Abstract
Background:
We have limited evidence for the relationship of high sugar intake with dementia risk.
Objective:
To determine whether high sugar intake is associated with an increased risk of dementia in community-dwelling older adults
Methods:
This study included 789 participants of the Rush Memory and Aging Project (community-based longitudinal cohort study of older adults free of known dementia at enrollment), with annual clinical assessments and complete nutrient data (obtained by validated food frequency questionnaire). Clinical diagnosis of dementia is based on the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association. We used Cox proportional hazard models.
Results:
118 participants developed dementia during 7.3 ± 3.8 years of follow-up. Those in the highest quintile of total sugar intake were twice as likely to develop dementia than those in the lowest quintile (Q5 versus Q1:HR=2.10 (95%CI: 1.05, 4.19) when adjusted for age, sex, education, APOE ε4 allele, calories from sources other than sugar, physical activity, and diet score. Higher percent calories from sugar were positively associated with dementia risk (β=0.042, p = 0.0009). In exploratory analyses, the highest versus lowest quintile of fructose and sucrose in the diet had higher dementia risk by 2.8 (95%CI: 1.38, 5.67) and 1.93 (95%CI: 1.05, 3.54) times, respectively.
Conclusions:
A higher intake of total sugar or total calories from sugar is associated with increased dementia risk in older adults. Among simple sugars, fructose (e.g., sweetened beverages, snacks, packaged desserts) and sucrose (table sugar in juices, desserts, candies, and commercial cereals) are associated with higher dementia risk.
Keywords: Alzheimer’s disease, dementia, longitudinal, total sugar intake
INTRODUCTION
It is estimated that 6.2 million Americans age 65 and older are living with Alzheimer’s disease (AD) dementia [1], a chronic disease of aging associated with progressive loss of cognitive function and the ability to carry out activities of daily living [2]. Age is among the strongest known risk factors for AD dementia [3], and due to an aging population, the number of adults living with dementia is projected to more than double by 2060 [1]. AD is a costly disease that results in significant loss of lives annually. In 2022, the estimated total payment for health care, long-term care, and hospice care for 65 and older living with dementia is around $321 billion [1]. Various lifestyle preventive strategies including diet are of great public interest. Healthy dietary patterns such as Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) and a Mediterranean diet are associated with a decreased risk of AD dementia in community-dwelling older adults [4, 5]. One important aspect of these healthy dietary patterns is limiting foods high in sugar. Animal studies have demonstrated that higher sugar intake induces insulin resistance and is associated with memory impairment and more amyloid-β accumulation in the brain [6, 7]. Human studies investigating the relationship between sugar intake and the risk of AD dementia have been limited. Sugar intake is associated with poor cognitive performance [8, 9] and risk of AD dementia among women [10]. Studies have reported higher sugared beverage intake associated with increased risk of dementia, AD dementia, stroke [11], and total brain volume and episodic memory [12]. Artificially sweetened beverages were also associated with dementia [13], while a recent meta-analysis reported null findings for sugar-sweetened beverages and dementia [14]. In the present study, we investigated whether there is a relationship between total sugar intake or percent calories from sugar and the risk of dementia in community-dwelling older adults (including both men and women). In additional analyses, we explored whether any specific simple sugars including sucrose, fructose, glucose, maltose, and lactose are associated with dementia risk.
METHODS
Data and sample
The study was conducted among Rush Memory and Aging Project (MAP) participants. MAP is an ongoing longitudinal cohort (began in 1997) study of chronic disease of aging in older adults from about 40 retirement communities, senior public housing units, and individual homes in the greater Chicago, Illinois area [15]. At the time of enrollment, participants consent to annual clinical assessments consisting of medical history, neurological exam, routine laboratory measures, and cognitive testing, more details about the recruitment and study methods have been published earlier [15, 16]. At the time of analysis, 2,160 participants without any known dementia had completed enrollment and baseline evaluation in MAP. The dietary assessments began in 2004, by then 83 MAP participants died, 9 withdrew from the study, and 64 were ineligible (dementia symptoms at the time of dietary assessments or English was not the primary language, as all the dietary assessments were conducted in English). Of those eligible for dietary assessments, 375 withdrew or died without completing a food frequency questionnaire (FFQ), the dietary assessment tool), 10 refused to fill out the FFQ, and 192 participants were excluded as their FFQ was incomplete or reported implausible calorie intake (<700 or >4,000 kilocalories for men and < 500 or >3,800 kilocalories for women). For another 401 participants, the derivation of dietary data was still in process and 191 had a missing value for total sugar, hence were also excluded from this study. Further out of 835 with complete dietary data, we excluded those without two cognitive assessments or clinical dementia at the time of FFQ assessment (n = 46). The analytical sample for this study was 789 (median (Inter quartile range) age: 80 years, (75, 84).
