Abstract
Background:
The serotonin (5-hydroxytryptamine (5-HT))-mediated system plays an important role in stress-related psychiatric disorders and substance abuse. Our previous studies showed that stress and drug exposure can modulate the dorsal raphe nucleus (DRN)-5-HT system via γ-aminobutyric acid (GABA)A receptors. Moreover, GABAA receptor-mediated inhibition of serotonergic DRN neurons is required for stress-induced reinstatement of opioid seeking.
Aim/methods:
To further test the role of GABAA receptors in the 5-HT system in stress and opioid-sensitive behaviors, our current study generated mice with conditional genetic deletions of the GABAA α1 subunit to manipulate GABAA receptors in either the DRN or the entire population of 5-HT neurons. The GABAA α1 subunit is a constituent of the most abundant GABAA subtype in the brain and the most highly expressed subunit in 5-HT DRN neurons.
Results:
Our results showed that mice with DRN-specific knockout of α1-GABAA receptors exhibited a normal phenotype in tests of anxiety- and depression-like behaviors as well as swim stress-induced reinstatement of morphine-conditioned place preference. By contrast, mice with 5-HT neuron-specific knockout of α1-GABAA receptors exhibited an anxiolytic phenotype at baseline and increased sensitivity to post-morphine withdrawal-induced anxiety.
Conclusions:
Our data suggest that GABAA receptors on 5-HT neurons contribute to anxiety-like behaviors and sensitivity of those behaviors to opioid withdrawal.
Keywords: α1-GABAA, dorsal raphe, opioid, anxiety, depression
Introduction
The dorsal raphe nucleus (DRN) in the brainstem contains the largest population of serotonergic (5-hydroxytryptamine (5-HT)) neurons and is involved in several functions such as sleep, temperature regulation, stress responses, and anxiety behaviors (Pourhamzeh et al., 2022). Emerging evidence shows that the 5-HT DRN system may also play a role in substance abuse and its behavioral risk factors (Fluyau et al., 2022; Kirby et al., 2011; Müller and Homberg, 2015; Taracha, 2021), especially the negative emotional aspects of opioid dependence and withdrawal which are likely to contribute to relapse (Goeldner et al., 2011; Koob, 2021; Pomrenze et al., 2022). Understanding the neurobiological mechanisms underlying the impact of the 5-HT DRN on stress and opioid-sensitive behaviors may provide novel insights to aid in the development of novel targeted treatments for opioid use disorders and relapse prevention.
The 5-HT DRN neurons receive tonic inhibitory inputs from γ-aminobutyric acid (GABA) interneurons as well as GABAergic projections from several brainstem regions such as hypothalamus, ventral tegmental area, and substantia nigra pars reticulata (Hernández-Vázquez et al., 2019). Moreover, projections using other neurotransmitters also form connections with GABAergic interneurons in the DRN and indirectly regulate 5-HT neuron activity (Hernández-Vázquez et al., 2019). For example, our work and others work have shown that opioids and the stress neurohormone corticotropin-releasing factor regulate GABAergic interneurons in an opposing manner, indirectly exciting and inhibiting 5-HT DRN neurons, respectively (Jolas et al., 2000; Kirby et al., 2008; Tao and Auerbach, 2005; Valentino et al., 2010). Much evidence has supported the physiological relevance of GABAergic inhibition in the DRN in drug-related stress (Hernández-Vázquez et al., 2019). For example, our studies show that GABAA receptor-mediated inhibitory post-synaptic currents (IPSCs) in the 5-HT DRN neurons are enhanced after swim stress-induced reinstatement of morphine-conditioned place preference (CPP), and the activation of GABAA receptors in the DRN is both necessary and sufficient for the reinstatement (Li et al., 2013; Staub et al., 2012).
Negative affective states including dysphoria, depression, anhedonia, irritability, and anxiety also contribute to substance use (Koob, 2000). Many of these negative affective behaviors are observed during drug withdrawal and can persist long after somatic withdrawal symptoms have resolved, increasing the risk of relapse in humans and reinstatement in rodent models (Koob and Volkow, 2016; Mantsch et al., 2016). GABAergic signaling in the DRN has also been implicated in these negative affective behaviors. Although the infusion of a cocktail of GABAA and GABAB receptor agonists into the DRN reduces conflict/anxiety associated with cocaine-seeking behavior (Ettenberg et al., 2011), a GABAA receptor agonist alone in the DRN escalates alcohol-heightened aggression (Takahashi et al., 2010). Moreover, GABAergic inhibition in the DRN contributes to drug dependence and withdrawal. For example, chronic morphine induces hypofunction of 5-HT DRN neurons via enhanced GABA tone (Jolas et al., 2000). Increased IPSCs in 5-HT DRN neurons are also associated with cocaine withdrawal-induced anxiety-like behavior in the open field test (Craige et al., 2015). Chemogenetic inhibition of DRN GABAergic neurons decreased hyperalgesia during spontaneous heroin withdrawal (Alvarez-Bagnarol et al., 2023). Protracted withdrawal from chronic morphine increases anxiety- and despair-like behaviors in mice while decreasing social interaction, effects reversed by the serotonin selective reuptake inhibitor fluoxetine, implicating hypofunction of the 5-HT system in the expression of these behaviors (Goeldner et al., 2011). Our earlier studies show that stress-induced reinstatement of morphine CPP sensitizes GABAA receptors on 5-HT DRN neurons, indirectly implicating hypofunction of 5-HT in opioid reinstatement (Staub et al., 2012). Collectively, these studies support the hypothesis that the 5-HT DRN system and its regulation by GABA impacts drug-seeking or behavioral risk factors for drug-seeking. To test this hypothesis, our current study used genetic mutational strategies to manipulate GABAA receptors selectively in the DRN or specifically in 5-HT neurons to examine the consequences of those genetic manipulations in models of stress-induced opioid reinstatement and opioid withdrawal-induced anxiety- and depression-like behaviors.
The GABAA receptor is a pentameric membrane ion channel composed of five individual subunits from seven main subunit families (α, β, γ, δ, ε, θ, and ρ). Among all the GABAA subtypes, the α1 β2γ2 composite is the most abundant subtype in the rodent brain (McKernan and Whiting, 1996) and α1 subunits are most abundantly expressed in 5-HT DRN neurons (Lemos et al., 2011). Our previous data further suggest that changes in α1 subunit expression in 5–HT DRN neurons are associated with hyperresponsiveness to stress (Lemos et al., 2011). Therefore, we chose to genetically delete the GABAA α1 subunit to manipulate GABAA receptors in the 5-HT DRN system. Moreover, the DRN is a heterogeneous nucleus containing several types of neurons (Commons, 2020), most of which express GABAA receptors as well (Corteen et al., 2015). It is possible that GABAA receptors on non-5-HT DRN neurons also contribute to drug- and stress-sensitive behaviors. To distinguish the contributions of all neurons in the DRN versus the entire 5-HT system, we employed ‘floxed’ α1-GABAA (Gabra1) mice that allowed us to perform both conditional region-specific (DRN 5-HT and non-5-HT neurons) and cell-type-specific Gabra1 gene deletions (all 5-HT neurons). To induce recombination of the Gabra1 gene in these mice, we introduced Cre recombinase (1) by DRN injection of an adeno–associated virus serotype 1/2 (AAV1/2) expressing Cre recombinase (DRN-specific gene deletion) and (2) by crossing Gabra1 floxed mice with a tryptophan hydroxylase 2 (TPH2)-iCre mouse line, in which Cre expression is driven by a transgene under the control of an inducible 5-HT–specific promoter. We predicted that by deleting the predominant GABAA receptor subtype within the DRN or in the entire population of 5–HT neurons, the resulting mice would exhibit alterations in stress-induced opioid reinstatement or opioid withdrawal-induced anxiety and depression-like behaviors.
Materials and methods
Animals
Floxed Gabra1 mice (B6.129(FVB)–Gabra1tm1Geh/J) breeders on a C57BL/6J background (Arain et al., 2012) were generously supplied by Dr. Martin Gallagher (Vanderbilt University). TPH2-iCreER mice (Tg(TPH2-icre/ERT2)6Gloss/J) were obtained from The Jackson Laboratory. R26R-EYFP mice (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J) were obtained from The Jackson Laboratory. Animal protocols were approved by the Temple University Institutional Animal Care and Use Committee and were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
Experiment 1: DRN-specific knockout of α1-GABAA
Stereotaxic surgery and virus injection.
