration. J. Mol. Biol. 2014;426(4):785–792. doi: 10.1016/j.jmb.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideline I.H.T. Validation of analytical procedures: text and methodology. Q2 (R1) 2005;1(20):05. [Google Scholar]
- Hysmith N.D., et al. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group a Streptococci. J. Pediatric. Infect. Dis. Soc. 2017;6(2):187–196. doi: 10.1093/jpids/piw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., et al. Development of an opsonophagocytic killing assay for group a streptococcus. Vaccine. 2018;36(26):3756–3763. doi: 10.1016/j.vaccine.2018.05.056. [DOI] [PubMed] [Google Scholar]
- Kabanova A., et al. Evaluation of a group a Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine. 2010;29(1):104–114. doi: 10.1016/j.vaccine.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Keeley A.J., et al. Development and characterisation of a four-Plex assay to measure Streptococcus pyogenes antigen-specific IgG in human sera. Methods Protoc. 2022;5(4) doi: 10.3390/mps5040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley A.J., et al. Streptococcus pyogenes colonization in children aged 24-59 months in the Gambia: impact of live attenuated influenza vaccine and associated serological. responses. 2023;228(7):957–965. doi: 10.1093/infdis/jiad153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J.A.A.B., James Julie, Hines Taylah B., Chen Benjamin S., Lee Tiffany, Sika-Paotonu Darren, Anderson Dianne, Harwood Anneka, Tong Matire, Baker Steven Y.C., Williamson Michael, Moreland Deborah, Nicole J. 2023. A Worldwide Population of Streptococcus Pyogenes Strains Circulating Among School-Aged Children in Auckland, New Zealand: A Genomic Epidemiology Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R.C. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 1933;57(4):571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbago B., et al. Role of Streptolysin O in a mouse model of invasive group a streptococcal disease. Infect. Immun. 2000;68(11):6384–6390. doi: 10.1128/iai.68.11.6384-6390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B.B., et al. Effect of a Russian-backbone live-attenuated influenza vaccine with an updated pandemic H1N1 strain on shedding and immunogenicity among children in the Gambia: an open-label, observational, phase 4 study. Lancet Respir. Med. 2019;7(8):665–676. doi: 10.1016/S2213-2600(19)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod C.K., et al. Neglecting the neglected: the objective evidence of underfunding in rheumatic heart disease. Trans. R. Soc. Trop. Med. Hyg. 2019;113(5):287–290. doi: 10.1093/trstmh/trz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palafox B., et al. The WHF roadmap for reducing CV morbidity and mortality through prevention and control of RHD. Glob. Heart. 2017;12(1):47–62. doi: 10.1016/j.gheart.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Paton J.C., Lock R.A., Hansman D.J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 1983;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Hernandez T., et al. Differing efficacies of Lead group a streptococcal vaccine candidates and full-length M protein in cutaneous and invasive disease models. mBio. 2016;7(3) doi: 10.1128/mBio.00618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Hernandez T., et al. An experimental group a Streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. mBio. 2019;10(2) doi: 10.1128/mBio.00693-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salie M.T., et al. Serum immune responses to group a streptococcal antigens following pharyngeal acquisitions among children in Cape Town, South Africa. mSphere. 2023;8(3) doi: 10.1128/msphere.00113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekuloski S., et al. Evaluation of safety and immunogenicity of a group a streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0198658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewell L.K., et al. The cholesterol-dependent cytolysins pneumolysin and streptolysin O require binding to red blood cell glycans for hemolytic activity. Proc. Natl. Acad. Sci. U. S. A. 2014;111(49):E5312–E5320. doi: 10.1073/pnas.1412703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P., et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J. Infect. Dis. 2005;192(5):771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- Uchiyama S., et al. Streptolysin O rapidly impairs neutrophil oxidative burst and antibacterial responses to group a Streptococcus. Front. Immunol. 2015;6:581. doi: 10.3389/fimmu.2015.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sorge N.M., et al. The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe. 2014;15(6):729–740. doi: 10.1016/j.chom.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcombe A.L., et al. An eight-plex immunoassay for group a streptococcus serology and vaccine development. J. Immunol. Methods. 2022;500 doi: 10.1016/j.jim.2021.113194. [DOI] [PubMed] [Google Scholar]
- Whitcombe A.L., et al. Increased breadth of group a Streptococcus antibody responses in children with acute rheumatic fever compared to precursor pharyngitis and skin infections. J. Infect. Dis. 2022;226(1):167–176. doi: 10.1093/infdis/jiac043. [DOI] [PMC free article] [PubMed] [Google Scholar]