The study was approved by an Institutional Review Board of Rush University Medical Center. All subjects signed informed consent and an Anatomical Gift Act. More detailed descriptions of study procedures are available [16]. MAP data can be requested at https://www.radc.rush.edu.
Dietary sugar intake
Dietary assessments were done using a validated FFQ based on the modified-Harvard FFQ consisting of 144 items [17]. Individual nutrient intakes were estimated using reported frequencies and age- and sex-standardized portion sizes from the US nationally representative food composition tables. Intakes of total sugar, sucrose, fructose, glucose, maltose, and lactose were estimated in grams/day [18] and modeled as quintiles. Total sugar includes all the soluble carbohydrates from food, fructose and glucose are the simple sugars, sucrose (glucose + fructose), maltose (glucose + glucose), and lactose (glucose + galactose) are the disaccharides, i.e., compound sugars. Calorie adjustment was carried out using the nutrient residual approach controlling for sex. Percent calories from sugar was also computed (calories from sugar ((total sugar (grams/day) multiplied by 4 kilocalorie (Kcal) / total calories)*100) [19].
Dementia and ADs dementia
A clinical diagnosis of cognitive status is rendered annually at each assessment using a three-step process. All participants undergo a uniform, structured, clinical evaluation including a batter of 21 tests, all scored on computer. Next a neuropsychologist experienced with cognitive testing of older adults reviews the test results and rates the severity of impairment in five cognitive domains using a 6-level rating ranging from normal (1) to severe (5). A final clinical evaluation and review of all data by a neuropsychologist, geriatrician, or geriatric nurse practitioner, who renders a final clinical judgment regarding the no impairment, presence of cognitive impairment and dementia. Clinical diagnosis of dementia and AD dementia is based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) and requires a meaningful decline in cognitive function based on at least two assessments, and deficits in memory and at least one other cognitive domain [20].
Covariates
Sociodemographic characteristics (age, gender, and education [years]) were measured at the baseline using a standard questionnaire. Non-sugar calories were computed by subtracting sugar calories (sugar in grams/day multiplied by 4 kcal) from total calorie intake (computed using the FFQ responses). Genotyping was performed by Polymorphic DNA Technologies (Alameda, California), using high throughput sequencing to determine apolipoprotein E (APOE ε4) carrier status by the two polymorphisms of rs429358 (codon 112) and rs7412 (codon 158) at exon 4 of the APOE gene [21]. Respondents with at least one allele were considered APOE ε4 positive. Physical activity (hours/week) was recorded based on self-reported time spent over two weeks on five activities (walking for exercise, yard work, calisthenics, biking, and water exercise) [22]. Diet quality was assessed using the MIND diet score (range: 0–15, a higher score indicates higher concordance, i.e., better diet). The MIND diet score as previously described has 15 dietary components including 10 brain-healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil, and wine) and 5 unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food) that were reverse coded, i.e., “0” if consumed more and “1” if consumed less [23]. Alcohol consumption was obtained from the computed output of the FFQ in grams/day. Diabetes was determined by self-report or diabetes medication use.