At 12 weeks of age, male floxed Gabra1 mice (hemizygous for the floxed Gabra1 allele) or wild-type (WT) littermates (WT for the floxed Gabra1 allele) were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and injected with 0.5 μl of AAV vectors (AAV1/2-Cre-GFP or AAV1/2-GFP (Vector BioLabs, Malvern, PA)) into DRN (AP −4.5; ML + 1.4; DV + 3.1; 26° angle) via 33-gauge injector at a speed of 0.1 μl/min. The analgesic ketoprofen (5 mg/kg) was given subcutaneously prior to surgery and on postoperative day 1. Three weeks were allowed for virus expression before other experiments were conducted. Quantitative loss of α1-GABAA in the DRN of the floxed Gabra1 mice was confirmed by Western blot, and functional loss of α1-GABAA in the DRN of the floxed Gabra1 mice was confirmed by electrophysiology. For the mice completing behavioral tests, the expression of Cre-GFP or GFP was visualized, and only those with expression of Cre-GFP or GFP confined to the DRN were used in behavioral analysis (Figure 1(a)).
Figure 1.
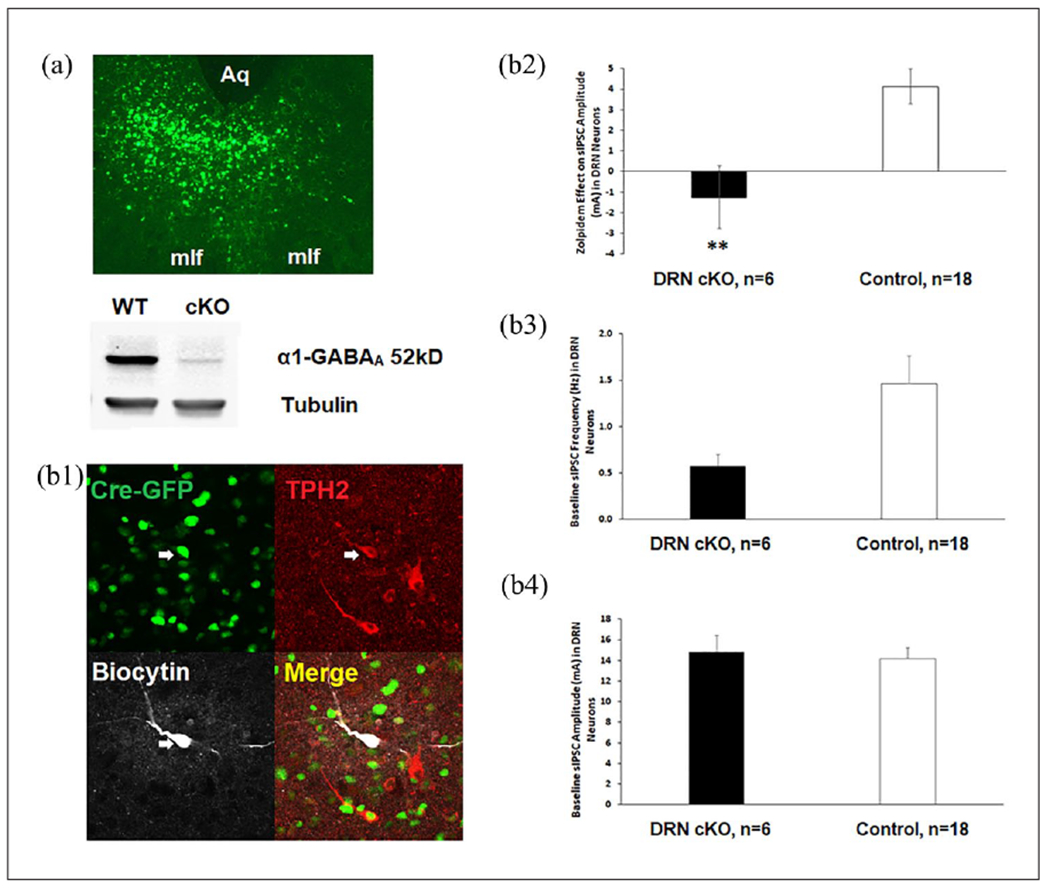
Verification of DRN-specific knockout of α1-GABAA. (a) Intra-DRN injection of 0.5 μl AAV-Cre-GFP (upper panel) in Gabra1 floxed mice produced a loss of α1-GABAA protein in DRN cKO (lower panel). Immunoblot of DRN injected with AAV-Cre-GFP showed α1-GABAA levels were reduced overall by 90% in the α1-GABAA floxed mice compared with WT littermates. Aq: aqueduct; mlf; medial longitudinal fasciculus, (b) Loss of α1-GABAA function and baseline GABA synaptic activity in DRN cKO. Immunohistochemical identification of the biocytin-filled recorded neuron (gray) with TPH2-IR (red) is shown in a Cre-GFP+ cell that is triple-labeled in the merged panel (b1). Zolpidem increased sIPSC amplitude in DRN 5-HT neurons in control groups (n = 18 cells from 12 male mice) but not in the floxed Cre+ group n = 6 cells from 5 male mice) (b2). However, the baseline sIPSC frequency (b3) and amplitude (b4) did not differ between floxed Cre+ and the controls.
Asterisks indicate significant differences from control by unpaired t-test (** p < 0.01).
5-HT: 5-hydroxytryptamine; AAV: adeno-associated virus; DRN: dorsal raphe nucleus; GABA: γ-aminobutyric acid; sIPSC: spontaneous inhibitory post-synaptic current; TPH2: tryptophan hydroxylase 2; WT: wild type.
Experiment 2: 5-HT-specific knockout of α1-GABAA
Floxed Gabra1 mice were crossed with the TPH2-iCreER mouse line to generate TPH2-iCreER (hemi)/floxed Gabra1. Both floxed Gabra1 and TPH2-iCreER mice were used as controls. To verify the expression of Cre, an R26R-EYFP reporter line was crossed with TPH2-iCre mice to generate TPH2-iCre/R26R-EYFP mice. To activate Cre, at 12 weeks of age, mice were given tamoxifen injections (i.p. 75 mg/kg) for 5 consecutive days, 2 weeks before other experiments were conducted. All control mice received the same tamoxifen treatment. Both sexes were included in the analysis.
Electrophysiology.
To verify the knockout of the α1-GABAA subunit and test the changes in GABAA receptors, animals were euthanized, and brain slices were prepared for electrophysiology as described previously (Kirby et al., 2008; Li and Kirby, 2016). Mice were euthanized by rapid decapitation and brains were placed into ice-cold artificial cerebrospinal fluid (ACSF) in which sucrose (248 mM) was substituted for NaCl. Slices (250 μm thick) were cut through the DRN using a Vibratome 3000 Plus (Vibratome, Bannockburn, IL) and placed in ACSF ((in mM): 124 NaCl, 3.0 KCl, 1.25 NaH2PO4, 3.0 CaCl2, 1.25 MgSO4, 10 dextrose, and 26 NaHCO3) with l-tryptophan (50 mM) at 35°C bubbled with 95% O2/5% CO2 for 1 h. Slices were then maintained at room temperature ACSF bubbled with 95% O2/5% CO2.
Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT, USA) and continuously perfused with ACSF at 1.5–2.0 ml/min. Only one cell was recorded per brain slice. DRN neurons were visualized using a Nikon E600 upright microscope (Optical Apparatus, Ardmore, PA). The resistance of the electrode was 4–6 MΩ when filled with an intracellular solution ((in mM): 70 K gluconate, 70 KCl, 2 NaCl, 4 EGTA, 10 HEPES, 4 MgATP, 0.3 Na2GTP, 0.1% biocytin, pH 7.3).
Spontaneous IPSC (sIPSC) recordings were made in cells located in the ventromedial subdivision of the DRN at mid-caudal levels that correspond to 4.36–4.96 mm caudal to bregma in Paxinos and Franklin (2001) where 5-HT neurons are most densely populated. Whole-cell recordings were conducted in voltage-clamp mode (Vm = −70 mV) with a HEKA patch-clamp EPC-10 amplifier (HEKA Elecktronik Lambrecht, Pfalz, Germany). Series resistance was monitored throughout the experiment. If the series resistance was unstable or exceeded four times the electrode resistance, the cell was discarded. Series resistance ranges were also noted for each experimental group to ensure consistent overlap across groups. Signals were filtered at 1 kHz and digitized at 10 kHz. The liquid junction potential was −9 mV between the pipette solution and the ACSF and was not subtracted from the data obtained.