Statistical methods
Descriptive analyses were carried out using simple means and standard deviations. Baseline differences in sample characteristics were presented for the overall sample and compared across quintile of total sugar intake at baseline. Cox proportional hazards models were used to test our primary hypothesis of the relationship between intake of total sugar, with incidence of dementia. In secondary models, we replaced intake of total sugar with total calories from sugar and with the specific simple sugars, sucrose, fructose, glucose, maltose, and lactose. The proportionality of the hazard’s assumption was verified (graphically by plotting survival probability). A log rank test was used to compare the survival curves. To examine linear trends of the sugar associations, we also employed variables of the sugar intake in which all records within a quintile were scored at the median value and report the p-value for the linear trend. Model 1 included age, education (years), sex, APOE ε4 allele status, and total energy from non-sugar sources. Model 2 further controlled for physical activity (hours/week). Model 3 was model 1 further controlled for the overall healthy dietary pattern (MIND diet score), and model 4 was controlled for physical activity and MIND diet score in the same model. As a secondary analysis, we further controlled the models for alcohol intake (grams/day). In sensitivity analyses, we examined associations by excluding individuals with diabetes at baseline and repeating the primary analyses. We also tested the interaction of sugar intake with MIND diet score for its association with dementia. The statistical significance was considered at p < 0.050. All the analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Selected sample characteristics are presented in Table 1. The analytical sample was predominantly white (93%), female (76%), and with the mean age of 79.0 (±7.1) years. Respondents with the highest intakes of sugar at baseline had less calories coming from other sources (Table 1), and the highest overall carbohydrate intake and lowest relative intakes of fat and protein (Supplementary Table 1). Respondents with the highest sugar intake consumed 34% of their total calories from sugar, as compared to 17% of the total calories from sugar consumed by those with the lowest intake. The average overall sugar intake was 106 (±1.5) grams/day (24% of total energy intake). There were 118 cases of incident dementia over 7.3 ± 3.8 years of follow-up. Out of these 118 cases, 90 were deceased with neuropathology data available. Of these 90, 84 (93%) had a diagnosis of AD dementia, and 82% (69 out of 84) met pathologic criteria for AD (based on NIA-Reagan criteria, high or intermediate likelihood).
Table 1.
Baseline characteristics of the analytical sample (N = 789)
| Variables | Overall | Quintile of Total Sugar Intake |
|||||
|---|---|---|---|---|---|---|---|
| First Q1 | Second Q2 | Third Q3 | Fourth Q4 | Fifth Q5 | p ∧ | ||
| N | 789 | 158 | 158 | 158 | 157 | 158 | |
| Total sugar intake, grams/day (median) | 102 | 71 | 89 | 102 | 115 | 133 | n/a |
| Percent calories from total sugar (median) | 24% | 17 % | 22% | 24% | 27% | 32% | <0.0001 |
| Age, y; mean ± SD | 79.0 ± 7.1 | 78.0 ± 7.8 | 78.8 ± 7.2 | 78.8 ± 7.1 | 80.0 ± 6.5 | 79.3 ± 6.7 | 0.07 |
| Female, % | 76 % | 82 % | 81 % | 72 % | 71 % | 75 % | 0.08 |
| Education, y; mean ± SD | 15.0 ± 3.0 | 14.6 ± 2.8 | 15.3 ± 3.1 | 15.2 ± 3.0 | 15.1 ± 3.1 | 14.7 ± 3.0 | 0.16 |
| APOE ε4, % | 21% | 21% | 23% | 20% | 18% | 22% | 0.86 |
| MIND diet score; mean ± SD | 7.9 ± 1.8 | 7.4 ± 1.2 | 7.6 ± 1.6 | 8.0 ± 1.9 | 8.0 ± 1.6 | 8.2 ± 1.9 | 0.008 |
| Total Calories from other sources/day*; mean ± SD | 1,308 ± 408 | 1,440 ± 537 | 1,365 ± 416 | 1,337 ± 359 | 1,545 ± 443 | 1,121 ± 292 | <0.0001 |
| Physical activity, hours/week; mean ± SD | 3.3 ± 3.9 | 3.3 ± 4.1 | 2.8 ± 2.7 | 3.1 ± 3.4 | 4.3 ± 5.5 | 3.4 ± 3.3 | 0.15 |
| Alcohol intake (grams/day); mean ± SD | 4.5 ± 8.8 | 7.8 ± 13.8 | 5.0 ± 8.4 | 4.4 ± 7.2 | 2.8 ± 5.6 | 2.5 ± 4.6 | 0.002 |
| Diabetes, % | 12% | 14% | 16% | 11% | 10% | 11% | 0.87 |
Total calories from sources other than total sugar.
(ANOVA or Kruskal-Wallis chi-square test).