The non-NMDA receptor antagonist 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (20 μM; Sigma-Aldrich, St. Louis, MO, USA) was bath applied to isolate GABAergic IPSCs. Baseline spontaneous sIPSCs were recorded for 200 s, then zolpidem (Tocris, Bristol, UK), a selective agonist to α1 subunit-containing GABAA receptors, was bath applied, and sIPSCs were recorded for 400 s. At the end of some experiments, the GABAA receptor antagonist bicuculline (20 μM) was applied to confirm the GABAergic nature of IPSC events. Following electrophysiology experiments, the neurochemical identity of the recorded cell was determined by post hoc immunohistochemistry described below. Only data from confirmed 5-HT DRN neurons were included in the analysis.
Western blot.
For experiment 1, to quantify the knockout of α1-GABAA in the DRN, a Western blot was performed as previously described (Shi et al., 2022). Floxed mice with AAV1/2-Cre-GFP injection or AAV1/2-GFP into the DRN were euthanized by rapid decapitation, and DRN was dissected, frozen on dry ice, and stored at −80°C. Tissue was then homogenized in ice-cold lysis buffer and the supernatant was collected by centrifugation. Samples were diluted in gel loading buffer and boiled for 5 min, 10 μg of protein was separated on 4–15% Tris–HCl Bio-Rad Readygels (Bio-Rad Laboratories, Hercules, CA, USA), and transferred to nitrocellulose membranes. Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) and incubated overnight at 4°C with the following primary antibodies: anti-α1-GABAA antibody (Abcam, Waltham, MA, USA) and anti-β-tubulin (1:600,000; Sigma-Aldrich). Membranes were washed in Tween-Tris-buffered saline and incubated with anti-rabbit (1:15,000) or anti-mouse (1:15,000) secondary antibodies conjugated to two different infrared dyes (LI-COR Biosciences). Membranes were visualized using the Odyssey infrared imaging system and proteins were quantitated using ImageJ software. Levels of α1-GABAA were expressed as a ratio to tubulin for each group (note: 2–3 pooled samples were used for each group due to the limited amount of protein abundance in each DRN sample).
Immunohistochemistry.
Following electrophysiology experiments, the neurochemical identity of the recorded cell was determined by post hoc immunohistochemistry. Brain slices were post-fixed in 4.0% paraformaldehyde for 20–24 h and kept in 30% sucrose solution until standard fluorescent immunohistochemical methods were performed to visualize the recorded biocytin-filled cell and TPH2 as a marker of 5-HT neurons. A rabbit-anti-mouse TPH2 antibody (1:1000; Millipore, Billerica, MA, USA) was visualized using an Alexa 594-conjugated donkey anti-rabbit secondary antiserum (1:1000; Life Technologies, Carlsbad, CA, USA). Biocytin was visualized using Alexa 647-conjugated streptavidin (1:1000; Life Technologies). The slices were coverslipped with SlowFade Gold mounting medium (Life Technologies) and visualized with Nikon A1R Confocal Scanning System with NIS Elements AR imaging software. Expression of Cre-GFP or GFP in the DRN and the recorded cell was confirmed at the same time.
For experiment 2, to verify the 5-HT neuron-specific expression of Cre, TPH2-iCre/R26R-EYFP and WT/R26-EYFP mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused with phosphate-buffered saline followed by 4% paraformaldehyde. Brains were removed, post-fixed for 24 h, and cryoprotected with 30% sucrose until sectioning. Mesencephalic slices containing the DRN were sectioned by cryostat at 40 μm thickness and stained with rabbit anti-TPH2 (1:500) primary antibody and donkey anti-rabbit Alexa Fluor 594 (1:1000) secondary antibody.
Elevated plus maze test.
Mice were tested for anxiety-like behaviors on a 50-cm high black Plexiglas elevated plus maze (EPM) as previously described (Huang et al., 2010). The exploration of mice in the EPM was videotaped, and the entries into open and closed EPM arms and EPM centers were scored for 5 min. The percentage (%) of time spent on the open arms (time in open arms/time in open + closed arms * 100) and % open arm entries (open entries/open + closed entries * 100) are considered anxiety measures, whereas the numbers of total and closed arm entries are considered locomotor measures (Lister, 1987).
Forced swim test.
A forced swim test (FST) was used to measure depression-like behavior as previously described (Porsolt et al., 1977) and as a stressor for reinstatement of CPP. Mice were placed in a cylindrical tank (46 cm tall × 20 cm diameter) of 23–25°C water filled to a depth of 15 cm for 6 min. The swim sessions were videotaped and the duration of immobility (minimum movements necessary to stay afloat) was measured for the last 4 min of the swim.
Splash test.
The splash test is a repeatable test of self-grooming in mice, and the decreased grooming time in this assay is associated with anhedonia and depression-like effects (Yalcin et al., 2008). On the test days, mice were given one habituation session before the experiment. A single mouse was placed in a transparent clean housing cage with standard bedding. After 1 min of habituation, approximately 0.7 ml of 10% (w/v) sucrose solution in water was sprayed on the dorsum of the mouse from a distance of 10 cm. A 5-min observation was started immediately, and the time spent in self-grooming was scored by an experienced observer under blinded experimental conditions.
Morphine CPP test.
The CPP procedure was adapted from our previous rat models (Li et al., 2013, 2021). The CPP apparatus was a Plexiglas chamber (12.7 cm × 34.7 cm × 12.7 cm) that consisted of two identically sized compartments with a guillotine door (5 cm × 5.9 cm) in-between. One compartment had walls and a floor with a black and white checker design and was illuminated with a blue light bulb (5 W). The other compartment had white walls and a floor and was illuminated by a red light bulb (5 W) (Xu et al., 2013).
In the preconditioning phase, mice were allowed to freely explore both sides of the chamber for 15 min, and the time they spent in each compartment was scored. The drug-paired side was then assigned to the least preferred side for each mouse to balance the initial bias.
The conditioning phase consisted of 4 days of conditioning and a conditioning test on day 5. Saline injections were given at 9 AM and morphine (10 mg/kg, s.c.) injections at 2 PM. After each injection, the mice were immediately confined to the corresponding compartment for 45 min. The conditioning test was conducted at 12 PM the day after the last conditioning session, and the mice were allowed to freely explore both compartments for a total of 15 min. The conditioning criterion was met if the time spent in the drug-paired side was at least 100 s greater than the time spent in that chamber during the preconditioning test (Nygard et al., 2016; Portugal et al., 2014).
The 7-day extinction phase of morphine CPP was structured similarly to conditioning, except that animals received only saline injections on the first 2 days, and no injections on the following 5 days. The extinction test was conducted on day 8 at 12 PM, and the mice were allowed to freely explore both compartments for 15 min.
For swim-stress-induced reinstatement, the next day after the extinction test, mice were placed into a cylindrical tank (46 cm tall × 20 cm diameter) of 23–25°C water filled to a depth of 15 cm for 6 min. The swim session was monitored and videotaped for later analysis. Following a 20-min drying period, mice were placed in the CPP chamber for 15 min free exploration. Swim stress-induced reinstatement of previously extinguished morphine CPP is considered to model opioid relapse triggered by stress exposure in humans (Mantsch et al., 2016).
Spontaneous morphine withdrawal.
To induce morphine dependence, mice were injected with escalating doses of morphine twice daily (20, 40, 40, 80, 80, 100, and 100 mg/kg morphine, s.c.; NIDA Drug Supply Program) over a period of 7 days, a regimen shown to induce significant weight loss over the course of injections, early somatic withdrawal signs followed by disruption of emotional-like behaviors during protracted abstinence (Goeldner et al., 2011; Zanos et al., 2016). Mice were left in the home cage for observation of spontaneous withdrawal. Body weight was monitored daily and compared to baseline. Animals were videotaped and jumping behavior (a sign of somatic withdrawal) was quantified over a 30-min period 24 and 48 h after the final injection. Tests of emotional-like behaviors were conducted 10 days after the final injection, with EPM and splash test on day 17 and FST on day 18. Post-withdrawal EPM and splash test scores were compared with baseline measurements in these assays which were conducted the day before the first morphine injection.