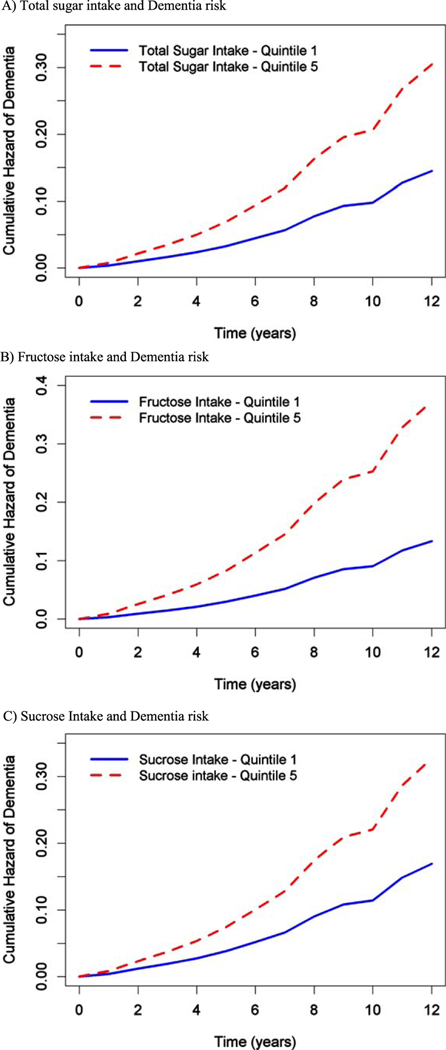
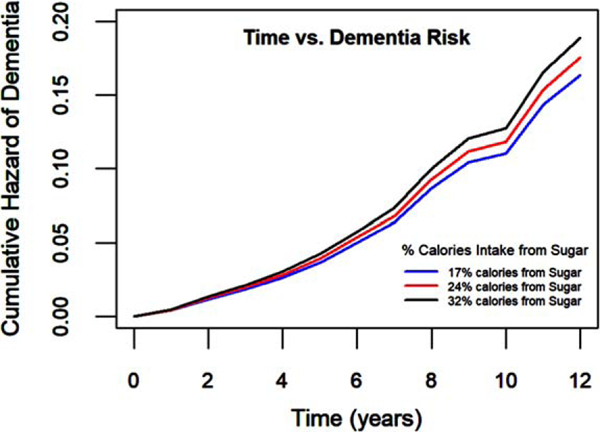
In the age-adjusted model, total sugar intake was not associated with dementia risk (Q5 versus Q 1: HR (95% CI)=1.41 (0.76, 2.62), p for trend = 0.54). However, when further controlled for other covariates including sociodemographic characteristics (age, sex, education), APOE ε4 allele status, total calories from non-sugar sources, we found a significant association of total sugar intake and dementia risk (Table 2). Further controlling for physical activity in model 2, (Supplementary Table 2) and MIND diet in model 3 (Table 2) indicated similar findings. In fully adjusted model when controlled for sociodemographic characteristics, genetic factor, calories, physical activity, and MIND diet score, we found those in the highest quintile of total sugar intake compared to those in the lowest quintile, have twice the risk of developing dementia (Q5 versus Q1: HR (95% CI)=2.1 (1.05, 4.19), p for trend = 0.08, Fig. 1A). We further assessed percentage calories coming from sugar as our exposure variable and had similar findings for 1 unit increase (HR (95% CI)=1.04 (1.01, 1.08). Thus, every 10% increase in calories from total sugar may increase dementia risk by almost 40%. This model was controlled for sociodemographic characteristics, genetic factor, physical activity, and MIND diet. Figure 2 shows that consuming a higher percentage of calories from sugar (lines showed include 17%, 24%, and 32% calories from sugar) had higher cumulative hazard of dementia with time.
Table 2.