Data analysis
For experiment 1, DRN-specific knockout of α1-GABAA, the effect of zolpidem was calculated as the maximum change of sIPSC amplitude after bath application of zolpidem compared to baseline. The differences in sIPSC frequency and amplitude in 5-HT DRN neurons between floxed Cre+ and controls (floxed Cre−, floxed GFP, and WT Cre+ combined for no statistically significant differences were observed among the three control groups) were analyzed using unpaired t-test. To determine the behavioral differences between floxed Cre and floxed GFP control, EPM and FST data were analyzed using unpaired t-test, CPP data were analyzed using two-way repeated measures ANOVA with the session as the within-subject variable and floxed Cre versus floxed GFP as the between-subject variable. For experiment 2, 5-HT-specific knockout of α1-GABAA, sIPSC frequency and amplitude at baseline, and the effect of zolpidem on sIPSC amplitude in 5-HT DRN neurons was compared between TPH2-iCre/floxed and WT/floxed groups by unpaired t-test. Body weight changes in the TPH2-iCre/floxed and control genotypes (WT/floxed, TPH2-iCre/WT, and WT/WT combined for no statistically significant behavioral differences were observed among the control genotypes) were compared during the morphine injection and withdrawal period by two-way repeated measures ANOVA (genotype as the between-subject variable, time as the repeated within-subject variable). Somatic withdrawal (jumping) and FST were analyzed using an unpaired t-test. Pre- and post-morphine EPM and splash test were analyzed using two-way repeated measure ANOVA with the session as the within-subject variable and genotype as the between-subject variable. Post hoc Tukey, Dunnett, and t-tests were performed where applicable. Data are presented as mean ± SEM and a probability of p < 0.05 was considered statistically significant. All data were screened for normality by the Shapiro–Wilk test and for equal variance prior to parametric statistical testing. Statistical analyses were performed by SPSS and SigmaStat.
Results
Experiment 1: DRN-specific knockout of α1-GABAA
Verifying the loss of α1-GABAA in the DRN
Western blot.
Immunoblot of DRN injected with AAV-Cre-GFP showed α1-GABAA levels were reduced overall by 90% (see Figure 1(a) and Supplemental Table 1) in the α1-GABAA floxed mice compared with WT littermates.
Electrophysiology.
Zolpidem increased sIPSC amplitude in DRN 5-HT neurons in the pooled control group (floxed Cre−, floxed GFP, and WT Cre+) but not in the floxed Cre+ group (DRN-specific deletion of α1-GABAA; Figure 1(b2)). Zolpidem effect on sIPSC amplitude was calculated as sIPSC amplitude after bath application of Zolpidem minus baseline sIPSC amplitude. Unpaired t-test showed that floxed Cre+ differed from control (t(22) = 3.144, p = 0.005). However, the baseline sIPSC frequency and amplitude did not differ between floxed Cre+ and the controls (Figures 1(b3) and (b4)), suggesting that knockout of α1-GABAA did not change the presynaptic release of GABA or the number or sensitivity of postsynaptic GABAA receptors.
DRN-specific knockout of α1-GABAA did not alter anxiety, depression, or opioid reward.
The timeline for behavioral studies in DRN cKOs is shown in Figure 2. For EPM (Figure 2(b)), no difference was revealed in % of open arm time (2b1), % open arm entries (2b2), number of closed (2b3), or total arm entries (2b4) between floxed Cre and floxed GFP groups, suggesting that DRN-specific knockout of α1-GABAA did not alter anxiety or locomotor behavior. The immobility time during FST (Figure 2(c)) did not differ between the two groups either, suggesting that DRN-specific knockout of α1-GABAA did not alter depression-like behavior. For morphine CPP (Figure 3), both floxed Cre and floxed GFP groups developed conditioning, extinction, and swim-stress induced reinstatement; however, there was no difference between the two groups. Two-way repeated measure ANOVA of morphine CPP data revealed a main effect of the session (F(3,54) = 16.026, p < 0.001) but not genotype or interaction. Pre-planned paired t-tests showed that conditioning was different from pre-conditioning (t(19) = −10.290, p < 0.001) and extinction (t(19) = 6.259, p < 0.001); meanwhile, reinstatement was different from extinction (t(19) = −2.81, p = 0.011).
Figure 2.
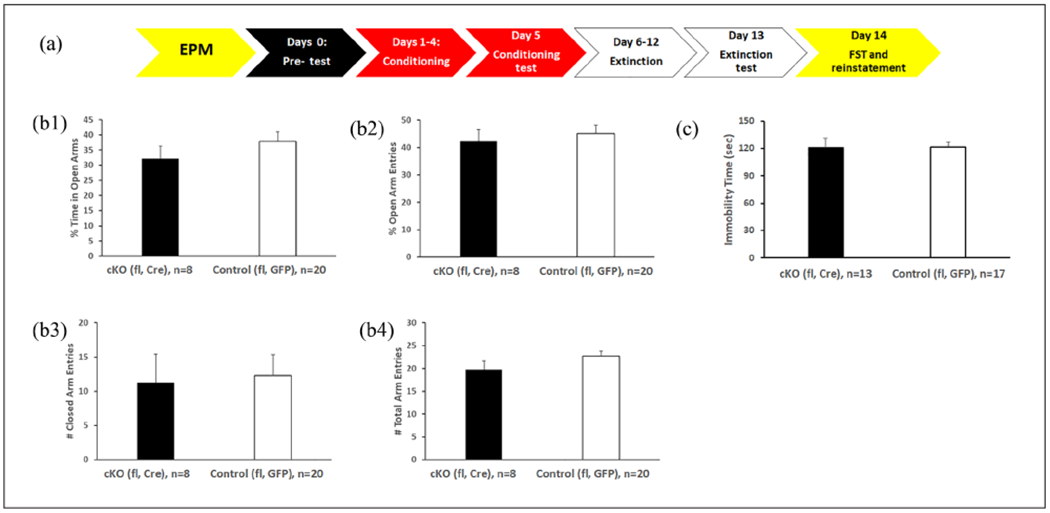
The DRN cKO mice exhibit similar stress responses as control mice. (a) Timeline of behavioral studies in DRN cKO. (b) Anxiety-like and motor behavior in EPM unaffected by DRN cKO. No difference was revealed in % of open arm time (b1), % open arm entries (b2), number of closed (b3) or total arm entries (b4) between Floxed Cre and floxed GFP groups. (c) Depression-like behavior in FST unaffected by DRN cKO. The immobility time during FST did not differ between Floxed Cre and floxed GFP groups. All DRN cKOs were generated in male mice.
DRN: dorsal raphe nucleus; EPM: elevated plus maze; FST: forced swim test.
Figure 3.