Sugar Intake by quintiles and Alzheimer’s disease dementia risk
| Median Intake; N (grams/day) | Model 1 | Model 1 + MIND diet | Model 1 + MIND diet + Physical activity | |
|---|---|---|---|---|
| Total sugar intake | ||||
|
| ||||
| Q1 | 71 grams/day; N = 158 | Ref | Ref | Ref |
| Q3 HR (95% CI) | 102 grams/day; N = 158 | 1.30 (0.67, 2.53) | 1.39 (0.71, 2.72) | 1.42 (0.72, 2.780) |
| Q5 HR (95% CI) | 133 grams/day; N = 158 | 1.77 (0.90, 3.46) | 2.04 (1.03, 4.06) | 2.10 (1.05, 4.19) |
| p-trend | 0.19 | 0.09 | 0.08 | |
|
| ||||
| Sucrose Intake | ||||
|
| ||||
| Q1 | 27 grams/day; N = 158 | Ref | Ref | |
| Q3 HR (95% CI) | 41 grams/day; N = 159 | 1.40 (0.75, 2.62) | 1.39 (0.74, 2.59) | 1.33 (0.71, 2.50) |
| Q5 HR (95% CI) | 58 grams/day; N = 159 | 1.95 (1.07, 3.59) | 1.90 (1.04, 3.48) | 1.93 (1.05, 3.54) |
| p-trend | 0.11 | 0.13 | 0.11 | |
|
| ||||
| Maltose Intake | ||||
|
| ||||
| Q1 | 1.0 grams/day; N = 158 | Ref | Ref | |
| Q3 HR (95% CI) | 1.6 grams/day; N = 159 | 0.97 (0.55, 1.71) | 0.98 (0.56, 1.72) | 0.97 (0.55, 1.70) |
| Q5 HR (95% CI) | 2.5 grams/day; N = 158 | 0.97 (0.54, 1.76) | 0.93 (0.51, 1.69) | 0.94 (0.52, 1.72) |
| p-trend | 0.92 | 0.78 | 0.80 | |
|
| ||||
| Lactose Intake | ||||
|
| ||||
| Q1 | 5 grams/day; N = 158 | Ref | Ref | Ref |
| Q3 HR (95% CI) | 15 grams/day; N = 158 | 1.27 (0.68, 2.38) | 1.87 (0.99, 3.51) | 1.39 (0.74, 2.63) |
| Q5 HR (95% CI) | 30 grams/day; N = 158 | 1.33 (0.68, 2.62) | 1.45 (0.73, 2.86) | 1.43 (0.72, 2.84) |
| p-trend | 0.69 | 0.53 | 0.60 | |
|
| ||||
| Fructose Intake | ||||
|
| ||||
| Q1 | 13 grams/day; N = 158 | Ref | Ref | Ref |
| Q3 HR (95% CI) | 22 grams/day; N = 158 | 1.96 (1.03, 3.72) | 2.26 (1.17, 4.34) | 2.26 (1.17, 4.35) |
| Q5 HR (95% CI) | 33 grams/day; N = 159 | 2.21 (1.12, 4.36) | 2.77 (1.37, 5.58) | 2.80 (1.38, 5.67) |
| p-trend | 0.14 | 0.033 | 0.028 | |
|
| ||||
| Glucose Intake | ||||
|
| ||||
| Q1 | 12 grams/day; N = 158 | Ref | Ref | Ref |
| Q3 HR (95% CI) | 19 grams/day; N = 157 | 1.65 (0.890, 3.03) | 1.85 (0.99, 3.43) | 1.77 (0.95, 3.31) |
| Q5 HR (95% CI) | 28 grams/day; N = 159 | 1.53 (0.77, 3.02) | 1.82 (0.91, 3.67) | 1.84 (0.91, 3.71) |
| p-trend | 0.42 | 0.18 | 0.17 | |
(N = 786; Cox proportional hazards models). Model 1 is controlled for age, sex, education, APOE ε4 status, total calories from non-sugar sources.
Fig. 1.
A) Total sugar intake and Dementia risk. B) Fructose intake and Dementia risk. C) Sucrose Intake and Dementia risk.
Fig. 2.
Those consuming a higher percentage of calories from sugar (lines include 17%, 24%, and 32%) had a higher risk of incident dementia. The model was controlled for age, sex, education), APOE ε4 allele status, physical activity, and MIND diet.
Additionally, we also investigated the associations of other sugars including sucrose, fructose, maltose, and lactose with dementia risk. In the age-adjusted models, none of the sugars were associated with dementia risk (data not shown). Whereas, when controlled for other covariates we found some significant relations. In fully adjusted model, those in the highest quintile intake of sucrose when compared to those in the lowest, had nearly twice the risk of developing dementia during the years of follow-up (HR (95%)=1.93 (1.05, 3.54), Table 2). Similarly, higher fructose intake was also associated with higher AD dementia risk. Maltose and lactose had no association with AD dementia risk (Table 2). The participants with the highest intakes of total sugar developed AD dementia an average of 7.1 years earlier than those with the lowest intake.