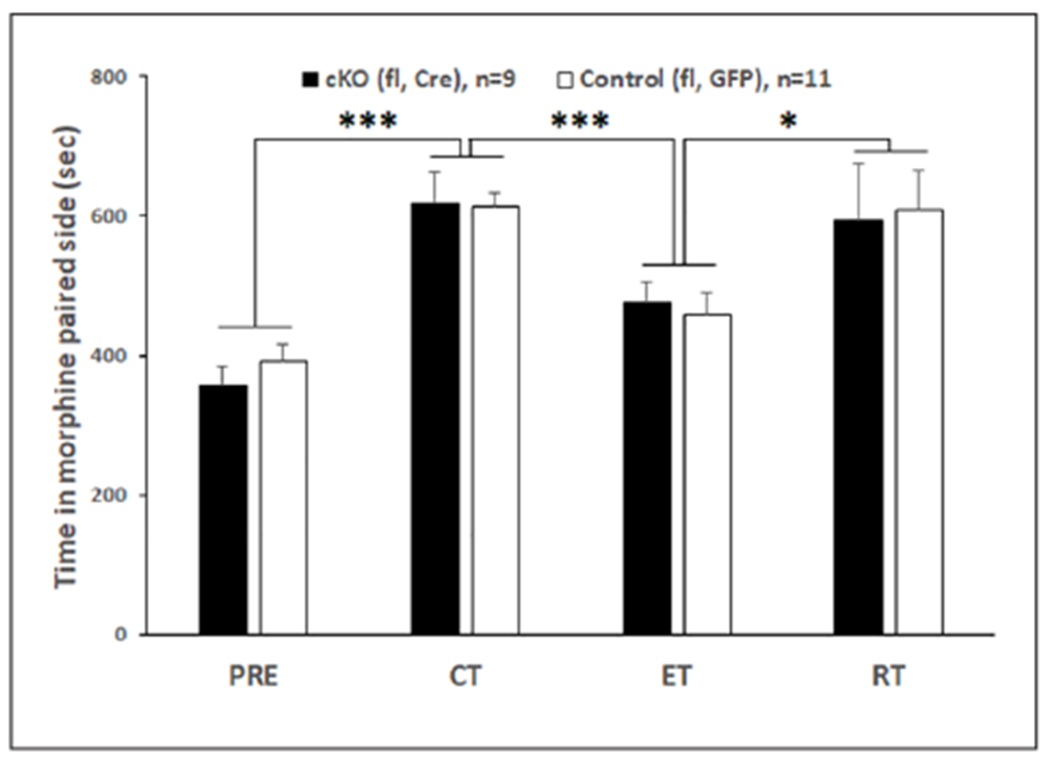
Morphine CPP with stress-induced reinstatement unaffected by DRN cKO. Both floxed Cre and floxed GFP groups developed conditioning, extinction, and swim-stress-induced reinstatement; however, there was no difference between the two groups. Mice spend more time on morphine paired side in conditioning test than precondition test. Extinction training significantly decreased the time in morphine paired side, which was reinstated to the conditioning level when mice were exposed to swim stress. All mice were male. Asterisks indicate significant differences by pre-planned paired t-tests.
(* p < 0.05; *** p < 0.001).
CPP: conditioned place preference; CT: conditioning test; DRN: dorsal raphe nucleus; ET: extinction test; PRE: preconditioning test; RT: reinstatement test.
Experiment 2: 5-HT-specific knockout of α1-GABAA
Verifying the loss of α1-GABAA in 5-HT neurons
Histology.
To verify the selectivity of Cre expression in 5-HT neurons in the TPH2-iCre mice, Cre mice and WT littermates were crossed with R26R-EYFP reporter mice to obtain TPH2-iCre-Rosa and WT-Rosa mice, respectively. Figure 4(a) shows that Cre-induced EYFP expression was only observed in the DRN of TPH2-iCre-Rosa but not WT-Rosa mice. Staining with TPH2 antibody showed extensive colocalization of EYPF and TPH2+ neurons in the DRN of TPH2-iCre-Rosa mice.
Figure 4.
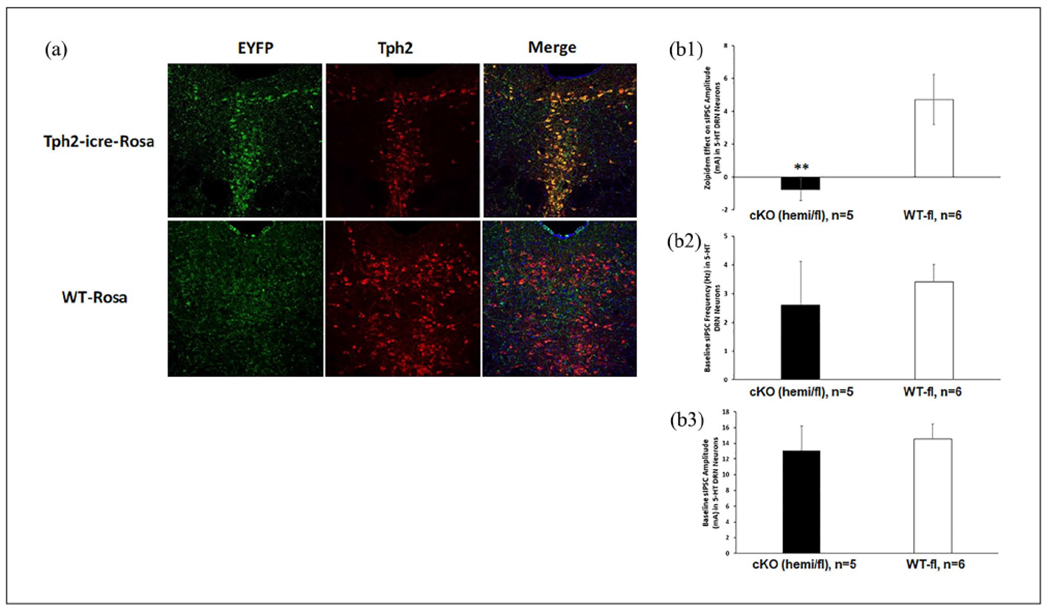
Verification of 5-HT-specific knockout of α1-GABAA. (a) Selectivity of Cre expression in TPH2-IR+ DRN neurons in the TPH2-iCre mouse. Cre-induced EYFP expression was only observed in the DRN of TPH2-iCre-Rosa but not WT-Rosa mice. Staining with TPH2 antibody showed extensive colocalization of EYPF and TPH2+ neurons in the DRN of TPH2-iCre-Rosa mice. (b) Loss of α1-GABAA function and baseline GABA synaptic activity in 5-HT cKO. Zolpidem increased sIPSC amplitude in DRN 5-HT neurons in control (WT/floxed; 6 cells from 3 male and 2 female mice) but not in cKO (TPH2-iCre/floxed; 5 cells from 1 male and 3 female mice) group (b1). However, the baseline sIPSC frequency (b2) and amplitude (b3) did not differ between cKO and the control. Asterisks indicate significant differences from WT/floxed by unpaired t-test (**p < 0.01).
5-HT: 5-hydroxytryptamine; GABA: γ-aminobutyric acid; DRN: dorsal raphe nucleus; TPH2: tryptophan hydroxylase 2; WT: wild type.
Electrophysiology.
Zolpidem increased sIPSC amplitude in DRN 5-HT neurons in control mice (WT/floxed) but not in 5-HT cKO mice (TPH2-iCre/floxed) (Figure 4(b1)). T-test on Zolpidem effect revealed a significant difference between genotypes (t(9) = 3.088, p = 0.013). However, the baseline sIPSC frequency (4b2) and amplitude (4b3) did not differ between the two genotypes, suggesting that 5-HT-specific knockout of α1-GABAA subunit did not change the presynaptic release of GABA or the number or sensitivity of postsynaptic GABAA receptors in 5-HT neurons.
5-HT-specific knockout of α1-GABAA disrupted pre- and post-withdrawal anxiety-like behavior.
The timeline for behavioral studies in 5-HT cKO mice is shown in Figure 5(a). Since no statistically significant sex differences were observed on any experimental endpoint, data were pooled across sex. Moreover, since no differences in behavioral endpoints were observed among the control genotypes (WT/floxed, TPH2-iCre/WT, and WT/WT), their data were pooled as a single control group. Figure 5(b) indicates that repeated morphine injections decreased body weight in both cKO (TPH2-iCre/floxed) and control groups, which returned to baseline at post-injection day 10. Two-way repeated measures ANOVA revealed a main effect of time (F(10,180) = 28.078, p < 0.001) but not genotype or interaction. Post hoc Dunnett’s tests showed that injection days 2–7, post-24 h, and post-48 h were all different from baseline (p < 0.001). This repeated injection protocol also induced reliable spontaneous withdrawal jumps 24 h after the last injection equally in both treatment groups (Figure 5(c)), indicating the similar magnitude of these two signs of somatic withdrawal.
Figure 5.