Although alcohol consumption was low in this cohort of older adults (mean of 4.5 ± 8.8 grams/day; one standard drink size is 10–14 grams), as a secondary analysis we further controlled the models for alcohol intake (grams/day). The effect estimates for any sugar associations with dementia risk did not change (Q5 versus Q1: total sugar- HR (95%)=2.09 (1.04, 4.21), sucrose- HR (95%)=1.91 (1.04, 3.51), fructose- HR (95%)=2.84 (1.37, 5.88). The results of sensitivity analyses are presented in Supplementary Table 3. When those with diabetes at baseline were excluded in the analyses (n = 98) the relationship between total sugar intake and risk of AD dementia was slightly stronger. In fully adjusted model, the hazard comparing those in the fifth quintile to those in the first was 2.30 (95% CI: 1.08, 4.94). Additionally, we also assessed whether the MIND diet score modified the association of total sugar intake with dementia; we found no significant interaction (p = 0.35).
DISCUSSION
In this study of community-dwelling older adults, we found that high total sugar intake was associated with a higher risk of AD dementia when controlled for demographic factors, genetic risk of AD, physical activity, and diet. Compared to those with the lowest intake of total sugar, the participants with the highest intake developed AD dementia an average of 7.1 years earlier or in other words they had twice the risk of developing AD dementia. Overall, a higher percent calorie from sugar were associated with higher risk of AD dementia. We are aware of no prior study examining the relationship between percent calories from sugar intake and AD dementia. Among different forms of sugar, fructose and sucrose were associated with AD dementia risk. Those in the highest quintile of fructose intake when compared to those in the lowest quartile had 2.8 times higher risk of dementia. After removing participants with baseline diabetes (who may have decreased their sugar intake after diagnosis) the estimated association between sugar and dementia risk was slightly stronger. These simple sugars such as fructose (primary sources include high fructose corn syrup in soda, juice, sweetened yogurt, fast food, packaged desserts, snack foods) and sucrose (disaccharide of fructose and glucose, i.e., table sugar primarily found in juices, desserts, candies, and sweetened commercial cereals) are widely used in the commercially available food products and should be further investigated among older adults for their roles in neurodegenerative disorders such as AD and related dementia. These findings among older adults suggest that consuming less calories from sugar, and lesser intake of fructose and sucrose in diet may reduce the risk of dementia.
Sugared beverages, typically sweetened with high-fructose corn syrup, have been demonstrated to be the leading source of added sugar in the diets of Americans, and their consumption is associated with increased risk of obesity, diabetes, heart disease, and stroke [24, 25]. A previous study of data from the Framingham Heart Study found that respondents with the highest intake of sugared beverages had lower total brain volume and poorer performance on episodic memory than those with the lowest intake [12]. In the same cohort, recent findings indicated higher intake of sugar in beverages associated with higher risk of dementia [11]. Another study (Women Health Initiative, WHI) recently reported higher total sugar intake associated with reduced risk of AD among women [10]. They also found lactose intake association with increased AD risk. Our findings support the existing literature for total sugar intake among both men and women, suggesting that community-dwelling older adults with higher total sugar intake or more calories from sugar compared to those with lower intake may have higher risk of dementia. As reported in previous literature, we did not find any association between lactose and dementia risk; however, we found association of higher fructose and sucrose intakes with increased dementia risk. We speculate there can be various reasons for this difference ranging from different study populations to different methods of outcome assessment. The previous study included women only with an average age around 66 years and AD diagnosis was obtained from the reports using a standard questionnaire, whereas in our study we included both men and women, average age was 79 years, and the dementia diagnosis was based on criteria of the joint working group of the NINCDS/ADRDA.