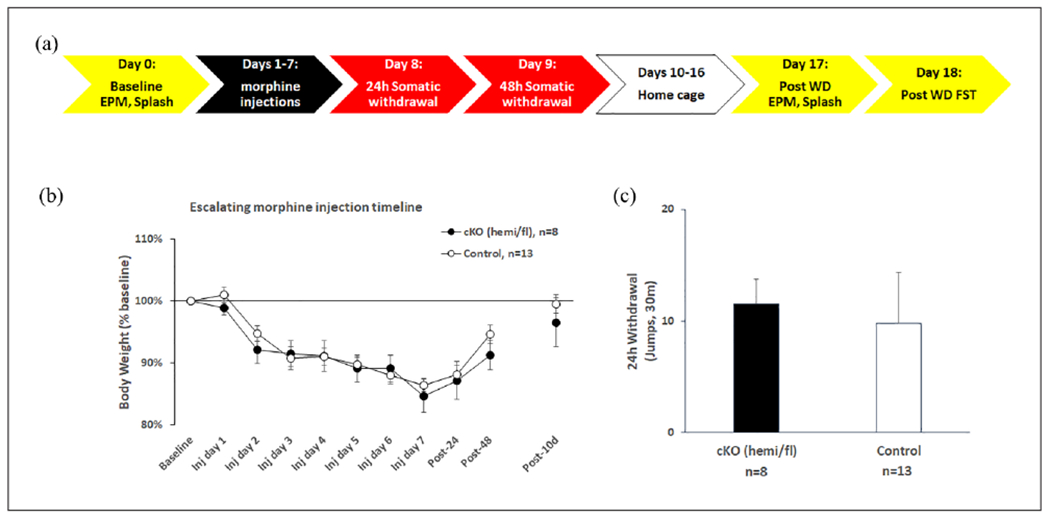
Spontaneous morphine withdrawal is unaffected by 5-HT cKO. (a) Timeline of behavioral studies in 5-HT cKO. (b) Repeated morphine injections decreased body weight in both cKO (TPH2-iCre/floxed) and control groups, which went back to baseline at post-injection day 10, and induced reliable spontaneous withdrawal jumps 24 h after the last injection that did not differ between cKO and control mice (c). cKO (n = 8: 3 male TPH2-iCre/floxed, 5 female TPH2-iCre/floxed), controls (n = 13: 1 male WT/WT, 4 female TPH2-iCre/WT, 5 male WT/floxed, 3 female WT/floxed).
TPH2: tryptophan hydroxylase 2; WT: wild type.
For EPM (Figure 6), 5-HT-specific knockout of α1-GABAA produced an anxiolytic phenotype at baseline, which was normalized after opioid exposure (6a1). Two-way repeated measures ANOVA on % of time in open arms revealed a significant effect of time (pre- vs post-withdrawal) (F(1,28) = 8.529, p = 0.007) and an interaction between genotype and time (F(1,28) = 4.756, p = 0.038). Post hoc Tukey tests show that cKOs showed an increased % of the time in open arms compared with control (p = 0.035) at baseline. cKOs also spent more % of the time in open arms before morphine exposure than after (p = 0.003). These findings indicate an anxiolytic phenotype in the cKO that reverses after morphine withdrawal, potentially indicating the sensitivity of the cKO to post-withdrawal anxiety, a sign of negative affect (Welsch et al., 2020). No other behavioral measures in the EPM (6a2, 6a3, 6a4) or in the splash test (6b) or FST (6c) were different between cKO and control groups. Morphine withdrawal did not change the behavior of the control group in either the EPM or splash test, suggesting that, contrary to cKOs, controls show no development of affective signs of withdrawal in these paradigms at the 1-week post-withdrawal timepoint.
Figure 6.
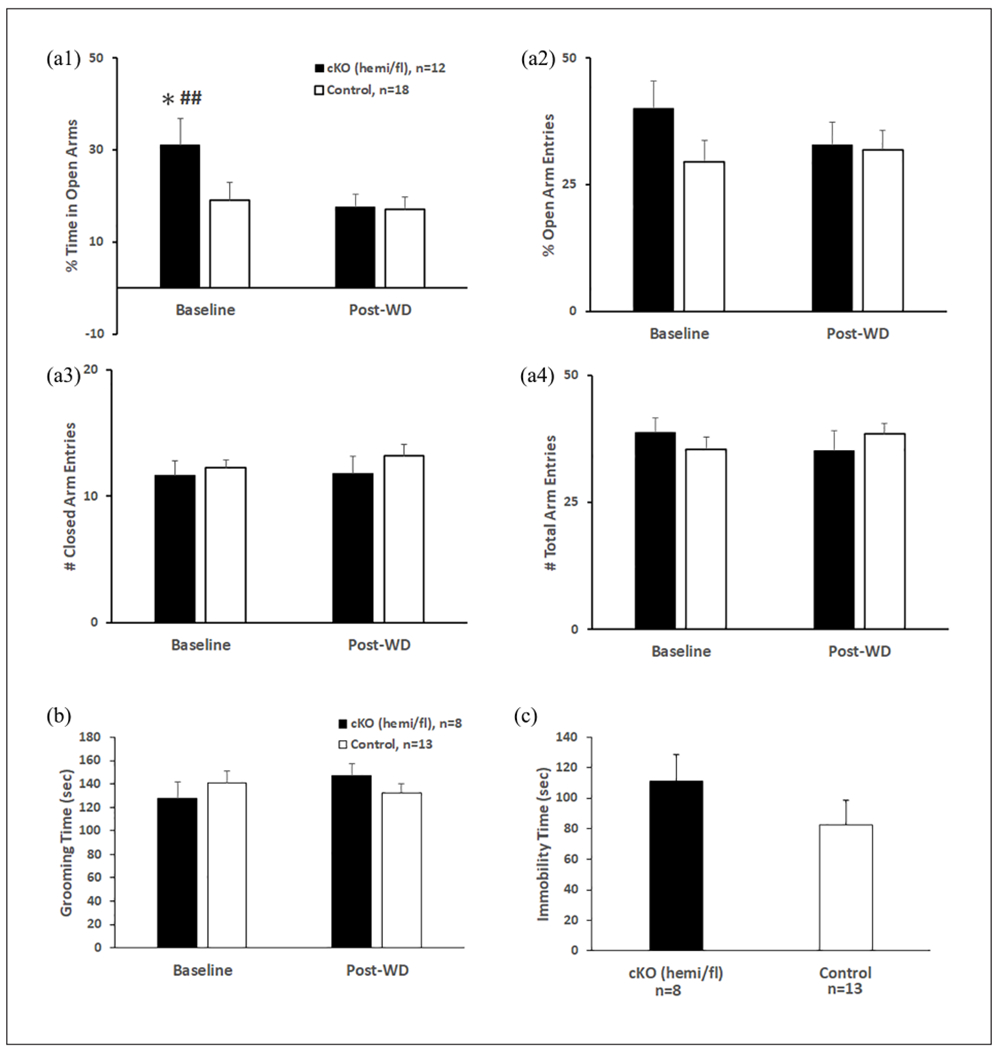
5-HT-specific knockout of α1-GABAA disrupted pre- and post-withdrawal anxiety-like behavior. (a) The anxiolytic phenotype of 5-HT cKO in EPM at baseline normalizes after morphine withdrawal. (a1) cKOs spent more % time in open arms before morphine exposure than control, which decreased significantly post-morphine withdrawal (a1). % open arm entries (a2), closed (a3), and total arm (a4) entries were similar in both groups, before and after morphine exposure. The asterisk indicates a significant difference from control at baseline by post hoc Tukey test (*p < 0.05). Pound signs indicate significant differences from post-withdrawal cKOs by post hoc Tukey test (###p < 0.01). (b) Anhedonia-like behavior in splash test unaffected by 5-HT cKO at baseline and post-morphine withdrawal. The grooming time did not change with morphine exposure and did not differ between cKO and control groups. (c) Depression-like behavior in FST unaffected by 5-HT cKO at post-morphine withdrawal. The immobility time during FST did not differ between cKO and control groups. For EPM test: cKO (n = 12: 3 male TPH2-iCre/floxed, 9 female TPH2-iCre/floxed), controls (n = 18: 1 male WT/WT, 7 male WT/floxed, 4 female TPH2-iCre/WT, 6 female WT/floxed). For splash test and FST: cKO (n = 8: 3 male TPH2-iCre/floxed, 5 female TPH2-iCre/floxed), controls (n = 13: 1 male WT/WT, 4 female TPH2-iCre/WT, 5 male WT/floxed, 3 female WT/floxed).
5-HT: 5-hydroxytryptamine; GABA: γ-aminobutyric acid; EPM: elevated plus maze; FST: forced swim test; TPH2: tryptophan hydroxylase 2; WT: wild type.
Discussion
Mice with an inducible knockout of α1-GABAA receptors in either the DRN or in the entire population of 5-HT neurons have been generated in this study, which provides new tools to further understand the role of GABA-5-HT interactions in opioid addiction and stress disorders. Mice with DRN-specific knockout of α1-GABAA receptors showed normal behavior in tests of anxiety, depression as well as swim stress-induced reinstatement of morphine CPP. By contrast, mice with 5-HT-specific knockout of α1-GABAA receptors showed an anxiolytic phenotype at baseline and increased sensitivity to morphine withdrawal-induced anxiety.
Global constitutive knockout of the α1 subunit produces a large and widespread loss of GABAA receptors in mouse brains (Sur et al., 2001), especially in the brain regions and neuronal types with predominant α1-GABAA receptor expression. For example, knockout of the α subunit abolished most GABAA-mediated inhibition in cerebellar Purkinje neurons (Nietz et al., 2020; Sur et al., 2001). However, in our study, inducible knockout of α1 subunit in either DRN or 5-HT neurons did not change GABAA synaptic activity in the DRN 5-HT neurons as shown by electrophysiology, suggesting compensatory alterations may exist in the DRN 5-HT neurons. Several compensatory alterations are observed in the mouse brain with global constitutive αl subunit knockout, including overexpression of other α subunits in brain regions where α1 subunits are normally distributed, and may explain why these mice do not show profound physiological or behavioral deficits despite losing 60% of GABAA receptors (Kralic et al., 2002, 2006). In the DRN, evidence shows that in addition to the α1 subunit, the majority of 5-HT neurons also co-express α2 and α3 subunits (Corteen et al., 2015). Expression of the α3 subunit in the DRN is highly dynamic (Corteen et al., 2015), and increases significantly in global constitutive α1 subunit knockout mouse brain (Kralic et al., 2006). Therefore, the unchanged GABAA synaptic activity in the DRN could be due to the replacement of α1 by α3 subunit in GABAA receptors. Moreover, pharmacological studies suggest that selective activation of α3-containing GABAA receptors has an anxiolytic effect (Atack et al., 2005; Dias et al., 2005). Thus, the replacement of α1 by α3 subunit may explain the anxiolytic phenotype at baseline in mice with knockout of α1-GABAA receptors in the entire population of 5-HT neurons. Though these compensatory mechanisms should be minimized by the inducible, time-limited nature of the gene deletion, GABAA receptor subunit switching has been demonstrated on timescales as short as 1 day (Brussaard et al., 1997). Further studies will be required to quantify the changes in other subunits of GABAA receptors in the DRN- and inducible 5-HT-specific knockout.
DRN-specific knockout influenced not only 5-HT neurons but also other cell types expressing GABAA receptors in the DRN. The DRN is a heterogeneous nucleus with a diversity of cell types that differ with respect to developmental origin, anatomical connectivity, electrophysiological properties, and functional roles (Commons, 2020; Okaty et al., 2019). Serotonin neurons make up only ~50% of the neuronal population, and other types of neurons include GABAergic, glutamatergic, and dopaminergic, as well as a variety of peptide-releasing neurons (Commons, 2020; Fu et al., 2010; Huang et al., 2019), some of which also express α1 subunits (Corteen et al. 2015). Injecting Cre into the DRN of floxed Gabra1 mice would knockout α1-GABAA receptors from those neurons as well. Such effects, particularly at GABAergic interneurons, would modulate the excitability of 5-HT neurons via local inhibitory circuits, potentially confounding the net effect in the DRN cKO.
On the other hand, 5-HT-specific knockout influenced the entire 5-HT system beyond the DRN. The DRN has been the focus of our studies because of its large population of 5-HT cell bodies and extensive forebrain projections (Commons, 2020; Jacobs and Azmitia, 1992). However, there are multiple raphe nuclei with ascending and descending 5-HT projections including median raphe nucleus (MRN), pontine raphe, nucleus raphe obscurus, and nucleus raphe magnus (Jacobs and Azmitia, 1992; Okaty et al., 2019). While these different nuclei subserve a number of distinct physiological and behavioral functions (Okaty et al., 2019), other functions are shared, such as the contributions of both DRN and MRN to different aspects of stress- and anxiety-related behaviors (Andrade et al., 2013; Zangrossi and Graeff 2014). In our 5-HT-specific knockout mice, the α1 subunit was deleted from all 5-HT neurons and thus could achieve a broader impact on the 5-HT system than DRN-specific knockout. This might explain why the mice with 5-HT-specific knockout of the GABAA α1 subunit showed an anxiolytic phenotype at baseline and were more sensitive to morphine withdrawal than the controls. Further studies are needed to examine how knockout of the α1 subunit affects GABAA-related functions in other 5-HT nuclei such as the MRN.
Previous preclinical studies have shown that opioid withdrawal produces both acute physical/somatic symptoms and more delayed affective symptoms including anxiety-, depression-, and anhedonia-like behaviors (Monroe and Radke, 2023). The time course of withdrawal-induced anxiety differs in the literature based on the opioid regimen employed such that some studies have shown it at the 1-week post-withdrawal timepoint that we tested and others have shown a more delayed expression (Welsch et al., 2020). Our study showed that mice with 5-HT-specific knockout of α1-GABAA are more sensitive to morphine-induced affective but not physical withdrawal signs. Specifically, cKOs had an anxiolytic profile at baseline (more % times in EPM open arms than controls) that was significantly diminished to control levels 1 week following withdrawal. By contrast, controls showed less % time in open arms at baseline which was unchanged following withdrawal. These data indicate less sensitivity of the controls to withdrawal-induced anxiety. Alternatively, it is possible that the low baseline % time in open arms of controls reflected a floor effect that could not be further reduced following withdrawal. However, our control mice spent comparable time in the open arms as the baseline in other morphine withdrawal studies, which is further decreased by prolonged morphine withdrawal (Ma et al., 2018; Zanos et al., 2014, 2016). Thus, the lack of changes in the control mice was less likely due to a floor effect. Another potential confound of the EPM test is its sensitivity to changes following repeated testing, typically demonstrated at intervals of 24–48 h (Cnops et al. 2022). Our EPM data are less likely to be impacted by this effect as the EPM sessions were separated by 17 days. Furthermore, the decreased % time in open arms in cKOs following withdrawal is unlikely due to a carryover effect of repeated exposure to the EPM as the % time in open arms did not change between the two EPM tests in controls.
The early onset of anxiety-like behavior in the knockout mice compared with controls, reversing their initial anxiolytic-like phenotype, suggests that losing α1-GABAA receptors may worsen neuronal adaptations of the 5-HT system that have been observed following morphine exposure, which persist and incubate with morphine abstinence, mediating affective withdrawal signs (Goeldner et al., 2011; Lutz et al., 2011). Altering subunit composition of GABAA receptors also impacts agonist affinity, gating, and pharmacological properties (Barker and Hines, 2020). Studies on global constitutive α1-GABAA knockout mice show that despite no significant behavioral deficits at baseline, the responses to drug challenges can be dramatically changed in knockouts compared with WTs. For example, global α1-GABAA knockout mice are more sensitive to the sedative effects of diazepam than WTs but show no hyper-locomotor response following amphetamine or cocaine treatment (Reynolds et al., 2003). Withdrawal from multiple drugs of abuse can change the expression and composition of GABAA receptors as well (Barker and Hines, 2020). For example, morphine withdrawal selectively increases GABAA ε subunit mRNA expression in locus coeraleus noradrenergic neurons (Heikkilä et al., 2001) but decreases the expression of most GABAA subunits in the nucleus accumbens (Spijker et al., 2004). Electrophysiology studies show that morphine withdrawal increased the frequency but not the amplitude of GABAergic IPSCs in 5-HT DRN neurons, which is associated with an upregulation of the adenosine 3′,5′-monophosphate pathway (Jolas et al., 2000). On the other hand, an earlier study in our laboratory showed that stress-induced reinstatement of morphine CPP increases the amplitude but not frequency of GABAergic IPSCs in 5-HT DRN neurons (Staub et al., 2012), suggesting that GABAA receptors are naturally dynamic, sensitive to both opioids and stressors. Therefore, together with our data, it suggests that changes in the subunit composition of GABAA receptors may contribute to sensitivity to morphine-induced affective withdrawal. Mice with an inducible knockout of α1-GABAA receptors in either the DRN or in the entire population of 5-HT neurons provide new tools to further understand the role of GABA-5-HT interactions in opioid addiction and stress disorders.