Impaired glucose metabolism is related with cognitive function and AD [26, 27]. Studies linking AD directly to insulin resistance have been limited, but obesity, diabetes, and abnormal levels of glucose and insulin levels are risk factors for AD [28]. Several plausible biological pathways may link sugar intake to risk of dementia. Importantly, most sugars are readily converted to glucose, placing them under homeostatic regulation by insulin. Evidence suggests that brain insulin resistance may play a role in the development of AD [27, 29, 30]. The insulin-mediated glucose transporter, GLUT4, is highly expressed in the hippocampus [31]. Blocking translocation of the GLUT4 transporter in the hippocampus has been shown to impair memory in an animal model [32, 33]. Animal studies have also linked intakes of both fructose and glucose with impaired glucose metabolism [34], and indicate fructose, driving the metabolic complications [35].
Although glucose and fructose are metabolized differently, both have been shown to promote inflammation and the formation of reactive oxygen species [36, 37], and it is well established that both inflammation and oxidative stress are related to AD. Further studies are needed to fully understand the role of sugar, and specifically simple sugars and AD.
Our study has various strengths and weaknesses. The strengths include large community-based sample of older adults who were prospectively followed for multiple years, via standardized tests, structured annual neurological assessments, clinical diagnosis of dementia and clinical AD dementia based on NINCDS/ADRDA criteria, and a dietary assessment tool validated in older adults. However, the study has some limitations. The observational study design implies that we can neither establish causation nor ensure that there are no unaddressed confounders. The FFQ is a self-reported tool to capture diet and may suffer recall bias. Total sugar used in the study includes sugar coming from various food sources and not just high sugar foods. Future studies specifically investigating added sugars, sweetened beverages, foods with high fructose corn syrup and artificial sweetener are needed to understand their roles in dementia risk. Additionally, as the analytical sample was majority non-Hispanic Whites and older, the study results may not generalize to other diverse or younger populations.
Findings from this study suggest that overall high sugar intake or a higher percentage of calories from sugar is associated with increased dementia risk. Those in the highest quintile of sugar intake were twice more likely to develop dementia during the years of follow-up compared to those who were in lowest quintile. Exploratory analysis indicated higher fructose (a monosaccharide) and sucrose (disaccharide composed of glucose and fructose moieties) intakes were positively associated with dementia risk. As both fructose and sucrose are readily used in commercially available food, these results should be further investigated for its role in AD and related dementias.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participants and the staff of Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center. We also thank the biostatistician Dominika Burba who worked on this project. Lastly, we thank Dr. Martha Clare Morris for her ground-breaking work on the MIND Diet and for providing initial support for this study.
FUNDING
This study was supported by grants from the National Institute of Health (R01AG17917, R01AG 054476, R01AG058679, and R01AG073627).
This paper is dedicated to Dr. Chris N. Ford. We would like to acknowledge Dr. Ford’s contribution to the development of this research idea, and the design of this paper before his untimely passing.
Footnotes
CONFLICT OF INTEREST
KD is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
All other authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230013.
DATA AVAILABILITY
MAP data can be requested at http://www.radc.rush.edu.
REFERENCES
- [1].(2021) 2021. Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [2].Martyr A, Clare L (2012) Executive function and activities of daily living in Alzheimer’s disease: A correlational meta-analysis. Dement Geriatr Cogn Disord 33, 189–203. [DOI] [PubMed] [Google Scholar]
- [3].Guerreiro R, Bras J (2015) The age factor in Alzheimer’s disease. Genome Med 7, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O (2019) The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease-a review. Adv Nutr 10, 1040–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cao D, Lu H, Lewis TL, Li L (2007) Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282, 36275–36282. [DOI] [PubMed] [Google Scholar]
- [7].Francis HM, Stevenson RJ (2011) Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci 125, 943–955. [DOI] [PubMed] [Google Scholar]
- [8].Chong CP, Shahar S, Haron H, Din NC (2019) Habitual sugar intake and cognitive impairment among multi-ethnic Malaysian older adults. Clin Interv Aging 14, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ye X, Gao X, Scott T, Tucker KL (2011) Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br J Nutr 106, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu L, Volpe SL, Ross JA, Grimm JA, Van Bockstaele EJ, Eisen HJ (2022) Dietary sugar intake and risk of Alzheimer’s disease in older women. Nutr Neurosci 25, 2302–2313. [DOI] [PubMed] [Google Scholar]