Supplementary Material
Acknowledgements
We would like to thank Martin Gallagher (Vanderbilt University) and Shin Kang (Temple University) for generously sharing Gabra1 floxed and R26R-EYFP mice, respectively.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Morphine was generously provided by the NIDA Drug Supply Program. This work was supported by R21 DA037523 (LK), P30 DA013429, and T32 DA007237 (EU).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- Alvarez-Bagnarol Y, García R, Vendruscolo LF, et al. (2023) Inhibition of dorsal raphe GABAergic neurons blocks hyperalgesia during heroin withdrawal. Neuropsychopharmacology 48: 1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade TG, Zangrossi H and Graeff FG (2013) The median raphe nucleus in anxiety revisited. J Psychopharmacol 27: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Arain FM, Boyd KL and Gallagher MJ (2012) Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor α1 subunit. Epilepsia 53: e161–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Hutson PH, Collinson N, et al. (2005) Anxiogenic properties of an inverse agonist selective for alpha3 subunit-containing GABAA receptors. Br J Pharmacol 144: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JS and Hines RM (2020) Regulation of GABA receptor subunit expression in substance use disorders. Int J Mol Sci 21: 4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, et al. (1997) Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron 19: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Cnops V, Iyer VR, Parathy N, et al. (2022) Test, rinse, repeat: A review of carryover effects in rodent behavioral assays. Neurosci Biobehav Rev 135: 104560. [DOI] [PubMed] [Google Scholar]
- Commons KG (2020) Dorsal raphe organization. J Chem Neuroanat 110: 101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corteen NL, Carter JA, Rudolph U, et al. (2015) Localisation and stress-induced plasticity of GABAA receptor subunits within the cellular networks of the mouse dorsal raphe nucleus. Brain Struct Funct 220: 2739–2763. [DOI] [PubMed] [Google Scholar]
- Craige CP, Lewandowski S, Kirby LG, et al. (2015) Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology 93: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, et al. (2005) Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci 25: 10682–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, et al. (2011) Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav 97: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluyau D, Mitra P, Jain A, et al. (2022) Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis. Eur J Clin Pharmacol 78: 931–942. [DOI] [PubMed] [Google Scholar]
- Fu W, Le Maître E, Fabre V, et al. (2010) Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol 518: 3464–3494. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, et al. (2011) Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä AT, Echenko O, Uusi-Oukari M, et al. (2001) Morphine withdrawal increases expression of GABA(A) receptor epsilon subunit mENA in locus coeruleus neurons. Neuroreport 12: 2981–2985. [DOI] [PubMed] [Google Scholar]
- Hernández-Vázquez F, Garduño J and Hernández-López S (2019) GABAergic modulation of serotonergic neurons in the dorsal raphe nucleus. Rev Neurosci 30: 289–303. [DOI] [PubMed] [Google Scholar]
- Huang KW, Ochandarena NE, Philson AC, et al. (2019) Molecular and anatomical organization of the dorsal raphe nucleus. Elife 8: e46464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu-Chen LY and Kirby LG (2010) Anxiety-like effects of SR141716-precipitated delta9-tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neurosci Lett 475: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL and Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72: 165–229. [DOI] [PubMed] [Google Scholar]
- Jolas T, Nestler EJ and Aghajanian GK (2000) Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience 95: 433–443. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, et al. (2008) Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci 28: 12927–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD and Winstanley CA (2011) Contributions of serotonin in addiction vulnerability. Neuropharmacology 61: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2000) Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci 909: 170–185. [DOI] [PubMed] [Google Scholar]
- Koob GF (2021) Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev 73: 163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2016) Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, et al. (2002). Molecular and pharmacological characterization of GABA(A) receptor alpha 1 subunit knockout mice. J Pharmacol Exp Ther 302: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, et al. (2006) Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol 495: 408–421. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Zhang G, Walsh T, et al. (2011) Stress-hyperresponsive WKY rats demonstrate depressed dorsal raphe neuronal excitability and dysregulated CRF-mediated responses. Neuropsychopharmacology 36: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C and Kirby LG (2016) Effects of cocaine history on postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Eur Neuropsychopharmacol 26: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, McCloskey N, Phillips J, et al. (2021) CRF-5-HT interactions in the dorsal raphe nucleus and motivation for stress-induced opioid reinstatement. Psychopharmacology (Berl) 238: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Staub DR and Kirby LG (2013) Role of GABA receptors in dorsal raphe nucleus in stress-induced reinstatement of morphine-conditioned place preference in rats. Psychopharmacology (Berl) 230: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 92: 180–185. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Pradhan AA, Goeldner C, et al. (2011) Sequential and opposing alterations of 5-HT(1A) receptor function during withdrawal from chronic morphine. Eur Neuropsychopharmacol 21: 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wang N, Wang X, et al. (2018) Wnt7a in mouse insular cortex contributes to anxiety-like behavior during protracted abstinence from morphine. Neuroscience 394: 164–176. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, et al. (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41: 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeman RM and Whiting PJ (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143. [DOI] [PubMed] [Google Scholar]
- Monroe SC and Radke AK (2023) Opioid withdrawal: Role in addiction and neural mechanisms. Psychopharmacology (Berl) 240: 1417–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP and Homberg JR (2015) The role of serotonin in drug use and addiction. Behav Brain Res 277: 146–192. [DOI] [PubMed] [Google Scholar]
- Nietz A, Krook-Magnuson C, Gutierrez H, et al. (2020) Selective loss of the GABA. J Neurophysiol 124: 1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard SK, Hourguettes NJ, Sobczak GG, et al. (2016) Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. J Neurosci 36: 9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Commons KG and Dymecki SM (2019) Embracing diversity in the 5-HT neuronal system. Nat Rev Neurosci 20: 397–424. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates (2nd edn). San Diego: Academic Press. [Google Scholar]
- Pomrenze MB, Cardozo Pinto DF, Neumann PA, et al. (2022) Modulation of 5-HT release by dynorphin mediates social deficits during opioid withdrawal. Neuron 110: 4125.e6–4143.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Lepichon M and Jalfre M (1977) Depression - new animal-model sensitive to antidepressant treatments. Nature 266: 730–732. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Al-Hasani R, Fakira AK, et al. (2014) Hippocampal long-term potentiation is disrupted during expression and extinction but is restored after reinstatement of morphine place preference. J Neurosci 34: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhamzeh M, Moravej FG, Arabi M, et al. (2022) The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol 42: 1671–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, O’Meara GF, Newman RJ, et al. (2003) GABA(A) alpha 1 subunit knock-out mice do not show a hyperlocomotor response following amphetamine or cocaine treatment. Neuropharmacology 44: 190–198. [DOI] [PubMed] [Google Scholar]
- Shi X, von Weltin E, Fitzsimmons E, et al. (2022) Reactivation of cocaine contextual memory engages mechanistic target of rapamycin/S6 kinase 1 signaling. Front Pharmacol 13: 976932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S, Houtzager SW, De Gunst MC, et al. (2004) Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB J 18: 848–850. [DOI] [PubMed] [Google Scholar]
- Staub DR, Lunden JW, Cathel AM, et al. (2012) Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Psychoneuroendocrinology 37: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, et al. (2001) Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci 21: 3409–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, Debold JF, et al. (2010) GABA(A) receptors in the dorsal raphe nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology (Berl) 211: 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R and Auerbach SB (2005) mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res 1049: 70–79. [DOI] [PubMed] [Google Scholar]
- Taracha E (2021) The role of serotoninergic system in psychostimulant effects. Postep Psychiatr Neurol 30: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I and Van BE (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res 1314: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch L, Bailly J, Darcq E, et al. (2020) The negative affect of protracted opioid abstinence: Progress and perspectives from rodent models. Biol Psychiatry 87: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang Y, Ma Z, et al. (2013) l-Isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend 133: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Belzung C and Surget A (2008) Mouse strain differences in the unpredictable chronic mild stress: A four-antidepressant survey. Behav Brain Res 193: 140–143. [DOI] [PubMed] [Google Scholar]
- Zangrossi H and Graeff FG (2014) Serotonin in anxiety and panic: contributions of the elevated T-maze. Neurosci Biobehav Rev 46: 397–406. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Gonzalez LR, et al. (2016) Emotional impairment and persistent upregulation of mGlu5 receptor following morphine abstinence: Implications of an mGlu5-MOPr interaction. Int J Neuropsychopharmacol 19: pyw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, et al. (2014) The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 39: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