- [11].Miao H, Chen K, Yan X, Chen F (2021) Sugar in beverage and the risk of incident dementia, Alzheimer’s disease and stroke: A prospective cohort study. J Prev Alzheimers Dis 8, 188–193. [DOI] [PubMed] [Google Scholar]
- [12].Pase MP, Himali JJ, Jacques PF, DeCarli C, Satizabal CL, Aparicio H, Vasan RS, Beiser AS, Seshadri S (2017) Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimers Dement 13, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pase MP, Himali JJ, Beiser AS, Aparicio HJ, Satizabal CL, Vasan RS, Seshadri S, Jacques PF (2017) Sugar- and artificially sweetened beverages and the risks of incident stroke and dementia: A prospective cohort study. Stroke 48, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun Q, Yang Y, Wang X, Yang R, Li X (2022) The association between sugar-sweetened beverages and cognitive function in middle-aged and older people: A meta-analysis. J Prev Alzheimers Dis 9, 323–330. [DOI] [PubMed] [Google Scholar]
- [15].Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 64, S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS (2012) Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morris MC (2003) Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 158, 1213–1217. [DOI] [PubMed] [Google Scholar]
- [18].Peralta M, Heskey C, Shavlik D, Knutsen S, Mashchak A, Jaceldo-Siegl K, Fraser GE, Orlich MJ (2021) Validity of FFQ estimates of total sugars, added sugars, sucrose and fructose compared to repeated 24-h recalls in Adventist Health Study-2 participants. Nutrients 13, 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Joh HK, Lee DH, Hur J, Nimptsch K, Chang Y, Joung H, Zhang X, Rezende LFM, Lee JE, Ng K, Yuan C, Tabung FK, Meyerhardt JA, Chan AT, Pischon T, Song M, Fuchs CS, Willett WC, Cao Y, Ogino S, Giovannucci E, Wu K (2021) Simple sugar and sugar-sweetened beverage intake during adolescence and risk of colorectal cancer precursors. Gastroenterology 161, 128–142.e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS (2006) Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 27, 169–176. [DOI] [PubMed] [Google Scholar]
- [21].Yu L, Lutz MW, Wilson RS, Burns DK, Roses AD, Saunders AM, Gaiteri C, De Jager PL, Barnes LL, Bennett DA (2017) TOMM40’523 variant and cognitive decline in older persons with APOE epsilon3/3 genotype. Neurology 88, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA (2007) Physical activity and motor decline in older persons. Muscle Nerve 35, 354–362. [DOI] [PubMed] [Google Scholar]
- [23].Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT (2015) MIND diet slows cognitive decline with aging. Alzheimers Dement 11, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Malik VS, Popkin BM, Bray GA, Després JP, Hu FB (2010) Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121, 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malik VS, Hu FB (2019) Sugar-sweetened beverages and cardiometabolic health: An update of the evidence. Nutrients 11, 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Calsolaro V, Edison P (2016) Alterations in glucose metabolism in Alzheimer’s disease. Recent Pat Endocr Metab Immune Drug Discov 10, 31–39. [DOI] [PubMed] [Google Scholar]
- [27].Arvanitakis Z, Wang HY, Capuano AW, Khan A, Täïb B, Anokye-Danso F, Schneider JA, Bennett DA, Ahima RS, Arnold SE(2020) Brain insulin signaling, Alzheimer disease pathology, and cognitive function. Ann Neurol 88, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67, 505–512. [DOI] [PubMed] [Google Scholar]
- [29].Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU (2016) Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96, 1169–1209. [DOI] [PubMed] [Google Scholar]
- [31].Grillo CA, Piroli GG, Hendry RM, Reagan LP (2009) Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res 1296, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pearson-Leary J, McNay EC (2016) Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J Neurosci 36, 11851–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pearson-Leary J, Jahagirdar V, Sage J, McNay EC (2018) Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4. Behav Brain Res 338, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332, 145–159. [DOI] [PubMed] [Google Scholar]
- [35].Softic S, Stanhope KL, Boucher J, Divanovic S, Lanaspa MA, Johnson RJ, Kahn CR (2020) Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci 57, 308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bonnefont-Rousselot D (2002) Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 5, 561–568. [DOI] [PubMed] [Google Scholar]
- [37].Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, Li JS, Li M, Pan Y, Huang JJ, Kong LD (2015) Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22, 848–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MAP data can be requested at http://www.radc.rush.edu.